Abstract
Frequently, preparative high-performance liquid chromatography separations of complex natural product mixtures by adsorption chromatography are erratic to achieve full baseline separation. Purification of metabolites with similar or identical polarity, such as epimers, diastereoisomers, homologs in a series, and geometric or positional isomers, by a single chromatographic run, is not properly achieved. Consequently, recycling preparative high-performance liquid chromatography has been proposed with a closed-loop recycling valve designed to increase the capacity of separation of mixtures of low-resolution peaks by a series of consecutive passes through the same column. Thus, the sample zone is basically recycled back into the column to continue the separation process in a closed-loop system. A consequence of this recycling mode is the increment in the number of theoretical plates with each cycle and maintaining a minium peak dispersion in the resulting chromatogram with no additional solvent needed for recycling. Pure samples are collected when the baseline resolution is achieved. Therefore, compounds with comparable physicochemical characteristics are fully separated to provide pure single chemical entities suitable for structure elucidation and further biological assessments. This review article examines the essential fundaments of this efficient method and its advances in isolation of natural products that have made the laborious purification processes less demanding and less time-consuming. Several applications that show the purification of natural products from small to large bioactive natural molecules by recycling preparative high-performance liquid chromatography are revised.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural product chemists commonly confront serious problems during the isolation of specialized metabolites or natural products with comparable or equal polarity. Preparative high‐performance liquid chromatography (prep-HPLC) has been in use for separation and purification of natural products for high purity, resolution, and selectivity from complex mixtures due to the use of uniform stationary phases with small particle sizes of 5–20 μm (Latif and Sarker 2012; Niculau et al. 2022). Unfortunately, prep-HPLC requires the use of long columns and large amounts of solvents. Nevertheless, there are many examples for which a single prep-HPLC chromatographic run is erratic to achieve full baseline separation. In most of these cases, they include the separation of isomers (epimers, diastereoisomers, and geometric or positional isomers), homologs in a series, and structurally related or unrelated natural products by adsorption chromatography because the components in these mixtures have similar physicochemical properties. In addition, during the preparative separation of natural product mixtures, where peak systems are overloaded, achieving baseline separation can be practically impossible. Consequently, recycling chromatography was proposed and recycling preparative HPLC with a closed-loop recycling valve (Fig. 1) was designed to repetitively recirculate unresolved peaks into the same column in order to increase the capacity of separation, although maintaining a minium peak dispersion in the resulting chromatogram to purify compounds with comparable physicochemical characteristics and, therefore, a comparable retention time. This technique uses short columns and requires considerably less quantities of solvent for higher purity, resolution, and generous recovery yields of the target compounds, as well as being able to separate minor bioactive constituents with close similar structures.
Representation of a recycling HPLC system with a closed-loop recycling valve to recirculate unresolved peaks into the column with a semipreparative or preparative scale. Reproduced from Schlinge et al. (2010) with a license provided by Elsevier
Recycling chromatography has the following features (Fig. 2): (i) reduced column costs, as this process has the same separation capacity as using longer or multiple columns; (ii) reduces environmental loads and costs, because the same mobile phase solvent is used repeatedly during the whole separation process and, thus, reducing the overall solvent consumption; and (iii) achieves effective preparative purification since each constituent of the mixture is fractionated and collected only after improving the baseline separation.
Comparison of preparative HPLC systems equipped with a uniform stationary phase column of small particle sizes (5–20 μm). A When using a short colum. B When using a long column which can produce high back pressures. C Recycling technique when the sample flows through the same column repeatedly with no solvent consumption. The use of a 1/3 length of a long column, but in a recycle sequence of 3 times, the result is the same as using one long column with half of the solvent consumption. Reproduced from https://www.jai.co.jp/english/catalog/pdf/HPLC_e_2022.pdf
With the improvement in smaller particle sizes for the packing materials in columns, which has resulted in an enhanced performance of liquid chromatography, attention in recycling preparative chromatography has declined in parallel to the increasing interest in superficial particles for the purification of natural products. This situation explains the reduced number of applications of recycling prep-HPLC (Gritti 2021), which has been embraced by the pharmaceutical industry but overlooked by academia.
Reversed-phase HPLC (RP-HPLC) is the most widespread preparative mode for the purification of natural products, which accounted for more than 90% of all purifications. An approximation of the number of natural products purified by recycling prep-HPLC would be close to less than 0.01% of over 2,140,000 of known secondary metabolites (Thirumurugan et al. 2018). This procedure needs to be popularized among natural product chemists, so that the problems involved in the separation and purification of secondary metabolites may decrease since there is a natural tendency during the isolation of secondary metabolites to obtain complex mixtures of enantiomers, epimers, diastereoisomers, and regioisomers which might be unresolvable by conventional prep-HPLC. Thus, the isolation procedures demand additional efforts for the purification process of these complex mixtures to be completed and yield single pure chemical entities (purity > 99%). If a comparison is made between the conventional prep-HPLC separations of isomers and structurally related compounds and purification by recycling prep-HPLC, the latter offers higher resolution by significantly reducing the acquisition time per sample, i.e., amount of eluted material per unit of time (Majors 2004).
One noteworthy example is the application of recycling prep-HPLC coupled to a refractive index (RI) detector during the purification of individual bioactive acylsugars from the complex mixtures of morning glories resin glycosides (Pereda-Miranda et al. 2010), which reflect the hyperdiversity of acylsugars created by promiscuous acylating enzymes during the biosynthesis of these specialized metabolites (Kruse et al. 2022). The refractive index detector is the best choice if UV and fluorescent detectors are insensitive to poor UV-absorbing compounds, such as sugars, triglycerides, and lipids (Al-Sanea and Gamal 2022). Up to now, the application of recycling prep-HPLC for the purification of resin glycosides accounted for almost the 85% of all the natural products isolated by this technique.
In the present article, several chemical applications, mostly phytochemicals, that show the purification strategies of small to large bioactive natural molecules from complex mixtures by recycling preparative high-performance liquid chromatography, are reviewed. Accordingly, this review could help readers in the fields of phytochemistry, pharmacognosy, medicinal chemistry, phytopharmaceutical biotechnology, biochemical pharmacology, glycosciences, and the pharmaceutical industry, among others, for selecting a correct combination of approaches when confronting a specific problem during the isolation of bioactive natural products with comparable or equal polarity.
Search Strategy
The keywords used in the Google Scholar search, with recycling preparative liquid chromatography being present in all combinations, were natural product purification, high‐performance liquid chromatography, recycling chromatography, adsorption chromatography, and recycle valve. Relevant reviews and digital documents in English language were consulted to collect all existing literature to deliver an updated survey for the topic recycling prep-HPLC for the purification of natural products from 2000 to complete the information previously reviewed by Sidana and Joshi (2013).
Discussion
Recycle Chromatography Systems
The first reports on recycle chromatography occurred 70 years ago due to the fact that the particle sizes (30–100 µm) of the stationary phases during the middle of the past century could not overcome the resolution of some complex matrices to avoid the high cost and backdrop pressure requirements of long columns packed with non-uniform stationary phases (Gritti 2021); therefore, the recycling technique was developed with the purpose of increasing the column resolution. For recycling chromatography, two essential systems have been developed: single-column closed loop and alternate two-column switch recycling (Fig. S1). In 1959, the first and simplest recycling system was developed as a closed-loop (Fig. S1a), where the sample is recycled through the same chromatographic column by passing through the pump and the detector with the support of a recycling valve, simulating the use of a longer column (Porter and Johnson 1959). Bailly and Tondeur (1984) recommended different applications of recycling by separation of binary mixtures by ion-exchange chromatography and showed its potential advantages since extensions of these procedures to other chromatographic modes are straightforward (Baily and Tondeur 1982, 1984).
In order to overcome the significant band broadening caused by the pump, Biesenberger and collaborators (Biensenberg et al. 1971) described an alternate pumping recycle chromatography where two identical columns are connected through a multiport valve, and the solute bands are circulated in a closed-loop of the two columns by switching the valve without flowing through the pump (Gritti et al. 2017a, b; Wei et al. 2019). If further purification is required, the analyte can be passed back from the second column to the first column by means of the valve before the pure sample be collected. This process could be repeated as many times to complete the sample purification (Kimata et al. 2019). In this alternate column recycling, the analyte only passes in one occasion through the pump (Fig. S1b). As a result, the separation will be constantly enhanced as the number of cycle increases. In the next section, a brief historical background for the technique optimizations of the recycle chromatography systems is discussed with special reference to the purification of natural products.
Technique Optimizations
Single-Column Closed-Loop Recycling
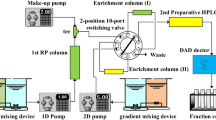
This is the simplest version that was developed, where the sample passes through the column, the pump, and the detector, while the sample constituents are separated and purified as they are recycled through the chromatographic column by an additional multiport valve that is included between the detector outlet and pump inlet (Fig. 1). Any preparative HPLC system could be adapted by connecting a recycle valve (available from different suppliers) in which an eluted selected unresolved peak is dispensed back into the same column repeatedly until the desired separation is accomplished. The mobile phase could also be directed into waste or could be recirculated (Fig. 1). Thus, the chromatographic separation takes place in the enclosed volume. The pressure developed for the mobile phase is almost constant and the dead volume in the recycle lines causes band spreading. It is important to mention that no fresh solvent is required in the conventional recycling mode (Fig. 2) and the sample is diluted in each volume of solvent, which each time generates the broadening of the band (Fig. 3). The selection for an adequate polarity of the solvent systems is not important to achieve the separation since the resolution by recycling chromatography depends only on the increment in theoretical plates (number of cycles). However, it is essential to select a mobile phase that provides a retention time of 10–20 min for the selected unresolved peak originally injected to avoid a time-consuming recycling process. In the chromatogram, each cycle would be identified by the injected unresolved mixture to be split into its constituents, or totally resolved, according to the number of passes in the column but maintaining the same retention time throughout the process (Fig. 3).
Separation of two low-resolution peaks (a and b) in a mixture of closely related natural products (5:1) by column overloading and recycling prepartaive HPLC. Column overloading is useful for the separation of minor components by application of peak saving and heart-cutting methodologies. A cut of the overloaded sample zone gives an enriched fraction in major compound a. After the first recycle of this fraction, the separation of the two constituents is satisfactory to collect minor peak b. When the eluate is returned to the column, peak a can then be isolated in pure state by achieving resolution with a sequence of separation cycles to eliminate a trace impurity (asterisk)
A closed-loop system can be operated in conventional elution or recycling mode, using the peak shaving technique or multiple feed injection followed by peak shaving (Fig. 3). If two peaks are involved, and there is some broadening, the techniques of peak shaving and peak cutting for recycling could be applied to avoid overlapping of the peaks, where the leading and tail ends of two merged peaks are collected as pure components, and the center merged portion is passed through the column to be recycled as many times as needed to be separated and purified by elimination of trace impurities. The sample is collected in the last cycle corresponding to a peak of higher purity with a Gaussian behavior (Fig. 3).
Chemical studies began to apply conventional recycling HPLC and its variant since the early 1980s for isolation of natural products. Closed-loop recycling has been the most widely used approach for preparative purifications with its modification of external recycle chromatography that includes the reinjection of unevaporated impure eluted fractions, as a single-volume overload injection, back onto the original column to enhance the total recovery and purity of sample components from a separation of adjacent overlapping peaks (Crary et al. 1989). Recycling in a single column means that only certain parts of the elution profile including the target components may be repeatedly recycled until the required separation is reached. Consequently, the separation efficiency is limited to samples containing a limited number of constituents. For the purification of complex mixtures, they must be prepared by the multiple pre-procedures mentioned above to inject each target fraction independently.
Early application of recycling HPLC in plant chemistry used normal phase chromatography with silica cartridges. Mixtures of isomeric labdadienes and labdatrienes were resolved by extensive use of the peak shaving-recycling (i.e., collect-recycle-collect) technique even though the HPLC chromatogram showed only one peak for all isomers in each case. There was a 98% recovery of starting material and, since hexane was used as the only solvent system, it was also recycled after each recycling routine (Mohanraj and Herz 1981). Tylolupenols A and B, pentacyclic triterpenoids, from the Chinese Vincetoxicum kerrii (Craib) A.Kidyoo, Apocynaceae, were purified by recycling HPLC in silica gel (mPorasil) with hexane saturated with H2O-i-propylether-i-propanol (97.75:2.00:0.25) (Kawanishi et al. 1985). The sesquiterpene isomeric mixture of curcumanolides A and B from the stem-distilled volatile oils of the roots from Curcuma heyneana Valeton & Zijp, Zingiberaceae, was resolved in a silica gel column with CHCl3 after a recycle sequence of 95 times (Firman et al. 1988). Likewise, guaiane-type sesquiterpenes characterized by the presence of a hydroxyperoxy function, were isolated from the wood of Viburnum awabuki K.Koch, Viburnaceae, and purified by recycling HPLC in silica with CHCl3 (Fukuyama et al. 1996).
What started in the early 1990s, close loop recycling in a reversed-phase chromatography with a C-18 column (ODS type), in which octadecyl groups are bonded to a silica base to deliver wide-ranging separation applicability, has become the most extensive system used for the purification of small natural products (see sections “Reversed-Phase HPLC” and “Applications in Natural Products”). The following chemical studies constitute examples of the initial applications of reversed-phase recycling chromatography: 4-quinolinone alkaloids from Esenbeckia leiocarpa Engl., Rutaceae, with antifeedant activities against the pink bollworm, Pectinophora gossypiella, were purified by recycling in a C-18 column with MeOH-H2O-MeCN (50:25:10) (Nakatsu et al. 1990). Purification of glycosphingolipids from the marine annelid Neanthes diversicolor was performed by means of recycling on a preparative C-18 column using MeOH-CHCl3 (10:1) (Naoki et al. 1993). Methyl 4-hydroxy-3-(3′-methyl-2′-butenyl)benzoate, the major insecticidal principle from Piper guanacostense C.DC., Piperaceae, was purified from its EtOH transesterification artifact by recycling through a semipreparative C-18 column with CH3CN-H2O (7:3) (Pereda-Miranda et al. 1997). The hepatotoxic mixtures of hydroxystilbene tetramers, vitisin A and cis-vitisin A, from Vitis coignetiae Pulliat ex Planch., Vitaceae, were resolved by recycling HPLC on a C-8 (octyl) column with MeOH-H2O (3:2) (Ito et al. 1998).
Finally, polyvinyl alcohol columns were also used for size exclusion chromatography to achieve the purification of phytoecdysteroids (Zhang et al. 1992) and anthraquinone glycosides (Kubo et al. 1992). Two polystyrene columns in tandem with CHCl3 (Fig. S2A) were unsatisfactory to separate the closely related macrolide alkaloids cathedulines E3, E4, and ES with insect growth inhibitory activity from the MeOH extract of the aerial parts of Catha edulis (Vahl) Endl., Celastraceae. However, recycling chromatography on polyvinyl alcohol resin column and methanol as an eluent was appropriate to provide the necessary resolution to achieve total purity of individual alkaloids. As illustrated in Fig. S2B, a principal peak with two shoulders was detected during the first pass through the polyvinyl alcohol column. However, after recycling two times, three individual peaks were observed which were resolved by applying the peak shaving-recycling technique. A single injection (95 mg) of the alkaloidal fraction was resolved within 5 h. Catheduline E5 (15 mg) was isolated after two cycles. Cathedulines E3 (43 mg) and E4 (22 mg) were purified after a third cycle (Kubo et al. 1987). An alternative closed-loop recycling technique with a periodic intra-profile is a binary chromatographic separation where the sample is injected into the interior of the circulating chromatographic profile and fractionation is achieved by collecting the leading and trailing edges during each cycle (Grill 1998). Closed-loop recycling is the most frequently used technique for natural product isolation (Sidana and Joshi 2013; Rohaity et al. 2017; Chen et al. 2019; He et al. 2020; Li et al. 2021).
Column Switch Closed-Loop Recycling
A variant of closed-loop recycling is the column switch recycling methodology but with the use of two coupled columns, where the sample passes from one column to the other through a recycling valve (Fig. S1b). The sample does not pass through the pump, and this can be used with isocratic or gradient mode, for the latter two valves are used (Wei et al. 2021). The impurities are removed using one two-position multi-port valve during the initial gradient step and the baseline resolution is guaranteed using a second two-position multi-port recycling valve (Gritti 2021). This technique has also allowed the separation of proteins in a wide molecular weight range by closed-loop recycling in a size exclusion chromatographic system (Yuan et al. 2009). Ten anthocyanins from red cabbage were isolated by recycling preparative HPLC using two twin Waters Sunfire preparative columns, which were used and manually switched by a 10 port-2 position valve. So, the unwanted low retention constituents were excluded, and the compounds of interest were redirected to column 2, until sufficient separation achieved (Chen et al. 2018).
On-column stopped-flow bidimensional recycling was designed for enantiomeric separation of chiral drugs as a variant of column switch recycling. In a chiral column, the two enantiomers are initially separated, and the pharmacologically active isomer is collected; then, the mobile phase flow in the chiral column is stopped and the other inactive isomer is trapped in the achiral column by switching the second valve, which was filled with an appropriate solvent where the sample undergoes acid catalyzed racemization. Then, the enantiomeric mixture is pushed back by the first valve to the chiral column at appropriate time where separation occurs. This process is repeated to obtain an enantiomeric enrichment to obtain about 95% of the pure pharmacologically active isomer. The procedure could be applied both in reverse-phase mode and in normal-phase mode (Cannazza et al. 2009, 2011). Until now, there are no reports on the separation of natural products by this technique.
Reversed-Phase HPLC
RP-HPLC is a technique using surface-modified silica, R(CH3)2OSi-OSi(OH)(CH3)2, where R is a covalently bonded alkyl group, such as C-4 (-C4H9), C-8 (-C8H17), C-18 (-C18H37), phenyl-hexyl, cyano (CN(CH2)3-), or amino (NH2(CH2)3-), to the surface of silica particles or closely related materials used as the stationary phase in order to create a non-polar hydrophobic stationary phase. The mobile phase is very polar and composed with water or mixtures of water with water-miscible polar organic solvents, such as methanol, acetonitrile, isopropanol, or tetrahydrofuran (their elution strength increases in this order). Isocratic elution with a fixed proportion of solvents is the most commonly used method at the semipreparative and preparative levels. Column characterization and selection systems in RP-HPLC have been recently reviewed (Žuvela et al. 2019). The analyte retention time increases with the increment of the (i) hydrophobicity of the solute, (ii) hydrophobicity of the stationary-phase surface, and (iii) polarity of the mobile phase. The interaction of the analyte and the stationary phase results in a reduction of the total hydrophobic area exposed to the mobile phase which represents the driving force for the reversed-phase retention (for a recent example, see Moreno-Velasco et al. (2024)).
Two physicochemical phenomena are involved in the course of separation, i.e., the partitioning process when the analyte molecules are totally immerse into the bonded phase and/or adsorption phenomenon at the bonded-phase/solvent interface. In addition, the logP value, the pKa value of the analyte, chromatographic parameters like mobile phase pH, and organic modifiers also influence the retention and selectivity of the analyte. It was found that hydrophobic, electrostatic, and hydrogen bonding and other specific interactions between the stationary phase and the solutes, along with the hydrophobicity of a molecule (logP), modify the retention behavior of the analytes. While the overall retention mechanism remains the same, subtle differences in the surface chemistries of the different stationary phases will lead to changes in selectivity. The mechanisms of retention and various factors that influence the retention behavior of analytes were recently revised with suitable examples by Ganesh et al. (2022). In addition, the basic principles of semi- and prep-HPLC (Surve et al. 2023), as well as several applications for the isolation of natural products from plants, bacteria, fungi, and marine organisms, were recently reviewed (Niculau et al. 2022; Queiroz et al. 2024).
Recycling Prep-HPLC
Scaling up the selected analytical conditions to prep-HPLC can be a relatively easy task if the only variables to be enlarged are the diameter and/or the length of the column. A direct linear scale-up can be achieved using the following equation:
where L is the length and Ac is the cross-sectional area of the (P) preparative and (A) analytical columns.
The direct scale factor allows the calculation of the scaled up flow rate and an estimation of the amount of sample that can be injected into the preparative column. Changes between the analytical and preparative systems require some modifications to be made at the preparative stage to achieve the optimal separation and avoid the development of high column backpressure. This situation could require a reduction of the flow rate and/or a change in the composition for the eluation mixture to maintain backpressure down. It is important to consider that in order to execute the calculations for the scale-up factors correctly, the analytical and preparative columns must contain the same stationary phases with equal particle sizes and preferably manufactured by the same company. The composition of the mobile phases must also remain the same. Concentration overload is normally used to inject a greater amount of sample (Niculau et al. 2022). Column overloading increases the capacity for peak detection and preparative-scale recycling work. If low-resolution peaks are sufficiently close together with minimal peak broadening and the preparative system is fitted with a recycle valve, the peaks can be passed through the column again and further separated as a consequence of the increment in the number of theoretical plates. Recently, state-of-the-art trends in preparative chromatography, which included gradient transfer from analytical to preparative scale HPLC, for the purification of natural products were reviewed (Queiroz et al. 2024).
Recycling prep-HPLC is used in the isocratic elution mode and low solvent flows are generally applied. In addition, recycling chromatography cannot proceed beyond the point where the sample is spread over the whole column. Further recycling from the optimum number of cycles would lead to a reduction in resolution by the overlapping of successive cycles and peak broadening. Application of peak shaving may further improve the performance of recycling chromatography since the overlap between the peaks stemming from two consecutive cycles can be avoided or delayed and, consequently, the purification can be enhanced in a cost-effective way (Teoh et al. 2003). Solvent gradients have been developed to solve purification problems encountered in the pharmaceutical industry for reinforcing the isolation of minor impurities in drugs (Gritti 2021; Wei et al. 2021). Recycling prep-HPLC is widely used in organic synthesis of therapeutical substances to separate impurities from target compounds and to collect large volumes of active ingredients in pharmaceutical developments (Gritti et al. 2018; Lamotte et al. 2017). Recycling prep-HPLC has also been applied during the isolation of natural products (Sidana and Joshi 2013).
Scale-up to Recycling Prep-HPLC
Some protocols for scaling up instrumental conditions to prep-HPLC in natural product isolation have been reviewed (Majors 2004; Latif and Sarker 2012). An isocratic scale-up method for recycling prep RP-HPLC to isolate resin glycosides (acylsugars) from Ipomoea purga Hayne, Convolvulaceae (Mexican jalap root), with laxative activity is shown in Fig. S3 to illustrate this process.
Reversed-phase chromatography with C-4, C-8, and C-18 has a stronger affinity for the hydrophobic portions in these amphipathic acylsugars, such as the aglycone (mono- and dihydroxylated C14-C18 fatty acids) and esterifying residues of the saccharide cores, which is evident from the time that the analyte spends in the column as a function of the hydrophobicity of each constituent of the resin glycoside matrix. Therefore, the longer retention time only depends on the adsorption via hydrophobic interactions. The stronger the interaction, the higher the resulting retention time (Moreno-Velasco et al. 2022, 2024).
Reversed-phase semiprep-scale up recycling HPLC afforded retention times that reflected the interaction of each analyte with the C-18 chains attached to the surface of silica. The initial optimized analytical conditions used to achieve the resolution of the resin glycosides from the jalap root were as follows (Fig. S3A): a Symmetry C-18 Waters column (4.6 × 250 mm, 5 µm), a mobile phase of MeOH at a flow rate of 0.4 ml/min with a refractive index detector and a sample injection volume of 1 ml (sample concentration, 0.1 mg/ml). Once these analytical conditions were found, they were extrapolated at the semipreparative level by the calculation of the scale flow rate and an estimation of the amount of sample to be injected without losing the appropriate resolution for the chromatogram to achieve the isolation and purification of the major individual constituents in each of the collected peaks. Scale-up was performed on a semiprep Symmetry C-18 Waters column (19 × 300 mm, 7 µm); mobile phase, MeOH; flow rate, 8.0 ml/min; and the sample injection volume of 500 ml (sample concentration, 0.1 mg/ml) (Fig. S3B). The following retention times for the hydrophobic purginoside series, pentaglycosidic macrolactones with the same oligosaccharide core, operculinic acid A, and their differences occur in the acylating residues at the positions C2, C3, and/or C4 of the last external rhamnose unit with units of (2S)-methylbutyric (mba), n-hexanoic (hexa), and trans-cinnamic acids (Cna), were recorded as follows: tR 22.0 min for purginoside I (1) and IV (4), 23.0 min for purginoside II (2) and 26.6 min for purginoside III (3), the retention properties of these analytes were detected as a linear function of the number of acylating substituents and the number of methylene groups in the fatty acid residues (Fig. S3B). Recycling of the selected unresolved peak (tR 20 min) for over 18 cycles assured 100% purity for purginoside IV (4) with a retention time of 345 min after the whole recycling process, while purginoside I (1) corresponded to the collected minor peak at 150 min by heart-cutting (Fig. 4). The purgin series (5–7) eluated first due to an increase in the hydrophilic area as a consequence of their ester-type dimeric structure with tR 16.6 min for purgin II (6), 18.3 min for purgin III (27), and 19.9 min for purgin I (5). Consequently, the longer the esterification groups, i.e., n-dodecanoic (dodeca) vs. n-decanoic (deca), the deeper the penetration into the ligand layer of the reversed phase.
Recycling semiprep-HPLC for the resolution of a fraction containing two related resin glycosides from the Mexican jalap root, Ipomoea purga. The unresolved peak (tR 20 min) was recycled to purify a major component by heart-cutting and elimination of impurities (asterisk) with a Waters C-18 Symmetry column (19 × 300 mm, 7 µm), a mobile phase of MeOH-CH3CN (9:1), and a flow rate of 8.0 ml/min for over 15 cycles to assure 100% purity for purginoside IV (4) with a retention time of 345 min after the whole recycling process. Purginoside I (1) corresponded to the collected minor peak at 150 min by heart-cutting. The purification process continued by recycling the remaining peak and the use of peak saving as indicated by the vertical longdashed lines, where the leading and tail ends were directed to waste, while the center portion was recycled

Structural elucidation of metabolites is always performed off-line following recycling prep-HPLC purification (Pereda-Miranda et al. 2010). Once the purification process is accomplished to yield single chemical entities, their structural elucidation and absolute configuration determinations are accomplished by the combination of NMR (Breton and Reynolds 2013), MS (Demarque et al. 2016), and chiroptical spectroscopy (Pereda-Miranda et al. 2023). Then, pure isolated compounds could be submitted to biological evaluations. For example, purgin II (2) enhanced vinblastine activity > 2140-fold when incorporated at 25 μg/ml against resistant human breast carcinoma cells (MCF-7) overexpressing glycoprotein (Pg-p) (Castañeda-Gómez et al. 2013).
Applications in Natural Products
Glycans
Glycoconjugates comprise complex carbohydrates, or glycans, linked to a protein, lipid, peptide, and secondary metabolites. They are the most profuse and multipurpose biopolymers mostly used for energy production and as structural materials by plants. Glycans are built by monosaccharides linked through glycosidic linkages. Significant biological roles of complex carbohydrates, carbohydrate polymers, and glycoconjugates, and their interactions with other biomolecules, such as carbohydrate-protein and carbohydrate-carbohydrate interactions, have been comprehensively documented (Bucior et al. 2009; Zeng et al. 2012). These interactions control and modulate a variety of cell processes, such as differentiation, proliferation and adhesion, inflammation, as well as the immune response (Townsend 2023) and as a main force to initiate cell-cell recognition (Bucior and Burger 2004). The great diversity of biochemical activities of glycoconjugates is a direct consequence of their three-dimensional architectural variability with a high conformational flexibility, exhibiting branched or linear arrangements, broadly ranging in oligo- and polysaccharide core sizes, and with the highest diversity of building blocks (sugar cores) of any other natural biopolymer (Perez and Makshakova 2022).
Successful purifications of glycoconjugates are extremely demanding in RP-HPLC since these polar compounds are commonly not sufficiently retained by the common stationary phases, such as alkyl-linked chromatographic packings ranging from methyl (C-1) to octadecyl (C-18) with a water/acetonitrile mobile phase. For closely related carbohydrates, such as anomers or regioisomers, an increase in peak broadening with a direct-pump design (a single column with a closed-loop recycling valve) may certainly not permit the complete separation from undesired impurities.
Three approaches have been developed to overcome these problems. Initially, an alternate-pump recycling HPLC increases the number of theoretical plates by “recycling” the glycan mixtures between two columns with a multi-port switching valve (Fig. S1B) without passing back through the mobile-phase solvent pump (Alley et al. 2013). A second method includes the use of hydrophilic interaction chromatography (HILIC), which was introduced for the separation of complex carbohydrates by the partition of analytes between hydrophilic stationary phases (polar: amino and amide) and a hydrophobic mobile phase, i.e., high proportion of acetonitrile (60–97%) and a low proportion of water or a volatile buffer (3–40%). The mechanisms involved in HILIC separation are somewhat complex, predominantly involving hydrophilic partitioning, dipole-dipole interactions, hydrogen bonding, and electrostatic interactions. Recently, a compilation of stationary phases used in HILIC has been published (Guarducci et al. 2023). The isolation of pure natural glycans and derivatives from complex biological matrix, including common glycoproteins and human breast milk, through the application of recycling HPLC equipped with twin columns in the HILIC mode of separation (amide-80 columns consisting of nonionic carbamoyl groups that are chemically bonded to the silica gel), have been described (Alley et al. 2013; Daniel and Nicola 2016). Isocratic mode separations with water/acetonitrile mobile phase and the use of an alternate-pump recycling HPLC method have been developed to allow purification of protected carbohydrates at levels of 99.5% purity, where the C-5 stationary phase was far inferior to either a pentafluorophenyl stationary phase for the resolution and fractionation of protected monosaccharides or a phenyl hexyl stationary phase for protected oligosaccharides (Nagy et al. 2016). A review of the chromatographic modes for the purification of protected and deprotected/natively unprotected carbohydrates was published by Nagy et al. (2017). Finally, based on the accomplishment with recycling HPLC in combination with HILIC modes, the application of recycle HPLC on size exclusion mode was introduced and greatly improved the resolution of glycans (Zhu et al. 2020). For example, the complete separation of human milk glycans with a single fucose difference using recycling size exclusion chromatography was outstanding, as illustrated by the resolution of a fraction containing three neutral oligosaccharides (tetra-, penta-, and hexasaccharides) by the use of a preparative Agilent PL aqua gel-OH 20 (100 Da–20 kDa range) column (300 mm × 25 mm, 8 mm). Resolution of the three major peaks was improved in relation to the increment of cycles through the column (Fig. S4). Baseline separation of the three constituents was achieved and was individually collected and further analyzed by MALDI-MS (Zhu et al. 2020).
Numerous glycans and carbohydrates contain a reducing end, where a suitable chromophore, such as 4-aminobenzamide or 4-(2-aminoethyl)-aniline, can be derivatized by a reductive amination of this portion of the oligosaccharides to increase UV detection from UV at 194 nm (native carbohydrate) to 298 nm for 4-aminobenzamide-derivatized sugars or 250 nm for 4-(2-aminoethyl)aniline-modified carbohydrates (Harvey 2011). The efficacy of recycling derivatized carbohydrates with subtle structural differences was demonstrated by the resolution of the linear trisaccharides maltotriose and cellotriose, which only differ in their α and β anomeric configurations (Alley et al. 2013). For this example, both carbohydrates were jointly injected, appearing as a single peak after one column passage. After two cycles through the amide-80 column, two distinct peaks began to emerge, and the analytes were fully resolved as their 4-aminobenzamide derivatives after 15 recycling passes (an effective column length of 3.75 m) in 3 h and detected with a satisfactory sensitivity at a wavelength of 298 nm (Fig. S5).
Resin Glycosides
Resin glycosides are complex mixtures of acylsugars or glicolipids composed of macrocyclic oligosaccharides of monohydroxylated and dihydroxylated C14-C18 fatty acids which are always isolated as complex mixtures of homologues where all individual components present the same glycosidic acid core but substituted with a different number of alkyl groups differing in chain length, as well as in the position of esterification (Pereda-Miranda et al. 2010). These unique secondary metabolites are confined to the Convolvulaceae, and they seem to play important roles in the natural pest resistance to microorganisms and insects, as well as in plant-plant interactions (Kruse et al. 2022). The macrolactone successfully restrains the oligosaccharide core into bioactive conformations as demonstrated for the cytotoxic tricolorin A (P-388: IC50 2.2 mg/ml), a tetrasaccharide of 11S-hydroxyhexadecanoic acid from Ipomoea tricolor Cav., the Mexican morning glory (Rencurosi et al. 2004). Therefore, the search for constrained oligosaccharides has been expanded to consider the lipophilicity/hydrophilicity balance for cytotoxicity in resin glycosides as demonstrated by variations in the number and type of esterifying residues and the IC50 values described for the natural resin glycosides against many cancer cell lines to discover examples of potent chemotherapeutics (Lira-Ricárdez and Pereda-Miranda 2020).
The most successful and commonly employed phases for HPLC purification of these amphipathic mixtures are the C-18 and the amino (for carbohydrate analysis) columns. The HPLC techniques of column overloading, heart-cutting, and peak shaving, singularly or combined, using a semi- or preparative reversed-phase column in the recycle mode have been employed in the purification of individual resin glycosides with mixtures of CH3CN-MeOH-H2O to provide samples with a purity 99%, as demonstrated by the NMR and MS methods, as illustrated by the isocratic scale-up method for the prep-HPLC to purify the murucoidins from Ipomoea murucoides Roem. & Schult. (Fig. S6) (Chérigo and Pereda-Miranda 2006; Chérigo et al. 2008). Outstanding examples of the application of recycling semiprep-HPLC, for the purification of homologs in a series, are the tetrasacharides from the seeds of moon vine morning glory (Ipomoea alba L.) as potential chemosensitizers in breast carcinoma cells (Cruz-Morales et al. 2012, 2016), as well as the tricolorins from I. tricolor (Castañeda-Gómez et al. 2019). Profiling of resin glycoside mixtures based on MS (Castañeda-Gómez et al. 2017, 2019) has allowed the annotation of known tricolorins A–G from of I. tricolor, which was accomplished after fractionation by HPLC in a C-18 column through retention times, coelution experiments with pure samples, and m/z values for cations [M + H]+ and [M + Na]+ and ions [M – H]− in FAB and ESI MS (Fig. S7). The application of recycling semiprep-HPLC allowed the resolution of ten major constituents from the total crude extract, which produced a strong modulation of vinblastine cytotoxicity in the MCF-7/Vin + cells with a reversal fold value > 255-fold (25 μg/ml). The eluates with tR values of 13.73 min and 20.25 min showed non-previously registered m/z values which indicated the presence of new resin glycosides. Recycling HPLC was used for the purification of these novel tricolorins K–M (Fig. S8) with a Symmetry C-18 column (7 µm; 19 × 300 mm); mobile phase, ACN (100%); flow rate 6 ml/min; detection, refractive index (Castañeda-Gómez et al. 2019). Without the application of recycling chromatography, the purification of diastereoisomeric hexasaccharides of 3S,12S-dihydroxyhexadecanoic acid from the purgative root of Operculina macrocarpa Urb., Convolvulaceae (Brazilian jalap), would be an extremely difficult accomplishment since their structural differences are the position of substituents, such as isovaleroyl, tigloyl, and exogonoyl or (3S,9R)-3,6:6,9-diepoxydecanoyl, as the esterifying residues (Lira-Ricárdez et al. 2019). Recycling HPLC was also essential for the purification of acylated macrocyclic bidesmosides from I. purga (Bautista et al. 2015, 2016), as well as ester-type dimers of branched oligosaccharides from the Mexican morning glory (Bah and Pereda-Miranda 1997), the jalap root (I. purga) (Castañeda-Gómez and Pereda-Miranda 2011; Castañeda-Gómez et al. 2013), the arborescent morning glory Ipomoea wolcottiana Rose (Corona-Castañeda et al. 2016), and sweet potato, Ipomoea batatas (L.) Lam. (Escalante-Sánchez and Pereda-Miranda 2007; Rosas-Ramírez and Pereda-Miranda 2013).
Finally, one additonal example is worth mentioning due to the remarkable separation of exceptionally complex diastereoisomeric mixtures of the orizabins from the Mexican scammony or false jalap, Ipomoea orizabensis (G.Pelletan) Ledeb ex Steud., one of the members of the pre-Hispanic purgative jalaps (Pereda-Miranda et al. 2006). All tetrasaccharides from the orizabin series were esterified by isobutiric (iba), tiglic (tga), and (±)-3-hydroxy-2-methylbutanoic acids (nilic acid, nla) which were responsible for their lipophilicity and the difference in their retention times (Pereda-Miranda and Hernandez-Carlos 2002). The chloroform-soluble portion of the Mexican scammony crude resins was fractionated by reversed-phase prep-HPLC with a Waters Spherisorb C-18 column (250 × 19 mm, 10 mm) with an elution of CH3CN-H2O (88:12) and a flow rate of 8 ml/min which afforded three fractions across peaks with tR of 23.8 min, 26.7 min, and 33.4 min collected by heart-cutting. Each collected fraction was independently resolved by application of HILIC mode on a Waters μBondapak NH2-propylmethylsilyl Si gel column (150 × 19 mm, 10 mm). Elution was isocratically performed with CH3CN-H2O (95:5; flow rate 4 ml/min) and the system was controlled in the recycle mode. The resolution and purification of each individual constituent were monitored with a refractive index detector (Pereda-Miranda and Hernandez-Carlos 2002). Each of these mixtures was composed of two pairs of diastereomeric niloyl ester derivatives. Preparative-scale recycling HPLC of each subfraction afforded two major diastereomeric pairs which were resolved into pure compounds as illustrated for fraction with 23.8 min in Fig. 5. Their final resolution into pure compound 8–11 was accomplished by peak shaving and recycling through the same amino column. Saponification of these pure acylsugars demonstrated that each diastereomeric pair resulted from esterification of the oligosaccharide core by (2R,3R) and (2S,3S) enantiomers of 3-hydroxy-2-methylbutanoic acid (nla). Saponification of orizabins X (8) and XI (9) yielded the levorotatory (2R,3R) and dextrorotatory (2S,3S) niloyl residues, respectively. Alkaline hydrolysis of orizabins XII (10) and XIII (11) individually afforded the same enantiomers of nilic acid, i.e., threo (-)- and (+)-nilic acid. Mosher’s method (Pereda-Miranda et al. 2023) was used to confirm the absolute configuration of each liberated nilic acid residue (Pereda-Miranda and Hernandez-Carlos 2002). The isomerism between the two pairs of diastereomeric niloyl esters (8/9 and 10/11, peaks 1/2 and 3/4 in Fig. 5) was a result of the interchange of the esterifying residues at positions C-2 of the rhamnose unit and C-6 of glucose. Consequently, orizabins X (8) and XI (9) represented a positional isomeric pair in relation to orizabins XII (10) and XIII (11) by interchanging the position of the nilic and isobutyric acids.
Recycling semiprep-HPLC resolution of two mixtures of diastereomeric niloyl (nla) ester pairs. Purification of the unresolved mixtures of peaks 1/2 and 3/4 (orizabins X/XI and XII/XIII, respectively) was accomplished by application of the heart-cutting and peak shaving methods in the hydrophilic-interaction chromatography mode. Column: 150 × 19 mm, μBondapak-amino, 10 μm; mobile phase: CH3CN–H2O, 95:5; flow rate 4 ml/min; sample injection: 10 mg/500 μl. Abbreviation: tga, tiglyl; iba, isobutiryl. Assignments: peak 1, orizabin X (8); peak 2, orizabin XI (9); peak 3, orizabin XII (10); and peak 4, orizabin XIII (11)

Alkaloids
Adina eurhyncha (Miq.) Å.Krüger & Löfstrand (syn. A. rubescens Hemsl.), Rubiaceae, is a species distributed in Western Indonesia and used to treat jaundice. Secorubenine A (12) was isolated from the MeOH extract of heartwood of this tree as a pentacyclic monoterpenoid indole alkaloid glycoside and purified from impurities by recycling SEC-HPLC (column: Asahipak GS-510 20G and GS-310 20G) with MeOH and UV detection (Nakashima et al. 2022).

Anthocyanins
Red cabbage (Brassica oleracea L.) is a vegetable that belongs to the Brassicaceae family and is used as food throughout the world because it contains vitamins, inorganic elements, β-carotene, and proteins. Due to its anthocyanin content, it can be used as a pH indicator and colorant in the food industry. Ten anthocyanins were purified from five fractions identified by HPLC-MS/MS of the crude anthocyanin extract obtained with MeOH-H2O (1:1 v/v, with 1% formic). The complex mixture of anthocyanins, based on cyanidin cation, 2-(3,4-dihydroxyphenyl)chromenylium-3,5,7-triol, is a consequence of the number and position of glycosides linked to the aglycone that can be acylated with groups. For example, 2 ml (50 mg/ml) of the fraction containing Cy-3-(caff-pC)-diGlc-5-Glc, Cy-3-(glucofer)-diGlc-5-Glc, and Cy-3-(glucosin)-diGlc-5-Glc, was injected into the recycling system with MeOH-H2O (3:7) and 3% formic acid until a 97 to 99% of purity was achieved by three to five recycling cycles (Chen et al. 2018).
Coumarins
Khellactone-type isomers, produced by differences in the substitution pattern by acetoxyl, angeloyl, senecionyl, tigloyl, isovaleryl, 2-methylbutyroyl substituent, such as the isomeric pair of (−)-3′,4′-diisovalerylkhellactone (13) and (−)-3′-isovaleryl-4′-(2-methylbutyryl)khellactone (14), were purified by recycling HPLC from the EtOH extract of roots of Peucedanum japonicum Thumb., Apiaceae, used in traditional Asian medicine for the treatment of cold, gout, cough, headache, spasms, neuralgic diseases, fever, rheumatoid arthritis, and other inflammatory diseases. The ethanolic extract was extracted with hexanes and H2O. Then, the hexane layer was separated by medium-pressure liquid chromatography using an XbridgeTM Prep C-18 column eluted with MeOH-H2O in a concentration gradient for fractionation. Each fraction was purified in a simple step by recycling HPLC with acetonitrile/water/formic acid (0.1%) as the optimum gradient system at 254, 280, and 360 nm by a UV-visible detector until complete purification of coumarins was achieved (Won et al. 2019).

Sphingolipids
The glycosphingolipids calyxoside B (15) and calyxoside (16) were isolated from MeOH and CHCl3-MeOH extracts of a marine sponge Cladocroce sp. The extracts were partitioned and subjected to C-18 flash column chromatography and reversed-phase HPLC to give a mixture of 1:3 of 15 and 16, which was not resolved as the natural products. Therefore, the amide mixture was derivatized with the Fmoc group (fluorenylmethoxycarbonyl protecting group) and purified by recycling HPLC with a C-18 UG-5 column (10 × 250 mm) through 20 cycles with 90% MeOH (Sugawara et al. 2021).

Flavonoids
Cajuputones A–C (17–19) are β-triketone flavanone hybrids that were isolated from the branches and leaves of Australian “tea tree,” Melaleuca cajuputi Maton & Sm. ex R.Powell, Myrtaceae. The volatile oil is used for the treatments of fungal skin infections, insect bites, sore throat, and wounds.
These flavones presented a rare 6/6/6/6 oxatetracyclic ring system fused between an acylphloroglucinol-derived β-triketone and a pinocembrin or strobopinin moiety via a pyran-like unit. The EtOH extract was partitioned and the CHCl3 extract was subjected to silica gel column chromatography and preparative HPLC with the mobile phase of MeOH-H2O (4:1) and repeatedly purified using MeCN-H2O (76:24) to give cajuputone A (17). The cajuputones B (18) and C (19), (C-2 epimers), were separated by RP-medium pressure liquid chromatography with isocratic 75% aqueous MeOH and further purified by recycling prepHPLC with MeCN-H2O (4:1) (Xu et al. 2020).

In 2020, Saito and collaborators published the isolation of genistein from soybean, Glycine max (L.) Merr., Fabaceae, an isoflavone, as a novel inhibitor of the ATP-binding cassette membrane transporter ABCC11 (also MRP8) with a IC50 61.5 µM. The water extract was separated on C18 column using different gradients. The most active subfraction was purified by a recycling preparative HPLC through a gel permeation column and methanol at 5 ml/min between 120 and 176 min. The detection was with a refractive index system and UV at 254 nm (Saito et al. 2020). Alkylated flavones and dihydrobenzoxanthones are responsible for the inhibitory effect against human neutrophilelastase (HNE), associated with inflammation (IC50 14.8 ~ 18.1 and 9.8 ~ 28.7 μM, respectively) that were isolated from the MeOH extract of Artocarpus elasticus Reinw., Moraceae, root barks by partition with CHCl3-H2O, column chromatography, and recycling HPLC (C-18 column and Sephadex-LH20) (Ban et al. 2020). The rhizome of Kaempferia parviflora Wall. ex Baker, Zingiberaceae (black ginger), is used in folk medicine in Thailand as a tonic, anticancer, anti-inflammation, as well as food for its dietary properties. From its MeOH extract, flavones, flavanones, diarylheptanoids, chalcones, and a stilbene were isolated by partition with H2O-EtOAc-BuOH, column chromatography on SiO2, medium-pressure liquid chromatography (MPLC), HPLC, recycling HPLC (Jaigel GS-310 + Jaigel GS-320, MeOH), and crystallization techniques. Most of the compounds showed inhibitory activity in the production of NO in vitro, for example, the chalcones flavokavin B, flavokavin A, 5,7-dimethylluteolin, 5,7,4-trimethoxyflavone, 5,7-dimethoxyflavanone, and 5,7,3′-trimethoxy-4′-hydroxyflavanone showed a IC50 of 2.51, 7.82, 9.03, 14.2, 14.3, and 16.1 μM, respectively (Fuchino et al. 2018).
Terpenoids and Steroids
Two pairs of epimeric lindenane monoterpene heterodimers fused by a 1,2-dioxane moiety and named as sarcaglarols A-D (20–23) were purified from the dried leaves of Sarcandra glabra (Thunb.) Nakai, Chloranthaceae. The aqueous EtOH extract (95%) was dissolved in warm water and partitioned with petroleum ether and dichloromethane. The dichloromethane fraction was subjected to a silica gel CC, followed by HW-40C gel, MCI gel, LH-20 gel, and purified by recycling preparative HPLC and normal preparative HPLC (Wang et al. 2021).

Twenty neo-clerodane diterpenoids, scutefolides G1-S, were isolated from the aqueous acetone extract (70%) of the aerial parts of Scutellaria violacea var. sikkimensis Hook.f. (syn. S. coleifolia H.Lév., Lamiaceae). These diterpenes represented ten pairs of C-13 epimers with variation in the substitution pattern by acetyl, tigloyl, isobutyryl, and 2-methylbutyryl groups, which were identified as moderately cytotoxic agents against human cancer cell lines (MCF7: IC50 36.2 to 82.5 μg/ml; A549 cells: IC50 61.0 ± 5.6 μg/ml). These principles were isolated by partition and column chromatography of the EtOAc-soluble extract. Each fraction was subjected to recycling reversed-phase HPLC. For example, the fraction rich in the epimeric mixture of scutefolide H1 (24) and scutefolide H2 (25) was purified from their diastereomers scutefolide J (26) by HPLC on COSMOSIL πNAP with MeCN-H2O (4:1) to give 26 and a mixture of 24 and 25, which was further purified by a recycling HPLC with the same chromatographic conditions (Kurimoto et al. 2016).

Euphorbia factors L2a (27) and L2b (28; IC50 0.87 mM against U937 cell line) were obtained from seeds of Euphorbia lathyrism with ethanol (95%) as a pair of cis/trans geometric isomers of a lathyrane-type diterpenoid with an unusual trans-gem-dimethylcyclopropane and purified by partition with H2O-petroleum ether-dichloromethane-ethyl acetate by column chromatography on silica gel and recycling semiprep-HPLC on C-18 (5 mm × 150 × 10 mm) with MeOH-H2O (85:15, 4 ml/min) (Li et al. 2021).

Triterpenes, cycloeucalenone (29), and 31-norcyclolaudenone (30), ergostene-type diatereoisomers, were obtained from banana peel by bioassay-guided fractionation and recycling HPLC (C-18 HG-5 column, 20 × 250 mm; 100% MeOH, flow rate, 8.0 ml/min; and UV detector, 254 nm) as effective inhibitors of α-glucosidase with IC50 values of 31.83 and 38.85 μM, respectively, and α-amylase, of 20.33 μM and 27.63 μM, respectively, being the carbonyl group at C-3 and the double bond in the side chain of the active sites of both compounds. Cycloeucalenone induced a parabolic mixed-type inhibition with a Ki value of 73.86 μM in the α-glucosidase inhibitory assay (Shang et al. 2021).
Ficutirucins A–I, tirucallane-type triterpenoids, were isolated from the EtOH extract of the fruit of Ficus carica used to treat inflammation, diarrhea, and indigestion. Diastereoisomeric ficutirucins C (31), D (32), and G (33) were purified by recycling prep-HPLC on a C-18 column (20 × 250 mm) with MeOH-H2O (9:1) and a DAD detector. These triterpenoids exhibited moderate cytotoxic activities against MCF-7, HepG-2, and U2OS with IC50 values of 11.67–45.61 μM (Jing et al. 2015).

Steroids derived from the anabolic androgenic steroid mestanolone were obtained by microbial transformation with Macrophomina phaseolina and Cunninghamella blakesleeana and purified by recycling preparative HPLC-LC-908 (Japan) with JAIGEL-ODS-L-80 column and MeOH-H2O as the mobile phase. Some compounds exhibited moderate cytotoxicity against human cervical carcinoma with values from IC50 12.8 to 27.6 μM (Farooq et al. 2018).
Gradient twin column recycling semiprep-HPLC was advantageously used to isolate, and baseline separate, the vitamins D2 (34, ergocalciferol) and D3 (35, cholecalciferol) present in a milk extract mixture with the following analytical conditions (Fig. S9): 0.3 ml sample injection volume, 7.8 mm × 150 mm Sunfire-C column, MeOH-H2O eluent mixtures at 65 °C, 14 cycles needed in 1.5 h (Gritti 2021).

δ-Lactones
Dihydro-furanones or δ-lactones are bioactive principles purified from plants, marine fungi, and sponges. 6-Heptenyl-5,6-dihydro-2H-pyran-2-ones have displayed antimicrobial and cytotoxic properties and are bioactive chemical markers of Hyptis species, tribe Hyptidinae (Lamiaceae, the mint family) and accountable for the curative properties of species with importance to the Latin-American traditional medicine (Martínez-Fructuoso et al. 2019, 2020). From Hyptis brevipes Benth., five 6-(6′-cinnamoyloxy-2′,5′-epoxy-1′-hydroxyheptyl)-5,6-dihydro-2H-pyran-2-ones, the brevipolides K-O (36–40), with cytotoxic activity (IC50 1.7–10 μM against six cancer cell lines) were isolated and purified by recycling semiprep-HPLC with the following conditions: C-18 column, 19 × 300 mm, 7 μm; mobile phase, CH3CN-H2O (7:3), 9 ml/min; detection, diode-array detection (λ 254 nm); injection volume, 500 μl (50 mg/100 μl), as illustrated in Fig. S10 (Suárez-Ortiz et al. 2017).

From the CH2Cl2-soluble fraction of Hyptis monticola Mart. Ex Benth., a combination of high-speed countercurrent chromatography with the biphasic solvent system hexane:EtOAc:MeOH:H2O (0.8:1:0.8:1), followed by reversed-phase recycling prep-HPLC, was applied for the purification of known monticolides A (41) and B (42) and four novel 5,6-dihydro-α-pyrones, monticolides C–F (43–46). Each of the collected fractions rich in δ-lactones was scanned by UHPLC-ESI-MS. Subsequently, recycling prep-HPLC was used to increase the separation power for each selected peak as illustrated in Fig. 6. The mixture of 42 and 43 collected from one of the CCC-fractions was resolved by application of heart-cutting, whereas peak shaving was employed to eliminate impurities. The complete resolution of the diastereomeric mixtures of diacetylated monticolides B (42) and C (43) was totally achieved after five passes for each separated component through an NH2-column (19 × 150 mm, 10 μm) with an isocratic flow of CH3CN; flow rate, 4.7 ml/min; DAD detector (290 nm); sample concentration, 30 mg/ml (da Silva et al. 2021).
Recycling semiprep-HPLC chromatogram for the resolution of the unresolved mixture of diacetylated monticolides B (42) and C (43) from a high-speed countercurrent chromatography-rich fraction. Conditions: mobile phase CH3CN; flow rate, 4.7 ml/min; column for carbohydrate analysis (19 × 150 mm, 10 μm); DAD detector (290 nm); sample concentration, 30 mg/ml

Quinones
Abruquinone A (47) and abruquinone B (48) were isolated from the methanol root extract of Abrus precatorius L., Fabaceae, as isoflavanquinones with antileishmanial activity against Leishmania major (IC50 6.35 ± 0.005 μg/ml and 6.32 ± 0.008 μg/ml, respectively) and Leishmania tropica (IC50 6.29 ± 0.015 μg/ml and 6.31 ± 0.005 μg/ml, respectively). The methanol extract was subjected to fractionation with different solvents, and the ethyl acetate fraction was subjected to bioassay-guided fractionation using SiO2 column chromatography, followed by recycling prep-HPLC on a normal phase column (LC–908 W) to removed impurities with Hex-EtOAc-MeOH (8:1.5:0.5) at a flow rate of 4 ml/min (Okoro et al. 2020).

Perspectives and Future Directions
Discovery and supply of bioactive natural chemicals depend on the availability of preparative-scale isolation and purification methods with the capability of resolving complex mixtures of natural secondary metabolites. The purification process must definitively provide a single chemical entity suitable for structure elucidation and identification of its relative and absolute configuration for further biological evaluations. Recycling semi/prep-HPLC for targeted and non-targeted isolation of natural products represents a purification method accesible, effortless, and less time-consuming than adsorption chromatography by conventional HPLC which generally is inconsistent to achieve full baseline resolution of components with similar physicochemical properties.
The potential of classical isocratic steps for the isolation and purification of target metabolites, after resolution from nearly co-eluting impurities by closed-loop recycling chromatography, which cannot be fully discarded by any established single-column semipreparative batch process, could be extended to twin column gradient recycling elution with modern reversed-phase chromatographic columns. Multiple mechanisms for the purification are compatible, including reverse phases, HILIC, gel-permeation, and size exclusion chromatography. Thus, this approach represents an alternative procedure for the preparative scale fractionation and resolution of complex natural product matrices by closed-loop recycle HPLC, as such found after bioassay-guided methodologies and metabolomic studies of microorganisms (Aguilar-Colorado and Rivera-Chávez 2023; Carrillo-Jaimes et al. 2024), marine sources (Panggabean et al. 2022), and unconventional poorly analyzed secondary metabolites, such as antimicrobial peptides (Barashkova and Rogozhin 2020), and glycans (Zhu et al. 2020), inter alia. The reviewed examples showed encouraging possibilities toward a successful purification of unknown targeted compounds before further physicochemical characterization, unequivocal structural elucidation, and biological evaluation.
Conclusions
Recycling preparative HPLC is a separation technique that provides a fast and elegant approach to purify milligrams to grams of bioactive metabolites from complex natural products matrices, in addition to the purification of starting materials, intermediates, and final semisynthetic products. This procedure permits to control the number of columns passes or “effective columns” that the target analytes experience by forwarding the effluent which is basically recycled back into the column to continue the separation process in a closed-loop system to provide a satisfactory number of column lengths for the resolution of closely related compounds with a high purity (98%) such as epimers, diastereoisomers, homologs in a series, and geometric or positional isomers. The applications of this methodology by academia in the pharmacognosy and phytochemistry fields have exemplified significant efforts in providing high-purity standards through the isolation of a high diversity of active secondary metabolites. The feasibility of this approach has been demonstrated through the isolation of novel plant natural products as comprehensively described in the present review.
Availability of Data and Material
Not applicable.
References
Aguilar-Colorado AS, Rivera-Chávez J (2023) Ants/nest-associated fungi and their specialized metabolites: taxonomy, chemistry, and bioactivity. Rev Bras Farmacogn 33:901–923. https://doi.org/10.1007/s43450-023-00417-3
Al-Sanea MM, Gamal M (2022) Critical analytical review: rare and recent applications of refractive index detector in HPLC chromatographic drug analysis. Microchem J 178:107339. https://doi.org/10.1016/j.microc.2022.107339
Alley WR Jr, Mann BF, Hruska V, Novotny MV (2013) Isolation and purification of glycoconjugates from complex biological sources by recycling high-performance liquid chromatography. Anal Chem 85:10408–10416. https://doi.org/10.1021/ac4023814
Bah M, Pereda-Miranda R (1997) Isolation and structural characterization of new glycolipid ester type dimers from the resin of Ipomoea tricolor (Convolvulaceae). Tetrahedron 53:9007–9022. https://doi.org/10.1016/S0040-4020(97)00607-8
Bailly M, Tondeur D (1982) Recycle optimization in non-linear productive chromatography—I mixing recycle with fresh feed. Chem Eng Sci 37:1199–1212. https://doi.org/10.1016/0009-2509(82)85063-X
Bailly M, Tondeur D (1984) Reversibility and performances in productive chromatography. Chem Eng Process: Process Intensificat 18:293–230. https://doi.org/10.1016/0255-2701(84)87006-3
Ban YJ, Baiseitova A, Nafiah MA, Kim JY, Park KH (2020) Human neutrophil elastase inhibitory dihydrobenzoxanthones and alkylated flavones from the Artocarpus elasticus root barks. Appl Biol Chem 63:63. https://doi.org/10.1186/s13765-020-00549-3
Barashkova AS, Rogozhin EA (2020) Isolation of antimicrobial peptides from different plant sources: does a general extraction method exist? Plant Methods 16:143. https://doi.org/10.1186/s13007-020-00687-1
Bautista E, Fragoso-Serrano M, Pereda-Miranda R (2015) Jalapinoside, a macrocyclic bisdemoside from the resin glycosides of Ipomoea purga, as a modulator of multidrug resistance in human cancer cells. J Nat Prod 78:168–172. https://doi.org/10.1021/np500762w
Bautista E, Fragoso-Serrano M, Pereda-Miranda R (2016) Jalapinoside II, a bisdesmoside resin glycoside, and related glycosidic acids from the officinal jalap root (Ipomoea purga). Phytochem Lett 17:85–93. https://doi.org/10.1016/j.phytol.2016.07.022
Biesenberger JA, Tan M, Duvdevani I, Maurer T (1971) Recycle gel permeation chromatography. 1. Recycle principle and design. J Polym Sci Part B Polym Lett 9:353–357. https://doi.org/10.1002/pol.1971.110090506
Bucior I, Burger MM (2004) Carbohydrate-carbohydrate interaction as a major force initiating cell-cell recognition. Glycoconj J 21:111–123. https://doi.org/10.1023/B:GLYC.0000044843.72595.7d
Bucior I, Burger MM, Fernández-Busquets X (2009) Carbohydrate–carbohydrate interactions. In: Gabius HJ (ed) The sugar code. Fundamentals of glycosciences. Wiley-VCH, Weinheim, pp 347–362
Breton RC, Reynolds WF (2013) Using NMR to identify and characterize natural products. Nat Prod Rep 30:501–524. https://doi.org/10.1039/C2NP20104F
Cannazza G, Carrozzo MM, Battisti U, Braghiroli D, Parenti C (2009) On-line racemization by high-performance liquid chromatography. J Chromatogr A 12165:655–659. https://doi.org/10.1016/j.chroma.2009.05.057
Cannazza G, Battisti U, Carrozzo MM, Brasili L, Braghiroli D, Parenti C (2011) Evaluation of stereo and chemical stability of chiral compounds. Chirality 23:851–859. https://doi.org/10.1002/chir.20941
Carrillo-Jaimes K, Fajardo-Hernández CA, Hernández-Sedano F, Cano-Sánchez P, Morales-Jiménez J, Quiroz-García B, Rivera-Chávez J (2024) Antibacterial activity and AbFtsZ binding properties of fungal metabolites isolated from Mexican mangroves. Rev Bras Farmacogn. https://doi.org/10.1007/s43450-023-00507-2
Castañeda-Gómez J, Pereda-Miranda R (2011) Resin glycosides from the herbal drug jalap (Ipomoea purga). J Nat Prod 74:1148–1153. https://doi.org/10.1021/np200080k
Castañeda-Gómez J, Figueroa-González G, Jacobo-Herrera N, Pereda-Miranda R (2013) Purgin II, a resin glycoside ester-type dimer and inhibitor of multidrug efflux pumps from Ipomoea purga. J Nat Prod 76: 64–71. https://doi.org/10.1021/np300739y
Castañeda-Gómez J, Rosas-Ramírez D, Cruz-Morales S, Fragoso-Serrano M, Pereda-Miranda R (2017) HPLC-MS profiling of the multidrug-resistance modifying resin glycoside content of Ipomoea alba seeds. Rev Bras Farmacogn 27:434–439. https://doi.org/10.1016/j.bjp.2017.05.003
Castañeda-Gómez J, Lavias-Hernández P, Fragoso-Serrano M, Lorence A, Pereda-Miranda R (2019) Acylsugar diversity in the resin glycosides from Ipomoea tricolor seeds as chemosensitizers in breast cancer cells. Phytochem Lett 32:77–82. https://doi.org/10.1016/j.phytol.2019.05.004
Corona-Castañeda B, Rosas-Ramírez D, Castañeda-Gómez J, Aparicio-Cuevas MA, Fragoso-Serrano M, Figueroa-González G, Pereda-Miranda R (2016) Resin glycosides from Ipomoea wolcottiana as modulators of the multidrug resistance phenotype in vitro. Phytochemistry 123:48–57. https://doi.org/10.1016/j.phytochem.2016.01.004
Chen YY, Yan XJ, Lu FL, Jiang XH, Friesen JB, Pauli GF, Chen SN, Li DP (2019) Preparation of flavone di-C-glycoside isomers from Jian-Gu injection (Premna fulva Craib.) using recycling counter-current chromatography. J Chromatogr A 1599:180–186. https://doi.org/10.1016/j.chroma.2019.03.030
Chen Y, Wang Z, Zhang H, Liu Y, Zhang S, Meng Q, Liu W (2018) Isolation of high purity anthocyanin monomers from red cabbage with recycling preparative liquid chromatography and their photostability. Molecules 23:991–1003. https://doi.org/10.3390/molecules23050991
Chérigo L, Pereda-Miranda R (2006) Resin glycosides from the flowers of Ipomoea murucoides. J Nat Prod 69:595–599. https://doi.org/10.1021/np0504457
Chérigo L, Pereda-Miranda R, Fragoso-Serrano M, Jacobo-Herrera N, Kaatz GW, Gibbons S (2008) Inhibitors of bacterial multidrug efflux pumps from the resin glycosides of Ipomoea murucoides. J Nat Prod 71:1037–1045. https://doi.org/10.1021/np800148w
Crary JR, Cain-Janicki K, Wijayaratne R (1989) External recycle chromatography: a practical method for preparative purifications. J Chromatogr A 462:85–94. https://doi.org/10.1016/S0021-9673(00)91338-4
Cruz-Morales S, Castañeda-Gómez J, Figueroa-González G, Mendoza-García AD, Lorence A, Pereda-Miranda R (2012) Mammalian multidrug resistance lipopentasaccharide inhibitors from Ipomoea alba seeds. J Nat Prod 75:1603–1611. https://doi.org/10.1021/np300414d
Cruz-Morales S, Castañeda-Gómez J, Rosas-Ramírez D, Fragoso-Serrano M, Figueroa-González G, Lorence A, Pereda-Miranda R (2016) Resin glycosides from Ipomoea alba seeds as potential chemosensitizers in breast carcinoma cells. J Nat Prod 79:3093–3104. https://doi.org/10.1021/acs.jnatprod.6b00782
Daniel EK, Nicola LB (2016) Protocol for the purification of protected carbohydrates: toward coupling automated synthesis to alternate-pump recycling high-performance liquid chromatography. Chem Commun 52:13253–13256. https://doi.org/10.1021/10.1039/c6cc07584c
da Silva AS, Martínez-Fructuoso L, Simas RC, Leitão GG, Fragoso-Serrano M, Barros YS, de Souza DR, Pereda-Miranda R, Leitão SG (2021) Distribution of 5,6-dihydro-α-pyrones by electrospray ionization ion trap mass spectrometry in different aerial parts of Hyptis monticola. Phytochemistry 185:112706. https://doi.org/10.1016/j.phytochem.2021.112706
Demarque DP, Crotti AE, Vessecchi R, Lopes JL, Lopes NP (2016) Fragmentation reactions using electrospray ionization mass spectrometry: an important tool for the structural elucidation and characterization of synthetic and natural products. Nat Prod Rep 33:432–455. https://doi.org/10.1039/c5np00073d
Escalante-Sánchez E, Pereda-Miranda R (2007) Batatins I and II, ester-type dimers of acylated pentasacharides from the resin glycosides of sweet potato. J Nat Prod 70:1029–1034. https://doi.org/10.1021/np070093z
Farooq R, Hussain N, Yousuf S, Ahmad MS, Choudhary MI (2018) Microbial transformation of mestanolone by Macrophomina phaseolina and Cunninghamella blakesleeana and anticancer activities of the transformed products. RSC Adv 8:21985–21992. https://doi.org/10.1039/c8ra01309h
Firman K, Kinoshita T, Itai A, Sankawa U (1988) Terpenoids from Curcuma heyneana. Phytochemistry 27:3887–3891. https://doi.org/10.1016/0031-9422(88)83038-3
Fuchino H, Fukui N, Iida O, Wada H, Kawahara N (2018) Inhibitory effect of black ginger (Kaempferia parviflora) constituents on nitric oxide production. Japn J Food Chem Saf 25:152–159. https://doi.org/10.18891/jjfcs.25.3_152
Fukuyama Y, Minami H, Ichikawa R, Takeuchi K, Kodama M (1996) Hydroperoxylated guaiane-type sesquiterpenes from Viburnum awabuki. Phytochemistry 42:741–746. https://doi.org/10.1016/0031-9422(96)00042-8
Ganesh V, Poorna Basuri P, Sahini K, Nalini CN (2022) Retention behaviour of analytes in reversed‐phase high‐performance liquid chromatography: a review. Biomed Chromatogr 31:e5482. https://doi.org/10.1002/bmc.5482
Grill CM (1998) Closed-loop recycling with periodic intra-profile injection: a new binary preparative chromatographic technique. J Chromatogr A 796:101–113. https://doi.org/10.1016/s0021-9673(97)01047-9
Gritti F, Besner S, Cormier S, Gilar M (2017a) Application of high-resolution recycling liquid chromatography: from small to large molecules. J Chromatogr A 1524:108–120. https://doi.org/10.1016/j.chroma.2017.09.054
Gritti F, Leal M, McDonald T, Gilar M (2017b) Ideal versus real automated twin column recycling chromatography process. J Chromatogr A 1508:81–94. https://doi.org/10.1016/j.chroma.2017.06.009
Gritti F, Basile M, Cormier S, Fogwill M, Gilar M, McDonald T, Riley F, Yan Q (2018) Semi-preparative high-resolution recycling liquid chromatography. J Chromatogr A 1566:64–78. https://doi.org/10.1016/j.chroma.2018.06.055
Gritti F (2021) Rebirth of recycling liquid chromatography with modern chromatographic columns: extension to gradient elution. J Chromatogr A 1653:462424. https://doi.org/10.1016/j.chroma.2021.462424
Guarducci MA, Fochetti A, Ciogli A, Mazzoccanti GA (2023) Compendium of the principal stationary phases used in hydrophilic interaction chromatography: where have we arrived? Separations 10:22. https://doi.org/10.3390/separations10010022
Harvey DJ (2011) Derivatization of carbohydrates for analysis by chromatography; electrophoresis and mass spectrometry. J Chromatography B 879:1196–1225. https://doi.org/10.1016/j.jchromb.2010.11.010
He JM, Huang J, Wu WL, Mu Q (2020) Unlimited recycling counter-current chromatography for the preparative separation of natural products: naphthaquinones as examples. J Chromatogr A 1626:461368. https://doi.org/10.1016/j.chroma.2020.461368
Ito J, Gobaru K, Shimamura T, Niwa M, Takaya Y, Oshima Y (1998) Absolute configurations of some oligostilbenes from Vitis coignetiae and Vitis vinifera ‘Kyohou.’ Tetrahedron 54:6651–6660. https://doi.org/10.1016/S0040-4020(98)00332-9
Jing L, Zhang YM, Luo JG, Kong LY (2015) Tirucallane-type triterpenoids from the fruit of Ficus carica and their cytotoxic activity. Chem Pharm Bull 63:237–243. https://doi.org/10.1248/cpb.c14-00779
Kawanishi K, Hashimoto Y, Wang Q, Xu Z (1985) Separation of the pentacyclic triterpenes tylolupenols A and B from Tylophora kerrii. Phytochemistry 24:2051–2054. https://doi.org/10.1016/s0031-9422(00)83120-9
Kimata K, Hirose T, Kanao E, Kubo T, Otsuka K, Hosoya K, Yoshikawa K, Fukusaki E, Tanaka N (2019) Recycle reversed-phase liquid chromatography to achieve separations based on one H/D substitution on aromatic hydrocarbons. LCGC Supplements 32:14–19
Kruse LH, Bennett AA, Mahood EH, Lazarus E, Park SJ, Schroeder F, Moghe GD (2022) Illuminating the lineage-specific diversification of resin glycoside acylsugars in the morning glory (Convolvulaceae) family using computational metabolomics. Hortic Res 9:unhab079. https://doi.org/10.1093/hr/uhab079
Kubo I, Kim M, De Boer G (1987) Efficient isolation of insect growth inhibitory macrolide alkaloids using recycle high-performance gel permeation chromatography. J Chromatogr A 402:354–357. https://doi.org/10.1016/0021-9673(87)80036-5
Kubo I, Murai Y, Soediro I, Soetarno S, Sastrodihardjo S (1992) Cytotoxic anthraquinones from Rheum pulmatum. Phytochemistry 31:1063–1065. https://doi.org/10.1016/0031-9422(92)80078-S
Kurimoto S, Pu JX, Sun HD, Shibata H, Takaishi Y, Kashiwada Y (2016) Acylated neo-clerodane type diterpenoids from the aerial parts of Scutellaria coleifolia Levl. (Lamiaceae). J Nat Med 70:241–252. https://doi.org/10.1007/s11418-016-0967-3
Lamotte S, Gruendling T, Loeb U, Stritesky R, Gerhardt R, Schmidt M, Deeb AA (2017) Generic ultrahigh resolution HPLC methods: an efficient way to tackle singular analytical problems in industrial analytics. Chromatographia 80:763–769. https://doi.org/10.1007/s10337-017-3300-8
Latif Z, Sarker SD (2012) Isolation of natural products by preparative high performance liquid chromatography (Prep-HPLC). In: Sarker SD, Nahar L (eds) Natural products isolation. Methods in molecular biology, vol 86. Humana Press, New York, NY, pp 255–274. https://doi.org/10.1007/978-1-61779-624-1_10
Li H, Zhang F, Jin Q, Zhu T (2021) Preparative separation and purification of cyclosporin D from fungus Hypoxylon spp. by improved closed-loop recycling counter-current chromatography. J Chromatogr A 1649:462221. https://doi.org/10.1016/j.chroma.2021.462221
Lira-Ricárdez J, Pereda-Miranda R (2020) Reversal of multidrug resistance by amphiphilic morning glory resin glycosides in bacterial pathogens and human cancer cells. Phytochem Rev 19:1211–1229. https://doi.org/10.1007/s11101-019-09631-1
Lira-Ricárdez J, Pereda-Miranda R, Castañeda-Gómez J, Fragoso-Serrano M, Simas RC, Leitão SG (2019) Resin glycosides from the roots of Operculina macrocarpa (Brazilian jalap) with purgative activity. J Nat Prod 82:1664–1677. https://doi.org/10.1021/acs.jnatprod.9b00222
Majors RE (2004) The role of the column in preparative HPLC. LCGC Europe 17:512–520
Martínez-Fructuoso L, Pereda-Miranda R, Rosas-Ramírez D, Fragoso-Serrano M, Cerda García-Rojas CM, Soares Da Silva A, Guimarães Leitão G, Guimarães Leitão S (2019) Structure elucidation, conformation and configuration of cytotoxic 6-heptenyl-5,6-dihidro-2H-pyran-2-ones from Hyptis species and their molecular docking to alpha-tubulin. J Nat Prod 86:520–531. https://doi.org/10.1021/acs.jnatprod.8b00908
Martínez-Fructuoso L, Pereda-Miranda R, Fragoso-Serrano M, da Silva AS, Leitão SG (2020) Dihydro-furanones from Hyptis species: chemical correlations and DFT-NMR/ECD calculations for stereochemical assignments. Phytochemistry 179:112481. https://doi.org/10.1016/j.phytochem.2020.112481
Mohanraj S, Herz W (1981) Use of the peak shaving-recycle technique for of labdadiene and labdatriene isomers by HPLC. J Liquid Chromatogr 4:525–532. https://doi.org/10.1080/01483918108059951
Moreno-Velasco A, Flores-Tafoya PD, Fragoso-Serrano M, Leitão SG, Pereda-Miranda R (2022) Resin glycosides from Operculina hamiltonii and their synergism with vinblastine in cancer cells. J Nat Prod 85:2385–2394. https://doi.org/10.1021/acs.jnatprod.2c00594
Moreno-Velasco A, Fragoso-Serrano M, Flores-Tafoya P, Carrillo-Rojas S, Bautista E, Guimaraes-Leitao S, Castañeda-Gómez J, Pereda-Miranda R (2024) Inhibition of multidrug-resistant MCF-7 breast cancer cells with combinations of clinical drugs and resin glycosides from Operculina hamiltonii. Phytochemistry 217:113922. https://doi.org/10.1016/j.phytochem.2023.113922
Nagy G, Peng T, Kabotso DEK, Novotny MV, Pohl NLB (2016) Protocol for the purification of protected carbohydrates: toward coupling automated synthesis to alternate-pump recycling high-performance liquid chromatography. Chem Commun 52:13253–13256. https://doi.org/10.1039/c6cc07584c
Nagy G, Peng T, Pohl NL (2017) Recent liquid chromatographic approaches and developments for the separation and purification of carbohydrates. Anal Methods 9:3579–3593. https://doi.org/10.1039/C7AY01094J
Nakashima N, Sakamoto J, Rakumitsu K, Kitajima M, Juliawaty LD, Ishikawa H (2022) Secorubenine, a monoterpenoid indole alkaloid glycoside from Adina rubescens: isolation, structure elucidation, and enantioselective total synthesis. Chem Pharm Bull 70:187–191. https://doi.org/10.1248/cpb.c21-00931
Nakatsu T, Johns T, Kubo I, Milton K, Sakai M, Chatani K, Saito K, Yamagiwa Y, Kamikawa T (1990) Isolation, structure, and synthesis of novel 4-quinolinone alkaloids from Esenbeckia leiocarpa. J Nat Prod 53:1508–1513. https://doi.org/10.1021/np50072a017
Naoki N, Ryuichiro T, Kazumoto M, Toshio K (1993) Isolation and characterization of a novel type of glycosphingolipid from Neanthes diversicolor. Biochim Biophys Acta Lipids Lipid Metab 1169:30–38. https://doi.org/10.1016/0005-2760(93)90078-n
Niculau ES, Oliveira DAB, Almeida NF (2022) Preparative high-performance liquid chromatography: isolation of natural chemical compounds for identification and characterization. Sep Sci plus 5:602–615. https://doi.org/10.1002/sscp.202200040
Okoro EE, Ahmad MS, Osoniyi OR, Onajobi FD (2020) Antifungal and antileishmanial activities of fractions and isolated isoflavanquinones from the roots of Abrus precatorius. Antifungal and antileishmanial activities of fractions and isolated isoflavanquinones from the roots of Abrus precatorius. Comp Clin Path 29:391–396. https://doi.org/10.1007/s00580-019-03073-z
Panggabean JA, Adiguna SB, Murniasih T, Rahmawati SI, Bayu A, Putra MY (2022) Structure–activity relationship of cytotoxic natural products from Indonesian marine sponges. Rev Bras Farmacogn 32:12–38. https://doi.org/10.1007/s43450-021-00195-w
Pereda-Miranda R, Bernard CB, Durst T, Arnason JT, Sánchez-Vindas P, Poveda L, San Román L (1997) Methyl 4-hydroxy-3-(3’-methyl-2’-butenyl)benzoate, major insecticidal principle from Piper guanacastensis. J Nat Prod 60:282–284. https://doi.org/10.1021/np960601x
Pereda-Miranda R, Hernández-Carlos B (2002) HPLC isolation and structural elucidation of diastereomeric niloyl ester tetrasaccharides from Mexican scammony root. Tetrahedron 58:3145–3154. https://doi.org/10.1016/S0040-4020(02)00284-3
Pereda-Miranda R, Fragoso-Serrano M, Escalante-Sánchez E, Hernández-Carlos B, Linares E, Bye R (2006) Profiling of the resin glycoside content of Mexican jalap roots with purgative activity. J Nat Prod 69:1460–1466. https://doi.org/10.1021/np060295f
Pereda-Miranda R, Rosas-Ramírez D, Castañeda-Gómez J (2010) Resin glycosides from the morning glory family. In: Kinghorn A, Falk H, Kobayashi J (eds) Progress in the chemistry of organic natural products; Springer-Verlag: New York, vol 92, Chapter 2, pp 77–152. https://doi.org/10.1007/978-3-211-99661-4_2
Pereda-Miranda R, Bautista E, Martínez-Fructuoso L, Fragoso-Serrano M (2023) From relative to absolute stereochemistry of secondary metabolites: applications in plant chemistry. Rev Bras Farmacogn 32:1–48. https://doi.org/10.1007/s43450-022-00333-y
Perez S, Makshakova O (2022) Multifaceted computational modeling in glycoscience. Chem Rev 122:15914–15970. https://doi.org/10.1021/acs.chemrev.2c00060
Porter RS, Johnson JF (1959) Circular gas chromatograph. Nature 183:391–392. https://doi.org/10.1038/183391a0
Queiroz EF, Guillarme D, Wolfender JL (2024) Advanced high-resolution chromatographic strategies for efficient isolation of natural products from complex biological matrices: from metabolite profiling to pure chemical entities. Phytochem Rev 23. https://doi.org/10.1007/s11101-024-09928-w
Rohaity R, Nor Hadiani I, Nurhuda M (2017) Recycling HPLC for the purification of oligostilbenes from Dipterocarpus semivestitus and Neobalanocarpus heimii (Dipterocarpaceae). J Liq Chromatogr Relat Technol 40:943–949. https://doi.org/10.1080/10826076.2017.1386673
Rosas-Ramírez D, Pereda-Miranda R (2013) Resin glycosides from the yellow-skinned variety of sweet potato (Ipomoea batatas). J Agric Food Chem 61:9488–9494. https://doi.org/10.1021/jf402952d
Rencurosi A, Mitchell EP, Cioci G, Pérez S, Pereda-Miranda R, Imberty A (2004) Crystal structure of tricolorin A: molecular rationale for the biological properties of resin glycosides found in some Mexican herbal remedies. Angew Chem Int Edit 43:5918–5922. https://doi.org/10.1002/anie.200460327
Saito H, Toyoda Y, Hirata H, Ota-Kontani A, Tsuchiya Y, Takada T, Suzuki H (2020) Soy isoflavone genistein inhibits an axillary osmidrosis risk factor ABCC11: in vitro screening and fractional approach for ABCC11-inhibitory activities in plant extracts and dietary flavonoids. Nutrients 12:2452. https://doi.org/10.3390/nu12082452
Shang C, Gu Y, Koyama T (2021) Major triterpenes, cycloeucalenone and 31-norcyclolaudenone as inhibitors against both α-glucosidase and α-amylase in banana peel. Int J Food Sci Tech 56:3519–3526. https://doi.org/10.1111/ijfs.14978
Schlinge D, Scherpian P, Schembecker G (2010) Comparison of process concepts for preparative chromatography. Chem Eng Sci 65:5373–5381. https://doi.org/10.1016/j.ces.2010.06.030
Sidana J, Joshi LK (2013) Recycle HPLC: a powerful tool for the purification of natural products. Chromatogr Res Int 2013:509812. https://doi.org/10.1155/2013/509812
Suárez-Ortíz GA, Cerda-García-Rojas CM, Fragoso-Serrano M, Pereda-Miranda R (2017) Complementarity of DFT calculations, NMR anisotropy, and ECD for the configurational analysis of brevipolides K-O from Hyptis brevipes. J Nat Prod 80:181−189. https://doi.org/10.1021/acs.jnatprod.6b00953
Sugawara K, Watarai H, Ise Y, Yokose H, Moril Y, Yamawaki N, Okada S, Matsunaga S (2021) Mar Drugs 19:287. https://doi.org/10.3390/md19060287
Surve NS, Thomas AB, Bhole RP, Patil CY (2023) Flash chromatography and semi-preparative HPLC: review on the applications and recent advancements over the last decade. Eurasian J Chem 109:20–30. https://doi.org/10.31489/2959-0663/1-23-9
Teoh HK, Sorensen E, Titchener-Hooker N (2003) Optimal operating policies for closed-loop recycling HPLC processes. Chem Eng Sci 58:4145–4158. https://doi.org/10.1016/S0009-2509(03)00287-2
Thirumurugan D, Cholarajan A, Raja SSS, Vijayakumar R (2018) An introductory chapter: secondary metabolites. In: Vijayakumar R, Raja SSS (eds) Secondary metabolites. Intech Open, pp 1–21. https://doi.org/10.5772/intechopen.79766
Townsend SD (2023) Glycoscience in infectious diseases virtual special issue. ACS Infect Dis 9:1. https://doi.org/10.1021/acsinfecdis.2c00613
Wang Y, Cui Z, Chi J, Tang P, Zhang M, Li J, Li Y, Zhang H, Luo J, Kong L (2021) Sarcaglarols A-D, lindenane-monoterpene heterodimers from Sarcandra glabra based on molecular networks. Chin J Chem 39:129–136. https://doi.org/10.1002/cjoc.202000456
Wei F, Sang J, Zhao Y (2021) Theoretical study of twin-column recycling chromatography with a solvent-gradient for preparative binary separations. J Chromatogr A 1651:462306. https://doi.org/10.1016/j.chroma.2021.462306
Wei F, Yang Z, Zhao Y, Wang Q (2019) A twin-column recycling chromatography with solvent gradient for reinforcing the isolation of minor impurities. AIChE J 65:702–711. https://doi.org/10.1002/aic.16444
Won HJ, Lee SM, Kim D-Y, Kwon O-K, Park MH, Kim J-H, Ryu HW, Oh S-R (2019) Rapid securing of reference substances from Peucedanum japonicum Thunberg by recycling preparative high-performance liquid chromatography. J Chromatogr B 1133:121835. https://doi.org/10.1016/j.jchromb.2019.121835
Xu WJ, Xie X, Wu L, Tian XM, Wang CC, Kong LY, Luo J (2020) Cajuputones A-C, β-triketone flavanone hybrids from the branches and leaves of Melaleuca cajuputi. Chem Biodiversity 17:e2000706. https://doi.org/10.1002/cbdv.202000706
Yuan H, Zhanga L, Zhang W, Liang Z, Zhang Y (2009) Columns switch recycling size exclusion chromatography for high resolution protein separation. J Chromatogr A 1216:7024–7032. https://doi.org/10.1016/j.chroma.2009.08.065
Zhang M, Stout MJ, Kubo I (1992) Isolation of ecdysteroids from Vitex strickeri using RLCC and recycling HPLC. Phytochemistry 31:247–250. https://doi.org/10.1016/0031-9422(91)83046-N
Zeng X, Andrade CA, Oliveira MD, Sun XL (2012) Carbohydrate–protein interactions and their biosensing applications. Anal Bioanal Chem 402:3161–3176. https://doi.org/10.1007/s00216-011-5594-y
Zhu Y, Bowen TJ, Song X (2020) Preparative scale purification of natural glycans by closed-loop recycle HPLC. Anal Biochem 599:113702. https://doi.org/10.1016/j.ab.2020.113702
Žuvela P, Skoczylas M, Jay LJ, Ba̧czek T, Kaliszan R, Wong MW, Buszewski B (2019) Column characterization and selection systems in reversed-phase high-performance liquid chromatography. Chem Rev 119:3674–729. https://doi.org/10.1021/acs.chemrev.8b00246
Acknowledgements
The authors are grateful to all graduate students, postdoctoral researchers, and collaborators cited in the reviewed references for their significant contributions.
Funding
Financial support was provided by Dirección General de Asuntos del Personal Académico, UNAM (DGAPA: IN202123).
Author information
Authors and Affiliations
Contributions
RPM conceptualized, designed, and wrote the manuscript. JFCG searched for references and wrote the applications in natural products, except glycoconjugates and resin glycosides. MFS has extensively revised and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Competing Interests
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pereda-Miranda, R., Castañeda-Gómez, J.F. & Fragoso-Serrano, M. Recycling Preparative Liquid Chromatography, the Overlooked Methodology for the Purification of Natural Products. Rev. Bras. Farmacogn. (2024). https://doi.org/10.1007/s43450-024-00561-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43450-024-00561-4