Abstract
Osteosarcoma is the most common primary malignant bone tumor; the main treatment method is surgery and adjuvant chemotherapy, with a 5-year survival rate of less than 20% for metastatic patients. Schisandrin B is the most abundant and active ingredient found in the fruit of Schisandra chinensis (Turcz.) Baill., Schisandraceae, which has document properties such as liver protection, antioxidant, anti-inflammatory, antiaging, and antitumor. The present investigation explored the therapeutic effect of schisandrin B on osteosarcoma (MG63 cells). Cell proliferation and viability, scratch assay, and transwell migration analysis were used to detect the effects of schisandrin B on the growth activity, migration, and invasion of MG63 cells. The effects of schisandrin B on MG63 cell apoptosis were detected by flow cytometry and tunel staining. Western blot was used to detect the expression levels of autophagy and apoptosis related proteins. Immunofluorescence staining was used to detect schisandrin B effects of autophagy and apoptosis on MG63 cells. Schisandrin B inhibited the growth activity, migration ability, and invasion ability of osteosarcoma cells. In addition, schisandrin B induced apoptosis of MG63 cells through autophagy mediated by PI3K/AKT/mTOR signaling pathway.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteosarcoma is a tumor derived from bone forming mesenchymal stem cells; it is also the most common primary bone malignant tumor (Harrison et al. 2018). The highest incidence occurs in children and adolescents (median age: 18 years), followed by older adults over 60 years of age, with a bimodal age distribution. Globally, the incidence is about 1 to 3 cases per million people per year (Cersosimo et al. 2020). Osteosarcoma mainly occurs in long bones, near the growth plate of diaphysis, and less in skull, jaw, and pelvis. Typical symptoms are local pain followed by local swelling and limited joint mobility; however, pathological fractures at the disease site are a rare event (Yoshida 2021). Osteosarcoma is a highly metastatic tumor, and lung was the most common site of metastasis, followed by distal bone and lymph nodes. Metastatic disease and relapse remain the leading causes of death in patients with osteosarcoma (Chen et al. 2021). At present, the main treatment methods are neoadjuvant therapy combined with surgery and chemotherapy (Kager et al. 2017). The 5-year survival rate for localized disease in children and young adults after treatment is 78%, but the 5-year survival rate for metastatic patients at diagnosis or recurrence is still only 20% (ElKordy et al. 2018). Therefore, improving the treatment of osteosarcoma remains a primary goal of research at clinical communities worldwide.
Schizandrin B (1) is the most abundant, biologically active dibenzocyclooctadiene lignan found in the fruit of Schisandra chinensis (Turcz.) Baill., Schisandraceae, or “Bei Wu-Wei-Zi” a commonly used herb in traditional Chinese medicine. It has a variety of activities and plays an important role in hepatoprotection, and as antioxidant, antiaging, and antitumor agent (Nasser et al. 2020). The antitumor effects of schizandrin B are mainly manifested in blocking the cell cycle, promoting cell apoptosis, and enhancing oxidative stress and autophagy (Zhao et al. 2021a, b). Another study found that schizandrin B could inhibit the proliferation of prostate cancer cells and promote apoptosis by enhancing oxidative stress, inhibiting androgen receptors, and activating the autophagy pathway PI3K/AKT (Nasser et al. 2019). Similarly, Li and Lu studied the effect of schizandrin B on glioma cells in vitro and in vivo and found that it induced apoptosis of glioma cells in a concentration dependent manner in vitro. Also, schizandrin B significantly inhibited tumor growth in vivo after subcutaneous inoculation of U87 cells in nude mice without thymus (Li et al. 2015). Study has also shown that schizandrin B can inhibit glial cell proliferation and metastasis by regulating the H0TAIR-microRNA-125a-mTOR signaling pathway (Jiang et al. 2017). Inhibited the proliferation of lung cancer cells A549 by blocking cell cycle and promoting apoptosis (Zhuang et al. 2019). In patients with triple-negative breast cancer, schizandrin B plays a powerful antitumor role by inhibiting STAT3 expression (Dai et al. 2018). Currently, there are many studies on the inhibitory effects of schizandrin B on tumors (Nasser et al. 2020), but there are a few related to its effects on osteosarcoma and its mechanism of action. Therefore, the therapeutical potential on human osteosarcoma cells was investigated.

Materials and Methods
Cell Culture and Treatment
Human osteosarcoma cell line MG63 was purchased from the Shanghai Chinese Academy of Sciences Cell Bank. Schizandrin B powder (purity > 98%, Lot number: E1822053) was purchased from aladdin and dissolved in PBS. The cells were cultured in Minimum Essential Medium (MEM) (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc) and 1% (penicillin/streptomycin) at 37 °C in a humidified incubator with 5% CO2.
Cell Proliferation Assay
To investigate the cytotoxicity of schizandrin B, the cell counting kit-8 assay was used. MG63 cells (1 × 105 cells/well) were transferred in 96-well plates for 24 h and tested different concentrations (0, 1, 2, 5, 10, 20, 40, 80 and 160 μg/ml) of schizandrin B for 24 h. After the exposure period, cell counting was carried out.
Scratch Assay
MG63 cells with logarithmic growth were planted in 6-well plates with about 5 × 105 cells/well. When the cells grew to 100% confluence, they were carefully scraped with a 200 μl sterile gun tip to keep the straight line and single layer, and then gently washed with cold PBS to remove floating cells. Then different concentrations of schizandrin B, prepared with MEM containing 2% FBS, were added and incubated for 72 h. After incubation time, the floating cells were washed with PBS and representative images were captured under an inverted fluorescence microscope using ImageJ software (v1.8.0.112; National Institutes of Health). All experiments were performed at least 3 times.
Transwell Migration Assay
The transwell invasion assay was performed using a transwell chamber and an 8-micron aperture filter (EMD Millipore). Polycarbonate filter coated with matrigel (30 µg/well; BD matrix matrix) and incubated in a 37 °C incubator for 1 h before inserting the cavity into a 24-well culture plate. MG63 cells were starved overnight in FBS free MEM, and a single cell suspension was seeded into the upper compartment (5 × 104 cells/well, in FBS free MEM). Minimum essential medium (600 μl) containing 10% FBS was added into the lower cavity. After incubation in the 37 °C incubator for 24 h, the non-invasive cells above the filter were removed using cotton swabs. The invasive cells were fixed in methanol at room temperature for 30 min, stained with 0.1% crystal purple at room temperature for 15 min, and finally observed under a fluorescence microscope (Olympus, × 100 magnification). All experiments were performed at least 3 times.
Flow Cytometry
Annexin V-PE apoptosis assay kit (BD, Biosciences, Franklin Lakes, NJ, USA) was used to detect the apoptosis rate of osteosarcoma cells. Firstly, cells were inoculated into 6-well plates with about 5 × 105 cells per well. After the cells were adhered to the wall and grew to 80% full plate rate, they were treated with schizandrin B (0, 20 and 40 µg/ml) at 37 °C for 24 h. At the end of incubation time, they were washed twice with cold PBS, then resuspended in 100 µl, 1 × binding buffer and transferred to 1.5 ml culture tubes with 1 µl annexin V-PE and 1 µl 7-AAD. Incubate the mixture at room temperature in the dark for 15 min. Results were immediately evaluated by FACS flow cytometry (BD, Biosciences, Franklin Lakes, NJ, USA) and analyzed by Flowjo software. All experiments were performed at least 3 times.
Tunel Assay
MG63 cells were seeded on 6-well plates cell slides. After the cells adhered, they were stimulated with different concentrations of schizandrin B (0, 20 and 40 μg/ml) for 24 h. The slides were collected and used according to the manufacturer's instructions. Apoptotic cells were detected by a one-step Tunel kit (Beyotime, Shanghai, China), and nuclei were stained with DAPI (5 mg/ml) for 3 min at room temperature. It was then observed under a fluorescence microscope.
Western Blot Analysis
The MG63 cells were washed twice with PBS and lysed in RIPA buffer (Beijing Sora Biotechnology Co., Ltd.) containing 2 mM phenylmethylsulfonyl fluoride (PMSF). Protein concentrations were determined using the bicinchoninic acid method (CoWin Biosciences). Proteins were separated using 12% SDS-PAGE and transferred to 0.45 µm PVDF membranes and blocked with 10% nonfat milk for 2 h at room temperature. Membranes were then incubated overnight at 4 °C with the following primary antibodies: β-actin (1:1000, 4ab020185, 4A Biotech), LC3 (1:1000, NB100-2220, Novus Biologicals), P62 (1:1000, 88588S, Cell Signaling Technology), ATG5 (1:1000, 12994 T, Cell Signaling Technology), cleaved caspase-3 (1:1000, 9661, Cell Signaling Technology), Bax (1:1000, 50599–2-Ig, proteintech), Bcl-2 (1:1000, 26593–1-AP, proteintech), Beclin1 (1:1000, ab210498, abcam), AKT (1:1000, 4685, Cell Signaling Technology), p-AKT (1:1000, 4060, Cell Signaling Technology), PI3K (1:1000, 4255, Cell Signaling Technology), p-PI3K (1:1000, 17366, Cell Signaling Technology), mTOR (1:1000, 66888–1-Ig, proteintech), p-mTOR (1:1000, 67778–1-Ig, proteintech). Then, the membranes were mixed with HRP-labeled secondary antibodies (goat anti-rat, cat. no. A0192; goat anti-rabbit, A0208 (both 1:10,000) (both from Beyotime Institute of Biotechnology) for 1 h at room temperature. Protein bands were detected using a gel imaging and analysis system (Tanon Scuence and Technology Co., Ltd.). Densitometry was performed using ImageJ software (National Institutes of Health). 3-methyladenine (3-MA, 10 mM, MedChemExpress).
Immunofluorescence
In the culture plate, slides were washed the with PBS for 3 times, 3 min each time; the slides were fixed with 4% paraformaldehyde for 15 min, and washed 3 times in PBS, 3 min each time; 0.5% Triton X-100 (prepared with PBS) was permeabilized at room temperature for 20 min, the slides were soaked in PBS for 3 times, 3 min each time, the PBS was dried on absorbent paper, normal goat serum was added dropwise on the glass slides, and the glass slides were blocked for 30 min at room temperature; the blocking solution was removed by the absorbent paper without washing, a sufficient amount of diluted primary antibody was dropped into each slide and put it in a wet box, incubated at 4 °C overnight. The next day, fluorescent secondary antibody was added, slides were dipped in PBST 3 times, 3 min each time. Absorbent paper was used to dry up the excess liquid on the slides, diluted fluorescent secondary antibody dropwise was added and incubated at 20–37 °C for 1 h in a wet box; the slides were washed 3 times with PBST for 3 min each time; DAPI was added and incubated in the dark for 5 min, then the specimens were subjected to nuclei staining, and the excess DAPI was washed off with PBST 5 min × 4 times, the liquid on the slide was blotted with absorbent paper, and the slide was sealed with a mounting liquid containing anti-fluorescence quencher, and then the images were collected under a fluorescence microscope.
Statistical Analysis
SPSS 19.0 was used for data analysis and GraphPad Primer 8 was used for graph generation. The data conforming to the normal distribution were expressed as mean ± standard deviation; t test was used to compare two groups of data, and one-way ANOVA was used to compare more than two groups of data. p < 0.05 was considered statistically significant.
Results and Discussion
MG63 Cells Proliferation
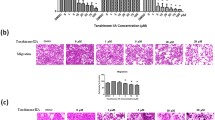
Initially, the toxic effect of schizandrin B (1) on the viability of MG63 cells. CCK-8 assay showed no significant difference in cell viability when cells were pretreated with schizandrin B 0 to 10 µg/ml. However, cell viability was gradually inhibited with increasing schizandrin B concentration (Fig. 1); therefore, the concentrations used in subsequent experiments were 20 and 40 µg/ml.
MG63 Cells Migration and Invasion
In order to confirm the effect of schizandrin B on the migration and invasion ability of MG63 cells, the migratory ability of the cells was first detected by scratch assay. The results are shown in Fig. 2A, after MG63 cells were stimulated with different concentrations of schizandrin B (0, 20 and 40 µg/ml) for 72 h, the number of cells migrating to the middle of the scratch decreased with increasing concentrations of the tested sample. In addition, the invasive ability of schizandrin B on MG63 cells was detected by the transwell assay. As shown in Fig. 2B, schizandrin B (0, 20 and 40 µg/ml) significantly inhibited the invasive ability of MG63 cells. The inhibitory ability was stronger when the concentration was 40 µg/ml. Statistical analysis was performed on the experimental results of the two groups (Fig. 2C and D), and the results were all statistically significant.
Effects of schizandrin B (1) on migration and invasion ability of MG63 cells. A MG63 cells were stimulated with different concentrations of schizandrin B for 72 h, and the migration ability of cells was detected by scratch test. B MG63 cells were incubated with different concentrations of schizandrin B for 24 h, and the invasive ability of the cells was detected by transwell assay. C and D Statistical analysis. Different data are expressed as the mean ± SD of three independent experiments
Cells Apoptosis
The effect of schizandrin B on apoptosis of MG63 cells was studied by flow cytometry. After 24 h of treatment with different concentrations of schizandrin B (0, 20 or 40 μg/ml), the early apoptosis rates of cells were 11.5, 17.2, and 23.9%, respectively, and increased significantly in a dose-dependent manner (Fig. 3C). Compared with the control group, there was statistical significance (p < 0.05). To further explore the potential mechanism of schizandrin B induced apoptosis of MG63 cells, the expression levels of apoptotic proteins were detected by western blot. The results showed that the expression levels of the pro-apoptotic proteins cleaved caspase-3 and Bax were increased, while the expression level of the anti-apoptotic protein Bcl-2 was decreased (Fig. 3A). All were found to be statistically significant (Fig. 3B). Finally, tunel staining was used to detect the number of apoptotic cells after stimulating MG63 cells with different concentrations of schizandrin B. The results were consistent with the results of flow cytometry (Fig. 3D). The number of apoptotic cells increased in a concentration dependent manner after schizandrin B stimulated MG63 cells.
Schizandrin B (1) induces apoptosis in MG63 cells. A Western blot analysis was used to determine the expression levels of apoptosis related proteins in cells treated with different concentrations of schizandrin B for 24 h. B Statistical analysis. C Apoptosis was detected by FITC-labeled Annexin V/7-AAD apoptosis detection kit and flow cytometry. D Tunel staining was used to detect the number of apoptotic cells. Different data are expressed as the mean ± SD of three independent experiments
Autophagy
In order to further explore the mechanism of apoptosis induced by schizandrin B in MG63 cells, different concentrations of schizandrin B (0, 20, and 40 µg/ml) were used to stimulate MG63 cells for 24 h, and autophagy related proteins were collected and detected by western blot. As shown in Fig. 4A, with the increase of schizandrin B concentration, the expression of autophagy related protein P62 was down-regulated, and the expressions of LC3II/LC3I, ATG5, and Beclin1 were up-regulated, demonstrating that schizandrin B can enhance autophagy with a statistical significance (Fig. 4B). Similarly, after different concentrations of schizandrin B stimulated MG63 cells for 24 h, the expression levels of autophagy related proteins P62 and LC3 were detected by immunofluorescence. Results are shown in Fig. 4C and D, the expression of autophagy protein LC3 was up-regulated, and the expression of P62 was down-regulated. It was also demonstrated that schizandrin B stimulated MG63 cells to enhance autophagy.
Schizandrin B (1) enhances autophagy in MG63 cells. A Western blot analysis was used to determine the expression levels of autophagy related proteins in cells treated with different concentrations of schizandrin B for 24 h. B Statistical analysis. C and D Immunofluorescence detection of the expression levels of autophagy proteins LC3 and P62. Different data are expressed as the mean ± SD of three independent experiments
Autophagy Mediated by PI3K/AKT/mTOR
The results illustrated in Fig. 5A represents the expressions of proteins p-PI3K, p-AKT, and p-mTOR, which were up-regulated, and the statistical analysis was significant, indicating that schizandrin B induced autophagy in MG63 cells mediated through the PI3K/AKT/MTOR pathway (Fig. 5B). After treating MG63 cells with the autophagy inhibitor 3-MA, it was found that the autophagy related protein P62 was up-regulated, LC3II/LC3I was down-regulated, the pro-apoptotic protein Bax was down-regulated, and the anti-apoptotic protein Bcl-2 was up-regulated (Fig. 5C) with a statistical significance (Fig. 5D). Similarly, the expression of apoptotic protein caspase-3 after 3-MA treatment was detected by immunofluorescence assay and the expression of this apoptotic protein decreased after autophagy inhibition (Fig. 5E). The above results confirm that schizandrin B promotes apoptosis of osteosarcoma cells by enhancing autophagy.
Schizandrin B (1) enhances autophagy and induces apoptosis in MG63 cells via PI3K/AKT/mTOR signaling pathway. A Western blot was used to analyze the expression levels of autophagy related pathways in cells treated with different concentrations of schizandrin B for 24 h. C Western blot was used to analyze the expression levels of autophagy and apoptosis proteins in MG63 cells treated with 3-MA. B and D Statistical analysis. E The expression of apoptotic protein caspase-3 after 3-MA treatment was detected by immunofluorescence method. Different data are expressed as the mean ± SD of three independent experiments
The main clinical manifestations of osteosarcoma are pain due to tumor tissue erosion and cortical dissolution (Harrison et al. 2018). Currently, the main treatment for osteosarcoma is surgery combined with neoadjuvant chemotherapy (Meltzer and Helman 2021). Although this combined approach improves the prognosis for patients with osteosarcoma, the prognosis for patients with metastatic or recurrent bone cancer remains unsatisfactory (Yoshida 2021).
Tumor cell death can be induced in a variety of ways, among which the most common way is to induce tumor cell apoptosis. Autophagic apoptosis is the most important form of apoptosis, which induces apoptosis by enhancing autophagy (Lin et al. 2019). Autophagy is a fundamental physiological process widely present in eukaryotic cells that maintains cellular homeostasis and homeostasis by removing harmful protein aggregates, damaged organelles, and certain pathogens through lysosomal degradation (Sciarretta et al. 2018; Kumariya et al. 2021 Li et al. 2021). Autophagy has a dual effect on tumor cells, proper autophagy is critical for maintaining cellular homeostasis, and excessive autophagy can lead to cell death (Xu and Hu 2022). Therefore, autophagy plays an important role in the process of antitumor drug treatment. There are many proteins related to autophagy, mainly including LC3、P62, ATG5, and Beclin1 (Levine and Kroemer 2019). LC3, which plays a key role in the maturation of autophagosomes, LC3 is modified by Atg4 in its C-terminal domain to become LC3-I, which is then combined with phosphatidyl, ethanolamine coupling becomes LC3-II (Nakamura et al. 2021). P62/SQSTM1 is the most well-known autophagy target and acts as a scaffold protein in various tissues (Jeong et al. 2019). It usually forms aggregates in the cytoplasm and is selectively degraded by autophagy (Kageyama et al. 2021). Beclin1, a homologue of the Atg6/vacuolar protein sorting (Vps)-30 protein in yeast, plays an important role in a key step in the autophagy process by interacting with inositol 3-kinase class III phosphate (Tran et al. 2021). The discovery of the autophagy gene (Atg) in yeast has had a huge impact on our understanding of the process and mechanism of autophagy (Nishimura and Tooze 2020). Most Atg genes are conserved in humans and play various roles, including autophagy initiation, autophagosome formation and maturation.
In our study, stimulation of MG63 cells with schizandrin B was performed and observed its effect on the growth activity of osteosarcoma cells. The results of proliferation and viability experiments showed that schizandrin B could inhibit the growth of MG63 cells. It is known that the main characteristics of tumor cells are local invasion and distant metastasis. Next, the effects of schizandrin B on the migration and invasion of MG63 cells were observed by scratch and transwell experiments, and it was found that schizandrin B could inhibit the migration and invasion of OS cells. Therefore, it was concluded that schizandrin B could inhibit the growth and metastasis of osteosarcoma cells. For tumor cells, autophagy is usually two-sided, it can recycle senescent organelles and nutrients to maintain energy reuse, but at the same time it can also be stimulated by stress or injury. It can inhibit the growth of tumor cells by activating the apoptosis signaling pathway, causing the cells to undergo autophagic cell death (Datan and Salman 2020; Schwartz 2021), which is also known as type II programmed death, compared with type I programmed death, its main feature is that a large number of autophagosomes and autophagolysosomes appear in the cytoplasm, and finally lysosome digestion and degradation cause cell death. In our study, after MG63 cells were treated with schizandrin B, the conversion of LC3-I to LC3-II could be clearly observed by Western blot assay, and the green fluorescence of autophagy protein P62 was weakened and the green fluorescence of LC3 was enhanced by immunofluorescence staining. These results all prove that schizandrin B can induce autophagy in osteosarcoma cells. In our study, an increase in early apoptotic cells was observed after schizandrin B treatment of MG63 cells, western bolt experiment observed that the expression of pro-apoptotic proteins Bax and caspase-3 increased, and the expression of anti-apoptotic protein Bcl-2 decreased. Tunel staining also indicated that green fluorescent spots increased after schizandrin B stimulated MG63 cells. The above three experimental methods all showed that schizandrin B could induce apoptosis of osteosarcoma cells. Finally, after inhibiting autophagy by using the autophagy inhibitor 3-MA, both western bolt assay and immunofluorescence assay observed a decrease in the expression of pro-apoptotic proteins and an increase in the expression of anti-apoptotic proteins.
Conclusion
In conclusion, schizandrin B, a bioactive principle from a traditional Chinese herbal medicine with multiple functions, can reduce the growth activity of osteosarcoma cells, inhibit their migration and invasion ability, and induce their apoptosis by enhancing autophagy, which was mediated by the PI3K/AKT/mTOR signaling pathway.
Data Availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Change history
19 February 2024
A Correction to this paper has been published: https://doi.org/10.1007/s43450-024-00522-x
References
Cersosimo F, Lonardi S, Bernardini G, Telfer B, Mandelli GE, Santucci A, Vermi W, Giurisato E (2020) Tumor-associated macrophages in osteosarcoma: from mechanisms to therapy. Int J Mol Sci 21:5207. https://doi.org/10.3390/ijms21155207
Chen C, Xie L, Ren T, Huang Y, Xu J, Guo W (2021) Immunotherapy for osteosarcoma: fundamental mechanism, rationale, and recent breakthroughs. Cancer Lett 500:1–10. https://doi.org/10.1016/j.canlet.2020.12.024
Datan E, Salman S (2020) Autophagic cell death in viral infection: do TAM receptors play a role? Int Rev Cell Mol Biol 357:123–168. https://doi.org/10.1016/bs.ircmb.2020.10.001
Dai X, Yin C, Guo G, Zhang Y, Zhao C, Qian J, Wang O, Zhang X, Liang G (2018) Schisandrin B exhibits potent anticancer activity in triple negative breast cancer by inhibiting STAT3. Toxicol Appl Pharmacol 358:110–119. https://doi.org/10.1016/j.taap.2018.09.005
ElKordy MA, ElBaradie TS, ElSebai HI, KhairAlla SM, Amin AAE (2018) Osteosarcoma of the jaw: challenges in the diagnosis and treatment. J Egypt Natl Canc Inst 30:7–11. https://doi.org/10.1016/j.jnci.2018.02.001
Harrison DJ, Geller DS, Gill JD, Lewis VO, Gorlick R (2018) Current and future therapeutic approaches for osteosarcoma. Expert Rev Anticancer Ther 18:39–50. https://doi.org/10.1080/14737140.2018.1413939
Jeong SJ, Zhang X, Rodriguez-Velez A, Evans TD, Razani B (2019) p62/SQSTM1 and selective autophagy in cardiometabolic diseases. Antioxid Redox Signal 31:458–471. https://doi.org/10.1089/ars.2018.7649
Jiang Y, Zhang Q, Bao J, Du C, Wang J, Tong Q, Liu C (2017) Schisandrin B inhibits the proliferation and invasion of glioma cells by regulating the HOTAIR-micoRNA-125a-mTOR pathway. NeuroReport 28:93–100. https://doi.org/10.1097/WNR.0000000000000717
Kageyama S, Gudmundsson SR, Sou YS, Ichimura Y, Tamura N, Kazuno S, Ueno T, Miura Y, Noshiro D, Abe M, Mizushima T et al (2021) p62/SQSTM1-droplet serves as a platform for autophagosome formation and anti-oxidative stress response. Nat Commun 12:16. https://doi.org/10.1038/s41467-020-20185-1
Kager L, Tamamyan G, Bielack S (2017) Novel insights and therapeutic interventions for pediatric osteosarcoma. Future Oncol 13:357–368. https://doi.org/10.2217/fon-2016-0261
Kumariya S, Ubba V, Jha RK, Gayen JR (2021) Autophagy in ovary and polycystic ovary syndrome: role, dispute and future perspective. Autophagy 17:2706–2733. https://doi.org/10.1080/15548627.2021.1938914
Li W, He P, Huang Y, Li YF, Lu J, Li M, Kurihara H, Luo Z, Meng T, Onishi M, Ma C, Jiang L, Hu Y, Gong Q, Zhu D, Xu Y, Liu R, Liu L, Yi C, Zhu Y et al (2021) Selective autophagy of intracellular organelles: recent research advances. Theranostics 11:222–256. https://doi.org/10.7150/thno.49860
Li Q, Lu XH, Wang CD, Cai L, Lu JL, Wu JS, Zhuge QC, Zheng WM, Su ZP (2015) Antiproliferative and apoptosis-inducing activity of schisandrin B against human glioma cells. Cancer Cell Int 15:12. https://doi.org/10.1186/s12935-015-0160-x
Lin MC, Lee YW, Tseng YY, Lin YW, Chen JT, Liu SH, Chen RM (2019) Honokiol induces autophagic apoptosis in neuroblastoma cells through a P53-dependent pathway. Am J Chin Med 47:895–912. https://doi.org/10.1142/S0192415X19500472
Levine B, Kroemer G (2019) Biological functions of autophagy genes: a disease perspective. Cell 11–42. https://doi.org/10.1016/j.cell.2018.09.048
Meltzer PS, Helman LJ (2021) New horizons in the treatment of osteosarcoma. N Engl J Med 385:2066–2076. https://doi.org/10.1056/NEJMra2103423
Nakamura S, Akayama S, Yoshimori T (2021) Autophagy-independent function of lipidated LC3 essential for TFEB activation during the lysosomal damage responses. Autophagy 17:581–583. https://doi.org/10.1080/15548627.2020.1846292
Nasser MI, Han T, Adlat S, Tian Y, Jiang N (2019) Inhibitory effects of schisandrin B on human prostate cancer cells. Oncol Rep 41:677–685. https://doi.org/10.3892/or.2018.6791
Nishimura T, Tooze SA (2020) Emerging roles of ATG proteins and membrane lipids in autophagosome formation. Cell Discov 6:32. https://doi.org/10.1038/s41421-020-0161-3
Nasser MI, Zhu S, Chen C, Zhao M, Huang H, Zhu P (2020) A comprehensive review on schisandrin B and its biological properties. Oxid Med Cell Longev 2020:2172740. https://doi.org/10.1155/2020/2172740
Schwartz LM (2021) Autophagic cell death during development, ancient and mysterious. Front Cell Dev Biol 9:656370. https://doi.org/10.3389/fcell.2021.656370
Sciarretta S, Maejima Y, Zablocki D, Sadoshima J (2018) The role of autophagy in the heart. Annu Rev Physiol 80:1–26. https://doi.org/10.1146/annurev-physiol-021317-121427
Tran S, Fairlie WD, Lee EF (2021) Beclin1: protein structure, function and regulation. Cells 10:1522. https://doi.org/10.3390/cells10061522
Xu HM, Hu F (2022) The role of autophagy and mitophagy in cancers. Arch Physiol Biochem 128:281–289. https://doi.org/10.1080/13813455.2019.1675714
Yoshida A (2021) Osteosarcoma: old and new challenges. Surg Pathol Clin 14:567–583. https://doi.org/10.1016/j.path.2021.06.003
Zhao B, Li GP, Peng JJ, Ren LH, Lei LC, Ye HM, Wang ZY, Zhao S (2021a) Schizandrin B attenuates hypoxia/reoxygenation injury in H9c2 cells by activating the AMPK/Nrf2 signaling pathway. Exp Ther Med 21:220. https://doi.org/10.3892/etm.2021.9651
Zhao N, Su X, Li H, Li Z, Wang Y, Chen J, Zhuang W (2021b) Schisandrin B inhibits α-melanocyte-stimulating hormone-induced melanogenesis in B16F10 cells via downregulation of MAPK and CREB signaling pathways. Biosci Biotechnol Biochem 85:834–841. https://doi.org/10.1093/bbb/zbaa100
Zhuang W, Li Z, Dong X, Zhao N, Liu Y, Wang C, Chen J (2019) Schisandrin B inhibits TGF-β1-induced epithelial-mesenchymal transition in human A549 cells through epigenetic silencing of ZEB1. Exp Lung Res 45:157–166. https://doi.org/10.1080/01902148.2019.1631906
Acknowledgements
Each author expresses his gratitude to their institutions for providing the opportunity to develop this manuscript. The authors sincerely thank the anonymous reviewers for the improvement in the quality of the manuscript.
Author information
Authors and Affiliations
Contributions
LQZ, LPZ, and HJM conducted the experiments; LQZ and LPZ contributed in writing the manuscript; JKZ edited the manuscript; HJM and JKZ contributed in collecting and analysis the references; DYL designed the study and revised the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, L., Zhou, L., Ma, H. et al. Schisandrin B Inhibits Osteosarcoma Cell Proliferation and Promotes Apoptosis Through PI3K/AKT/mTOR Pathway Mediated Autophagy. Rev. Bras. Farmacogn. 33, 945–953 (2023). https://doi.org/10.1007/s43450-023-00391-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43450-023-00391-w