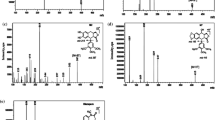
Abstract
A bioanalytical method was developed and validated for digoxin. The method was extremely sensitive and without adduct formation by ultra-performance liquid chromatography coupled with electro-spray ionization-tandem mass spectrometry. Internal standard was used as digoxin-d3. The chromatographic optimization was achieved by using an analytical column Kinetex biphenyl C18 and a mobile phase containing 1 mM NH4HCO2 in methanol in a binary gradient mode. The solid phase extraction technique was used for the extraction and cleaning of the analyte from plasma. Multiple reaction monitoring in positive polarity was applied for the quantification of digoxin. The analyzed concentrations ranged from 20 to 4000 pg/ml of digoxin which was used to plot a calibration curve; a linear regression was detected in the range of 0.9955 to 0.9992. The inter-day precision was found in the range of 2.76 to 8.33 for quality control samples. This validated method by using UHPLC-MS/MS can be applied for bioequivalence studies of digoxin.
Graphical Abstract




Similar content being viewed by others
References
Boulanger B, Rozet E, Moonen F, Rudaz S, Hubert P (2009) A risk-based analysis of the AAPS conference report on quantitative bioanalytical methods validation and implementation. J Chromatogr B 877:2235–2243. https://doi.org/10.1016/j.jchromb.2009.06.019
Campbell TJ, MacDonald PS (2003) Digoxin in heart failure and cardiac arrhythmias. Med J Aust 179:98–102. https://doi.org/10.5694/j.1326-5377.2003.tb05445.x
Erngren I, Haglöf J, Engskog MK, Nestor M, Hedeland M, Arvidsson T, Pettersson C (2019) Adduct formation in electrospray ionisation-mass spectrometry with hydrophilic interaction liquid chromatography is strongly affected by the inorganic ion concentration of the samples. J Chromatogr A 1600:174–182. https://doi.org/10.1016/j.chroma.2019.04.049
Hashimoto Y, Shibakawa K, Nakade S, Miyata Y (2008) Validation and application of a 96-well format solid-phase extraction and liquid chromatography–tandem mass spectrometry method for the quantitation of sigoxin in human plasma. J Chromatogr B 869:126–132. https://doi.org/10.1016/j.jchromb.2008.05.026
Iyer SS, Zhang ZP, Kellogg GE, Karnes HT (2004) Evaluation of deuterium isotope effects in normal-phase LC-MS-MS separations using a molecular modeling approach. J Chromatogr Sci 42:383–387. https://doi.org/10.1093/chromsci/42.7.383
Kirby BJ, Kalhorn T, Hebert M, Easterling T, Unadkat JD (2008) Sensitive and specific LC-MS assay for quantification of digoxin in human plasma and urine. Biomed Chromatogr 22:712–718. https://doi.org/10.1002/bmc.988
Klausner RE, Parra D, Kohl K, Brown T, Hill GD, Minich L, Godown J (2021) Impact of digoxin use on interstage outcomes of single ventricle heart disease (from a NPC-QIC registry analysis). Am J Card 154:99–105. https://doi.org/10.1016/j.amjcard.2021.05.048
Li X, Wang Y, Zhou Q, Yu Y, Chen L, Zheng J (2015) A sensitive method for digoxin determination using formate-adduct ion based on the effect of ionization enhancement in liquid chromatography–mass spectrometer. J Chromatogr B 978:138–144. https://doi.org/10.1016/j.jchromb.2014.11.023
Øiestad EL, Johansen U, Opdal MS, Bergan S, Christophersen AS (2009) Determination of digoxin and digitoxin in whole blood. J Anal Toxicol 33:372–378. https://doi.org/10.1093/jat/33.7.372
Patocka J, Nepovimova E, Wu W, Kuca K (2020) Digoxin: pharmacology and toxicology - a review. Environ Toxicol Pharmacol 79:103400. https://doi.org/10.1016/j.etap.2020.103400
Scalese MJ, Salvatore DJ (2017) Role of digoxin in atrial fibrillation. J Pharm Pract 30:434–440. https://doi.org/10.1177/0897190016642361
Shi S, Li Z, Chen H, Zeng F (2008) Development and validation of an LC–MS method with electrospray ionization for quantitation of digoxin in human plasma and urine: application to a pharmacokinetic study. J Chromatogr B 875:405–410. https://doi.org/10.1016/j.jchromb.2008.09.016
Wofford JL, Ettinger WH (1991) Risk factors and manifestations of digoxin toxicity in the elderly. Am J Emerg Med 9:11–15
Xue X, Huang M, Xiao H, Qin X, Huang L, Zhong G, Bi H (2011) Rapid and simultaneous measurement of midazolam, 1′-hydroxymidazolam and digoxin by liquid chromatography/tandem mass spectrometry: application to an in vivo study to simultaneously measure P-glycoprotein and cytochrome P450 3A activity. J Pharm Biomed Anal 55:187–193. https://doi.org/10.1016/j.jpba.2011.01.018
Acknowledgements
The authors are thankful to Ms. Swati Guttikar, Dr. Ajay Gupta, and Dr. Primal Sharma, Veeda Clinical Research, Ahmedabad, for their continuous technical support. The authors are also thankful to Dr. Nitin Bhatnagar Professor, GLA University, Mathura, for editing the manuscript.
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the paper as follows: study conception and design: GPA, KS; data collection: KS; analysis and interpretation of results: KS; draft manuscript preparation: GPA. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Agrawal, G.P., Soni, K. Development of a Bioanalytical Method by UHPLC-ESI-TQD-MS for the Quantification of Digoxin in Plasma. Rev. Bras. Farmacogn. 33, 747–755 (2023). https://doi.org/10.1007/s43450-023-00404-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43450-023-00404-8