Abstract
For the first time, the analytical electron microscopy has been used to determine the solute concentration profiles left behind the moving reaction front (RF) of the discontinuous precipitation (DP) reaction in a Fe-13.5 at.% Zn alloy. These profiles have been converted into grain boundary diffusivity (sδDb) values, using Cahn’s diffusion equation in its original form and the data of the growth rate of the discontinuous precipitates obtained from independent measurements. This approach has essentially removed existing difference in comparison to sδDb values obtained from Cahn′s simplified and Petermann–Hornbogen models relevant for the global approach to the DP. Simultaneously, the local values of sδDb have been up to 8–10 orders of magnitude higher than the data for volume diffusion coefficients and much greater than for diffusion at the stationary grain boundaries of Zn in pure Fe. This is clear indication that the rate controlling factor for DP reaction in the Fe-13 at.% Zn alloy is diffusion at the moving RF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Discontinuous precipitation (DP) belongs to a group of solid state diffusive phase transformations occurring at the moving interface called the reaction front (RF). During DP reaction, a supersaturated solid solution α° decomposes into colonies of alternate lamellae of a new solute-rich β phase and solute-depleted α phase. The DP is relevant example of nano-phase transformations as the solute redistribution leading to creation and growth of the lamellar product takes place at the moving RF which is ca. 0.5 nm in width and maximal diffusion distance is equal to half width of the α lamella. Moreover, the thickness of α phase lamellae is usually in order of 100–500 nm and the thickness of β lamellae even 7–10 times smaller.
Fe–Zn system is very suitable for the examination of mechanism and kinetics of DP reaction as it shows a large range of solid solubility (up to 42 at.% Zn) and DP occurs in a wide range of ageing temperatures and solute concentrations. According to Predel and Frebel [1,2,3], only at relatively high temperatures located ca. 20–50 °C below solvus line, continuous precipitation (CP) is the only one way of precipitation. On the other hand, a competition between CP and DP occurred at relatively low temperatures for the Zn content more than 25 at.%. Therefore, iron alloys with different content of Zn were the subject of extensive studies in the past. One of the first information, showing the typical DP morphology, was delivered by Schramm and Mohrnheim [4] and Hornbogen [5]. A few years later, Speich [6] performed systematic studies for four FeZn alloys focusing mainly on kinetic aspects associated with verification of Cahn′s [7] theory of DP while Predel and Frebel [1,2,3], Parthiban and Gupta [8] extended the studies to discontinuous coarsening (DC).
A detailed examination of Fe-13.5 at.% Zn alloy with scanning electron microscopy (SEM) made by Chuang et al. [9, 10] revealed the go- and -stop motion and formation of so-called “ghost images” of the former successive positions of the RF and the places where the zin-rich phase was previously located during discontinuous dissolution (DD). Chuang et al. [10] summarized all the data concerning diffusivity at the moving RF of three main discontinuous reactions, namely DP, DC, DD with the conclusion that there is no substantial differences between the rate of diffusion processes at the stationary and migrating grain boundaries providing that the data for the stationary boundaries are selected properly. Some years later, similar approach was used by Gupta [11] who additionally investigated diffusion induced grain boundary migration (DIGM) and grain growth. Applying different models of kinetics of discontinuous reactions, he predicted that the diffusion of Zn occurs through grain boundaries during DP, DC, DD, DIGM and grain growth in Fe–Zn system.
Most of the examinations, summarised above, were performed using light and scanning electron microscopy on etched specimens applying also surface replica technique. A few results were obtained also with transmission electron microscopy (TEM) [5, 6] but without any information concerning the chemistry of DP product. Therefore, the present study was undertaken to get more details about the solute concentration profiles accompanying the growth of discontinuous precipitates in a Fe-13.5 at% Zn alloy.
2 Experimental
The Fe-13.5 at.% Zn alloy was obtained with iron (99.8% purity) and zin (99.99% purity) in an autoclave under an argon pressure of 1.5 GPa. The grain size (~ 1–2 mm) of obtained rods (10 mm × 9 mm in diameter) was too large for TEM studies. Therefore, the samples were subjected to thermal cycling at 723 K for 40 h followed by 5 h at 1073 K in quartz capsules under vacuum of 10 Pa. This procedure enabled to reduce the grain size down to 50–250 μm. Then, the rods were cut into slices of 1.5 mm in thickness and aged in an evacuated silica capsule.
The foils for the TEM examinations were prepared from the slices mechanically thinned to 0.25 mm from which discs 3 mm in diameter were cut through a spark erosion. The final operation was dimpling and ion beam thinning using a GATAN Duomill instrument. The microstructure investigation was carried out using a Philips CM 20 Twin transmission electron microscope operated at 200 kV and equipped with a EDAX Phoenix energy-dispersive X-ray spectrometer (EDX) enabling a high spatial resolution chemical analysis of the solute content in the DP products. Some of the results, specifically selected area electron diffraction patterns, were obtained by using TECNAI G2 FEG super TWIN (200 kV) TEM.
The local reaction front velocities for the DP were assessed from TEM images. To accomplish this, a two-step ageing was applied. After the first step, the DP was present in most of the grain boundaries. The second stage was performed at a temperature 50 K higher than the previous one for further growth of discontinuous precipitates but with significantly larger inter-lamellar spacing. Then, the growth distance of the second stage was measured neglecting the transition period associated with the change of interlamellar spacing. Only those colonies, in which the arrays of cells were perpendicular to the growing front of discontinuous precipitates were taken into account. For such colonies, the thickness of the α lamellae was also measured and the EDX analysis of the zinc concentration was performed across the α lamellae.
3 Results and discussion
Figure 1a presents a colony of Zn-depleted (α) and Zn-rich lamellae growing into a supersaturated solid solution. The nearly constant lamellar spacing over a long distance proves that the steady-state period of growth has already been reached. The selected area electron diffraction (SAED) patterns revealed that the Zn-rich lamella is the intermetallic phase Γ(Fe3Zn10) (Fig. 1b, c). Simultaneously, the existence of Γ1(Fe5Zn21) phase at temperatures below 823 K was excluded. This result was of importance as the EDX analysis in this case was inconclusive, i.e. the high scatter of zinc concentration made presence of both phases possible.
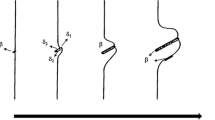
Figure 2a shows the results of two-step ageing used for assessment of the growth rate. The colonies with much larger inter-lamellar spacing are growing from primary colonies of DP developed at lower ageing temperature. Further ageing leads to an impingement of the colonies growing from different segments of the original grains (Fig. 2b) and termination of the DP process.
The decisive role of the grain boundary diffusion in the occurrence of DP reaction was confirmed by measuring the Zn-concentration profile across the reaction front (solid line in Fig. 3a). The characteristic abrupt change of Zn content (Fig. 3b) is the proof that the diffusion process leading to formation of the Γ phase is limited to a few nanometers or even less.
On the other hand, the measurements of Zn-content profiles within the α lamellae showed a characteristic reverse “U” shape, symmetrical with respect to the central axis of the α lamella (Fig. 4). This means that the growth of the single set of α and Γ lamellae was not disturbed by any crystallographic or chemical perturbation. Such measurements were made in five randomly selected α lamellae which did not belong to the same colony or grain. One should note that Zn content in the middle of the α lamella was noticeably higher than the concentration according to the solvus line in the equilibrium phase diagram [12]. The solid lines in Fig. 4 represent the least squares fit of the experimental data to the solution the solution of Cahn's [7] diffusion equation relevant for the DP reaction:
in which the dimensionless C parameter is expressed by:
Here, xo is the original solute content in the alloy, xe is the solute content in the α lamella in contact with the Γ lamella (assumed to be an equilibrium value), vins is the instantaneous growth rate of the discontinuous precipitates, λα is the thickness of the α lamella, and y is the normalised co-ordinate measured from the edge of the Γ lamella and running perpendicular to the α lamella.
The fitting procedure delivers values of the Zn-content at the α/Γ interface (xi) and C parameters given in Table 1. The acquired data indicate that the Zn content at the α/Γ interface (xi) does not correspond with the value for the α phase in equilibrium with the Γ phase (xe). This result is compatible with those obtained for the DP reaction in some other systems, like Al–Zn [13, 14], Mg–Al [15] and Ni–In [16]. One should emphasize here that all the EDX measurements were performed in the sufficient distance from α/Γ interfaces so that no X-rays were excited from the Γ phase. Moreover, the larger difference between xi and maximum Zn content in the middle of α lamellae (y(x = 0.5)) the higher C parameter. This means that an increasing part of each α lamella can form and grow at a smaller change in the composition from the initial state. In principle, the larger value of C parameter is attributed to the larger α lamella thickness (λα) which is in accord with the results obtained for Cu-4.5 at.% in alloy [17].
The scatter of the C values at the same temperature, changes of the α phase lamellae and growth rate indicate that the DP has to be considered as the local process relevant for an individual set of α and Γ lamellae which moves into the supersaturated solid solution with the instantaneous velocity vins. This confirms that the Cahn model can be applied in the form expressed by Eq. (1) with ultimate goal which is the determination of the diffusivity at the moving RF in terms of the triple product sδDb.
Until now, the information concerning the sδDb values in Fe–Zn system was derived applying global approach to the DP in which it is assumed that the it proceeds with an average velocity (vav) for an average α lamellae thickness (λα) and an average solute concentration (xav) within the α lamellae [2, 6, 10, 18,19,20]. This means that all the parameters represent average values for the whole population of the cells in the sample. In such a treatment, quantitative metallographic techniques are applied for the estimation of vav and λα, while X-ray diffraction analysis is used to estimate the value of the concentration xav.
The most frequently model which was used for the global approach to the kinetics of DP process in Fe-Zn system was that of Petermann–Hornbogen [21] who considered the dynamic equilibrium between the atomic jumps from one side of the RF to another. It means that the transfer of the atoms directly across the RF into α and Γ lamellae occurs much faster than that along the RF. This equilibrium is disrupted by the existence of a driving force. Statistical mechanical consideration of the jumps across the RF means that the velocity in the Petermann–Hornbogen (P–H) model describes the time-averaged behaviour of the system. This model leads to the following equation for the grain boundary (GB) diffusivity at the steady state period of the DP reaction:
where ∆G is the total driving force of the DP reaction, R is the gas constant, and T is the temperature of the process.
The second model departs from the integration of Eq. (1) for the average solute concentration xav in the α lamella.
After integration, the final result is given in the following form:
with
where QR is the saturation released due to DP reaction. Note, that right now the values of the α lamella thickness and the growth rate in Eq. (6) refer to the average values. Knowing xav from X-ray diffraction measurements, it is possible to find the parameter C by an iteration procedure and, in a consequence, the global value of the diffusivity for the average values of the growth rate and α lamellae thickness.
Determination of a local value for sδDb is straightforward once an optimum, local value of the parameter C has been determined by the fitting. The input data and results thus obtained are listed in Table 1. One can notice that the local diffusivities can vary up to a factor of 10 at the same temperature, whereas the experimental accuracy is expected to be 10–15% relative. This is mainly due to the fact that the kinetics of DP process is very sensitive as it concerns the GB characteristics. Recent examination revealed [22] that a large fraction of GBs did not undergo DP process, even with misorientations above 19°. Moreover, growth in one direction occurred for a relatively large fraction of GBs including all the GBs with special Coincidence Site Lattice misorientation. There was also no evidence that the GBs close to the special Σ misorientations exhibited a very low DP reaction rate. Last but not least is that determination of vins from in situ experiment failed due to Zn-evaporation from Γ lamellae during foil heating in the TEM which was confirmed by the EDX analysis. Therefore, the values of growth rate calculated based on the procedure applied in the present paper, even if it was done for short distance, may be influenced by the go- and -stop motion of the RF.
The calculated local sδDb values as well as data for the global approach using P–H [2, 10] and Cahn′s [6, 10] models are graphically presented in the form of an Arrhenius plot in Fig. 5. One can see that the local values of sδDb are located below the diffusivities calculated from the P–H model by Chuang et al. [10] for the alloy of the same composition. We should remark that the sδDb values reported by Chuang et al. [10] for the Fe-13 at.% Zn alloy are higher than for the alloys containing 17.6 [17], 20.0 [2], 22.16 [20] and 30 at.% Zn [2]. Therefore, they look to be rather overestimated and the trend set by the data for Fe-10 at.% Zn [6] alloy is more realistic.
Arrhenius diagram for chemical diffusion along moving reaction front of the DP reaction in FeZn alloys determined using Petermann–Hornbogen (P–H) [2, 10] and Cahn′s (C) [6, 10] models in comparison with results obtained in the present paper. The solid line is for diffusivities at stationary GBs of Zn in α-Fe [24]
Simultaneously, the local sδDb values are even two orders of magnitude higher than the diffusivities calculated by the use of global Cahn′s approach (Eq. 5) and the data for the Fe-13 at.% Zn alloy [10]. The discrepancy between the results of the global and local analysis using Cahn′s model is attributed to experimental errors. There are two main sources of errors. First one, is that the incubation period preceding the nucleation of Γ lamellae as well as non-cooperative growth of cells are not excluded in the measurements of growth distances. Also, it is not possible to distinguish still growing cells from those for which the growth was terminated. The second source, of lesser importance is that the determination of the average solute concentration in the α lamella (xav) obtained by X-ray diffraction included the areas of the non-cooperative growth stage.
One should emphasize, that the local sδDb values are ca. 10 orders of magnitude higher than the data for volume diffusion of Zn in α-Fe [23] after extrapolation to the temperatures less than 830 K and assuming δ = 5 × 10–10 m. They are also 2–3 orders of magnitude higher than GB diffusivities determined from diffusion couple experiments for the temperature range 623–998 K [24]-see solid line in Fig. 5. The differences up to four orders of magnitude are for the tracer diffusion of Zn in the stationary GBs of polycrystalline α-Fe [25] also extrapolated from temperatures above 865 K. This means that GB diffusion in the α phase of FeZn alloys is much faster than in pure iron. Similar tendency was found to be in the AlZn alloys containing from 4.33 to 49.4 at.% of Zn [26]. However, it does not mean that this diffusion is anomalously fast but that the ultimate conclusion can be made if the obtained data are compared to Zn tracer diffusion in FeZn alloys. Such data, unfortunately, are not available, as yet.
Finally, the slope of the curves which can be derived from the experimental points referring to the diffusivities for DP reported in Refs. [2, 6, 10] and the local sδDb values show the same tendency (Fig. 5). This means that the values of the activation energies for the DP should also be similar. Therefore, based on the above discussion, the clear message appears that the DP reaction in the Fe-13 at.% Zn alloy is controlled by the diffusion process at moving RF (which is considered as a high angle GB).
4 Conclusions
The application of high spatial resolution energy-dispersive X-ray microanalysis helped to determine the changes of the Zn concentration accompanying the formation of α and Γ lamellae during discontinuous precipitation (DP) reaction in the Fe-13.5 at.% Zn alloy.
The detected step-like change of the Zn concentration across the reaction front (RF) confirmed that the solute redistribution during DP is essentially limited to the RF. Furthermore, the measurements the Zn content across individual α lamellae in conjunction with a special two-step ageing procedure for the determination of the growth velocity of particular colonies made possible to use the Cahn′s diffusion model valid for the local treatment of the DP reaction. The resultant values of local diffusivities (sδDb) at the RF were more than two orders of magnitude higher than those obtained for the same model but using average or global values of the kinetic parameters. Simultaneously, the values of local sδDb were located just below the grain boundary diffusivities calculated based on the Petermann–Hornbogen global treatment of DP.
As the local values sδDb were almost ten orders of magnitude higher than respective values for volume diffusion and much greater than the rate of diffusion at the stationary grain boundaries of Zn in pure iron, one should conclude that there is no essential difference in the rate of the diffusion processes at the stationary and moving boundaries in the Fe-13.5 at.% Zn alloy.
References
Predel B, Frebel M. Die Gegenseitige Beeinflussung verschiedener Ausscheidungs-reaktionen in α-Fe-Zn-Mischkristallen. Acta Metall. 1972;20:1259–68.
Predel B, Frebel M. Zur Kinetik der feinlamellaren diskontinuierlichen Ausscheidung in α-Mischkristallen des Systems Eisen-Zink. Arch Eisenhüttenwes. 1972;43:839–44.
Predel B, Frebel M. Beitrag zur Klaerung des Ausscheidungsverhaltens der Alpha-Mischkristalle des Systems Eisen-Zink. Metall. 1973;27:460–6.
Schramm J, Mohrnheim A. Die Aushärtung von Eisen-Zink- und Kobalt-Zink-Legierungen. Z Met. 1948;39:71–8.
Hornbogen E. Two types of discontinuous precipitation in alpha iron solid solutions. Trans AIME. 1963;227:1411–8.
Speich GR. Cellular precipitation in Fe-Zn alloys. Trans AIME. 1968;242:1359–67.
Cahn JW. The kinetics of cellular segregation reactions. Acta Metall. 1959;7:18–28.
Gupta SP, Parthiban GT. Discontinuous precipitation and coarsening of lamellar cellular precipitate in Fe-Zn alloys. Metallography. 1988;21:11–32.
Chuang TH, Gust W, Predel B, Fournelle RA. Discontinuous coarsening and dissolution in an Fe-13.5 at% Zn solid solution. Mater Sci Eng. 1989;112:175–83.
Chuang TH, Fournelle RA, Gust W, Predel B. Drei diskontinuierliche Festkörperreaktionen in einer a-Fe-13.5 at-% Zn-Legierung. Z Met. 1989;80:318–26.
Gupta SP. A comparative study of the kinetics of interface diffusion controlled transformations in Fe-Zn alloys. Can Metall Q. 2001;40:127–42.
Burton BP, Perrot P. Fe-Zn (iron-zinc). Phase diagrams of binary iron alloys. Materials Park: ASM International; 1993. p. 459–466.
Solorzano IG, Purdy GR, Wheatherly GC. Studies of the initiation, growth and dissolution of the discontinuous precipitation product in aluminium–zinc alloys. Acta Metall. 1984;31:1709–17.
Zięba P. Recent progress in the energy-dispersive X-ray spectroscopy of the discontinuous precipitation and discontinuous dissolution reactions. Mater Chem Phys. 2000;62:183–21313.
Duly D, Cheynet MG, Brechet Y. Morphology and chemical nanoanalysis of discontinuous precipitation in Mg–Al alloys—I regular growth. Acta Metall Mater. 1994;42:3843–54.
Alexander KB. The growth kinetics of cellular precipitation, Ph.D. thesis, Carnegie-Melon University, Pittsburgh, PA; 1985.
Lopez GA, Zięba P, Gust W, Mittemeijer EJ. Discontinuous precipitation in a Cu –4.5 at-%In alloy. Mater Sci Technol. 2003;19:1539–45.
Nakkalil R, Gupta SP. Kinetics of discontinuous dissolution in an Fe-20 wt.% Zn alloy. Z Met. 1989;80:37–47.
Budurov S, Russev K, Zlateva G, Petrov R. Die Kinetik der diskontinuierlichen Ausscheidung in ferritischen Eisen-Zink-Legierungen. Z Met. 1973;64:372–6.
Gupta SP, Parthiban GT. Kinetics of discontinuous coarsening of cellular precipitate in Fe-Zn alloys. Z Met. 1985;76:505–11.
Petermann J, Hornbogen E. Drei Mechanismem der Ausscheidung in Blei-Natrium-Mischkristallen. Z Met. 1968;59:814–22.
Zięba P, Faryna M, Chronowski M. Combined in situ and EBSD studies of discontinuous precipitation in Al-22 at%.Zn alloy. Mater Char. 2019;157:109889.
Budurov S, Kovatchev P, Kamenova Z. Chemical diffusion of zinc into γ- and α-iron. Z Met. 1973;64:652–4.
Dohie JS, Cahoon JR, Caley WF. The grain boundary diffusion of Zn in α-Fe. J Phase Equilib Diffus. 2007;28:322–7.
Herzig C, Neuhaus P, Geise J. Solute diffusion in the grain boundary. In: Saimoto S, Purdy GR, Kidson GV, editors. Solute-defect interaction: theory and experiment. Toronto: Pergamon Press; 1986. p. 271–280.
Hässner A. Untersuchung der Korngrenzendiffusion von Zn-65 in α-Aluminium-Zink-Legierungen. Kristall Tech. 1974;9:1371–88.
Acknowledgements
The research was financially by the National Science Centre in Poland (Grant Mass transport during phase transformations at migrating boundaries of discontinuous precipitates-experiment vs. modelling, OPUS No. 2017/25/B/ST8/02198). Part of the research was performed within the Accredited Testing Laboratories possessing the certificate no. AB 120 issued by the Polish Centre of Accreditation according to European standard PN-ISO/IEC 321 17025:2005 as well as the EA-2/15.
Funding
Research was supported by the National Science Centre in Poland, Grant No. OPUS No. 2017/25/B/ST8/02198. All the authors received financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Ethical statements
As a corresponding author, on behalf of all the manuscript authors, I confirm that: the submitted work is original and it has not been published elsewhere in any form or language (partially or in full). The submitted work has not been submitted for consideration elsewhere, simultaneously. All the authors who contributed to the manuscript are familiar with its content and approve it.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zięba, P., Chronowski, M. & Morgiel, J. Micro-analytical studies of discontinuous precipitation in Fe-13.5 at.% Zn alloy. Archiv.Civ.Mech.Eng 20, 76 (2020). https://doi.org/10.1007/s43452-020-00082-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43452-020-00082-6