Abstract
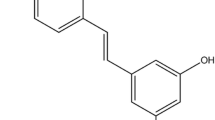
Resveratrol obtained in grape seed and skin is structurally similar to a synthetic estrogen diethylstilbestrol. The endogenous estrogen, 17β-estradiol, induces cellular responses by binding to the estrogen receptor alpha and beta. The bone fracture due to decreased bone mineral density in postmenopausal women is linked to reduced estrogen. The adverse drug reactions of hormone replacement therapy warrant identifying unique natural compounds with ER-subtype specificity to improve bone health. Resveratrol is considered a phytoestrogen; however, its isoform selectivity has not yet been established on osteoblast cell lines. Therefore, in vitro and in silico docking studies were performed to analyze the binding affinity and selectivity of resveratrol towards receptor alpha and β-isoforms. Resveratrol was evaluated for its actions on the proliferation and differentiation in the primary rat calvarial osteoblasts and bone marrow cells. Osteoblasts specifically increased receptor alpha expression in rat calvarial osteoblasts cells; however, there was no effect on receptor beta expression. In silico studies further confirmed receptor alpha isoform specificity. The observed differences in the orientation, interaction pattern, and binding affinity of resveratrol at the active site of receptor alpha and beta are supported by the western blot analysis. The estrogen mimetic action of resveratrol suggests its therapeutic potential as a bone anabolic agent for postmenopausal osteoporosis.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postmenopausal osteoporosis and fractures are mainly due to decreased estrogens. The symptoms include reduced bone mineral density and susceptibility for fractures, particularly in the hip, vertebrae, and wrist. The treatment option for postmenopausal osteoporosis includes mainly hormone replacement therapy (HRT), which includes estrogen as conjugated equine estrogen with or without a progestin to reduce the fracture risk by 30–40% (Cauley 2015). However, the regular use of HRT has limitations, such as risks for cardiovascular disease and breast cancer (Cagnacci and Venier 2019). The estrogens play a significant role in bone growth and development. Immunohistochemical analysis reveals the presence of ER-α and ER-β isoforms in osteoblasts (OB), osteocytes, and osteoclasts. Estrogen interacts with ER-α and ER-β and maintains the balance of osteoblastogenesis and osteoclastogenesis (Khalid and Krum 2016). The critical risk-benefit analysis associated with HRT represents an alternate use of phytoestrogens, non-steroidal natural products with estrogenic activity also found in several dietary sources (Glazier and Bowman 2001). These natural molecules induce proliferation and mineralization in osteoblast through the estrogen receptor–mediated pathway, which includes icariin (Song et al. 2013), ugonin K (Lee et al. 2012), genistein (Liao et al. 2014), puerarin (Wang et al. 2013), herba epimedii (Xiao et al. 2014a), and vanillic acid (Xiao et al. 2014b).
Resveratrol (1, RSV) is considered a phytoestrogen which shows a varying degree of estrogen receptor expression in different cells. RSV has anticancer activity in ER-positive MCF-7, ER-negative MDA-MB-231 breast cancer cells, and T47D cells (Gehm et al. 1997). The mixed agonist/antagonist estrogenic expression represents selective estrogen receptor modulators (SERMs). According to the molecular docking study carried out by Chakraborty et al. (2013), RSV displayed partial agonist actions on ER-α (Chakraborty et al. 2013). In a recent work, RSV and its derivatives were showed the highest correlation 0.797 for estrogen receptor by CoMFA/SIA (comparative molecular field analysis comparative molecular similarity index analysis) modeling (Pande et al. 2021).

As per reported literature, an in vitro evaluation on osteoblast, osteoclast, mesenchymal stem cells, and chondrocyte investigations, RSV (1) enhances bone mass by promoting osteoblastogenesis and inhibits osteoclastogenesis (Mobasheri and Shakibaei 2013). RSV inhibits miR-338-3p in human osteoblasts and subsequently enhances human osteoblast differentiation (Goel et al. 2015). In combination with other phytochemicals like genistein and quercetin, resveratrol showed an additive effect by decreasing adipogenesis and enhancing osteoblastogenesis (Rayalam et al. 2011). Tou JC (2014); had given a detailed insight into the pre-clinical studies on RSV associated with estrogen deficiency, age-related, bone acquisition and disuse bone loss conditions. RSV is responsible for the activation of SIRT1, which is a potent inhibitor of adipocyte development thereby increasing the expression of osteoblastic markers. It helps osteoblastic maturation and osteogenesis via ERK1/2 and p38-MAPK signaling pathways in human bone marrow stromal cell cultures (Baile et al. 2011). The potent antioxidant nature of RSV (Subramanian et al. 2014, 2016) displayed inhibition of osteoclastogenesis in RAW264.7 cells thus reducing the osteoclastogenesis (He et al. 2010). RSV similarly exhibits bone-protective potential as HRT without inducing any risk of breast cancer via Forkhead proteins. Therefore, RSV is the remedy for the treatment of postmenopausal osteoporosis with the safety profile in breast cancer (Su et al. 2007). With an increased risk of endometrial cancer by HRT (Crosignani 2003), our research group also assessed the safety profile of the RSV-SBE-β-CD inclusion complex (10 mg/kg/i.v./day) in rats for 3 months. It was safe without inducing any deliberate changes on the endometrium wall (Shah et al. 2021). HRT drugs have also demonstrated to cause serious adverse effects like stroke. RSV possesses cardioprotective effects against reperfusion-induced arrhythmias in ischemic reperfused myocardium via antioxidant and anti-inflammatory pathways (Sato et al. 2000). Apart from this, RSV has anticancer potential in cervical cancer cells through apoptosis and autophagy (García-Zepeda et al. 2013) and by inhibiting HPV E6 and E7 genes (Sun et al. 2020). The therapeutic role of RSV on female reproductive health is well explored in the case of endometriosis (Kolahdouz Mohammadi and Arablou 2017), polycystic ovary syndrome (Aquino and Nori 2014), infertility (Ochiai and Kuroda 2020), and preeclampsia (Ding et al. 2017). Maternal consumption of RSV has beneficial effects on both pregnant females and offspring due to its cardioprotective, antiobesogenic, antiatherosclerotic, and antidiabetic effects (Zheng et al. 2018). All these data suggest the advantages of RSV over HRT treatment.
RSV (1) has the dual effect of being an enhancer of osteoblast proliferation and an antagonist of osteoclast differentiation (Murgia et al. 2019). RSV is also a common target such as reactive oxygen species, lipid mediators, apoptosis, and pro-inflammatory mediators (Delmas et al. 2021) and therefore needs to be investigated further for its action on the estrogen receptor. RSV binds ER-α and ER-β with comparable affinity but with a 7000-fold lower affinity than 17β-estradiol (Bowers et al. 2000). However, its isoform selectivity has not yet been established on osteoblast cell lines. This study aims to understand the differences in the ability of RSV to activate the α- and β-isoforms of ER-receptors. We have investigated the effects of RSV on activation of the estrogen receptor isoforms in OB cells and its possible anabolic effect by using in vitro and computation modeling approaches.
Materials and Methods
Resveratrol compound (1) (purity: > 99.0% GC) was purchased from TCI Chemicals, India. For the in vitro studies, the rat calvarial OB cells were isolated and grown in α-MEM (Minimum Essential Medium Eagle) culture media purchased from Sigma-Aldrich (St. Louis, MO). The p-nitrophenyl phosphate was purchased from Calbiochem (San Diego, CA). Antibodies for the western blot analysis were procured from Cell Signaling Technology (USA) and Abcam (UK). All the computational studies like docking and MM-GBSA were performed by using the Maestro Molecular Modeling platform (version 11.4) (Schrodinger Inc.). The molecular docking studies were carried out for predicting intrinsic affinity towards isoforms of estrogen receptors and compared with the standard 17β-estradiol.
In Vitro Evaluation
Culture of Osteoblasts
The isolation and culturing of rat calvarial osteoblasts were performed as per reported literature by using a sequential enzymatic digestion method (Goel et al. 2015). Sprague-Dawley rat pups (both sexes, n = 3) of 1–2 days old were used to isolate the calvaria and processed for sequential enzymatic digestion. The cells released from digestions were pooled centrifuged and resuspended in the cell culture flasks containing a-MEM media supplemented with 10% FCS and 1% penicillin/streptomycin (complete growth medium).
Evaluation of Cell Viability
The safety profile of RSV (1) on OB was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The cells were seeded in a 96-well plate at about 1 × 105 cells. The cells cultured with medium alone served as a control, while the cells incubated with RSV (50 nM to 2.5 μM) for 48 h were considered treated groups (n = 3 experiments). The percentage cell viability was determined by % of cell survival = ([OD of the sample]/[OD of control]) × 100. Data were analyzed using GraphPad Prism 5.0 free trial version (GraphPad Software, San Diego, CA) for determination of half-maximal effective concentration (EC50).
ALP Activity Assay
Alkaline phosphatase (ALP) activity was determined by the enzymatic assay method as reported in the literature (Adluri et al. 2010). Osteoblast cells, at 90% confluence, were treated with 1 at 1 pM to 1 μM for 7 days at 39.5 °C in 96-well plates. After treatment, cells were washed with PBS, then lysed into 0.6 ml of buffer containing 10 mM Tris-HCl pH 7.5, 0.5 mM MgCl2, and 0.1% Triton X-100. The cell lysate was centrifuged at 470 × g, and the soluble fraction was used for enzyme assay. A sample volume of 50 μl each was added to 125 μl glycine buffer (25 mM, pH 9.4), containing 2 mM MgCl2 and 5 mM p-nitrophenyl phosphate (pNPP), and incubated at 37 °C for 50 min in a water bath. The enzymatic reaction was stopped by the addition of 125 μl 1 M NaOH. The final product (p-nitrophenol) was quantified at 405 nm in an ELx808 absorbance microplate reader (BioTek, USA) (n = 3).
Mineralization of Bone Marrow Cells
For mineralization assay, rat femora were excised aseptically, the epiphyses of femora were cut off, and the marrow was flushed out in a culture medium. Bone marrow cells (BMCs) were cultured in an osteogenic medium consisting of α-MEM, supplemented with 10% fetal bovine serum (FBS), 50 μg/ml ascorbic acid, and 10 mM β-glycerophosphate (Goel et al. 2015).
Alizarin Red Staining Assay
The alizarin red S stain (ARS) was used to stain and evaluate the calcium deposition in BMCs by a procedure described by Gregory et al. (2004); mineralization was induced on confluent BMC monolayers in osteogenic media. The cells were seeded in 6-well plates and incubated with 1 at 0.1, 0.5, and 1 μM concentrations (n = 3). The cultures were incubated at 37 °C for 14 days. Monolayers in 6-well plates (10 cm2/well) were washed with PBS and fixed in 10% (v/v) formaldehyde and then again washed with excess distilled water. Alizarin red stain of 40 mM (1 ml) was added to each well and incubated for 20 min at room temperature with gentle shaking. The wells were washed with distilled water, and stained monolayers were visualized by phase microscopy using an inverted microscope. The quantification of staining was performed with the acetic acid extraction method. Acetic acid 10% (v/v) (800 μl) was added to each well, and the monolayer was scraped. Then, ammonium hydroxide 10% (v/v) was added to neutralize the acid and to maintain the pH between 4.3 and 4.5. Aliquots (150 μl) of the supernatant were read in triplicate at 405 nm in 96-well format using an ELISA plate reader.
Western Blot Analysis
Rat calvarial osteoblast cells were grown to 60–70% confluence and treated with 1 (0.1 μM). The cells were incubated for 24 and 48 h then homogenized with Triton lysis buffer (Sigma-Aldrich). The whole-cell lysates (20 μg) were subjected to SDS-PAGE and then transferred to polyvinylidene fluoride (PVDF) membranes (GE Healthcare, USA). The membranes were probed with ER-α and ER-β primary antibodies and later incubated with corresponding horseradish peroxidase–labeled secondary antibodies. The bands were developed using a chemiluminescence kit. Quantitation of band intensity was performed by densitometry using Image-Pro Plus 4.0 (MD, USA) analysis software. Densitometric data are shown as a % change in protein expressions (n = 3).
Molecular Docking
To further validate the findings of western blot analysis results, compound 1 was docked with the active site of ER-α and ER-β using the Glide module of Schrodinger software. The crystal structures of ER-α (PDB: 4PP6) (Nwachukwu et al. 2014) and ER-β (PDB: 5TOA) (Souza et al. 2017) were retrieved from the PDB database. The docking studies were carried out under the default settings from Glide. β-Estradiol, a known agonist of ER, was used as a reference ligand in both the docking experiments.
Statistical Analysis
All data were represented as mean ± SEM. Data were analyzed by ANOVA, and post comparison was carried out by Dunnett’s multiple comparisons test. The p ≤ 0.05 is considered a significant difference.
Results and Discussion
Cell Viability Assay
In the MTT assay, the percent proliferation showed a non-toxic effect on osteoblast cells at doses ranging from 0.05 to 2.5 μM of RSV (1) as compared to the control group. RSV at 0.05 μM and 0.1 μM concentrations showed enhanced cell proliferation of about 123.7 and 121.4%, respectively (Fig. 1). At 0.25, 0.5, and 1 μM, the % cell viability was 113.1, 110.8, and 92.53%, respectively, compared to the control cells. RSV at 2.5 μM showed 92.02% cell viability. The EC50 calculated for RSV was found to be 0.39 μM.
Dose-response curve of resveratrol (1) on osteoblasts for the estimation of cell viability. Graph of % cell viability vs. final log10 concentration for 1 (0.05–2.5 μM) incubated for 48 h. All the data were expressed as a mean ± SD (n = 3). It was statistically analyzed by calculating the dose-response stimulation curve with log (agonist) vs. response variable slope nonlinear analysis using GraphPad prism software (ρ ≤ 0.05)
ALP Activity
Figure 2 shows the effect of RSV (1) on the ALP activity of OB treatment at different concentrations from 1 pM to 1 μM studied at 48 h. RSV at 1-, 10-, and 100-pM concentrations did not show any significant change after treatment. The mean absorbance for the control group was 0.218 ± 0.016, while RSV at 1, 10, and 100 pM was 0.253 ± 0.022, 0.222 ± 0.0234, and 0.226 ± 0.017, respectively. RSV at 1, 10, 100, and 1000 nM were 0.234 ± 0.0263, 0.251 ± 0.030, 0.228 ± 0.031, and 0.230 ± 0.0344, respectively. This result suggests that RSV did not affect osteoblast differentiation during the early phase of osteoblast differentiation.
Effect of resveratrol (1) on alkaline phosphatase activity. All the data were expressed as a mean ± standard error of the mean (n = 3). All data were statistically analyzed by one-way ANOVA test, and post comparison was carried out using Dunnett’s multiple comparisons test with “control group” (ρ ≤ 0.05)
Detection of Mineralization
ARS binds selectively to calcium salts which are the mineralized bone. Therefore, it is considered to detect the mineralization phase of bone formation. Figure 3a describes mineralization by BMCs with RSV (1) treatment at three different concentrations (0.1, 0.5, and 1 μM, respectively). The RSV treatment increases the number of calcified nodules as visualized by a bright red calcified nodule in the microscopic and as depicted in the figure compared with control. The RSV at 0.1 μM was found to be the most effective dose in exhibiting maximum mineralization and therefore considered for western blot analysis.
Detection (A) and quantification (B) of mineralization. Representative images of the calcified nodules in rat bone marrow cells. (a) Control and tested concentrations of resveratrol (1) as (b) 0.1 μM, (c) 0.5 μM, and (d) 1 μM by alizarin red staining after culturing in osteogenic medium for 14 days (n = 3). The reaction between calcium ions and alizarin forms the red-colored nodules visualized under a light microscope
Quantification of Mineralization
In the quantification, RSV (1) at 0.-1, 0.5-, and 1-μM concentrations showed a significant increase in the mineralization after 14 days of culture, as shown in Fig. 3b. The absorbance in the control group was 1.264 ± 0.012. RSV at various concentrations as 0.1, 0.5 and 1 μM demonstrated the absorbance as 1.929 ± 0.046; 1.560 ± 0.023 and 1.246 ± 0.054, respectively (mean ± SEM, n = 3). RSV at 0.1 μM revealed the enhanced mineralization potential and was subsequently responsible for late osteoblastic differentiation.
Western Blot
The western blot analysis was carried out based on the mineralization assay result. RSV (1) 0.1 μM was found to be potent and hence used for protein lysate preparation in estrogen receptor expression analysis. The possible osteogenic effect of RSV is seen by the activation of ER-α expression in calvarial osteoblast at 0.1 μM on 24- and 48-h incubation. The RSV treatment at 0.1 μM concentration increases the ER-α expression significantly in a time-dependent manner without affecting ER-β isoform expression (Fig. 4). An increase in the ER-α expression proved the rise in the osteoblastic differentiation and reveals the phytoestrogenic properties.
Representative western blots. The increase in ER-α expression was time-dependent (24 h, 48 h) in osteoblasts treated with resveratrol (1) at 0.1 μM. Densitometric data represented a % change in protein expression levels. Values are expressed as mean ± SEM, n = 3 (independent samples). β-actin—internal control
Molecular Docking
RSV (1) was docked with the active site of ER-α and ER-β using the Glide module of Schrodinger software shown in Fig. 5. The active site of ER-α and ER-β consists of polar as well as hydrophobic amino acids. The 17β-estradiol occupies the active site of both enzymes exactly in a similar fashion. The 3-OH substituent of 17β-estradiol forms H-bonding with the carbonyl oxygen of Glu353. The aryl ring also forms π-π stacking with the Phe404 residue (Fig. 5a). The 17-OH located at the other end of the 17β-estradiol forms the H-bond with the imidazole nitrogen. In ER-β active site, the 17β-estradiol displayed similar interactions like α-isoform. The 3-OH and 17-OH of 17β-estradiol form H-bonds with Leu339 and Hie475 residues, and the aromatic ring formed π-π stacking with the Phe356 residue (Fig. 5d). Docking of RSV with both the active sites revealed striking differences in the orientation pattern. RSV occupies the active site of ER-α with the p-OH containing aromatic ring mimicking the 17-OH H-bonding interaction of 17β-estradiol with Hie524 residues. The dihydroxy aryl ring mimicked the 3-OH interactions of 17β-estradiol with Glu353 residue. Besides, one of the OH of the dihydroxy ring of RSV also displayed additional H-bonding with the Leu387 residue (Fig. 5b). Unlike β-estradiol, RSV does not orient in the same way in the active site of the ER-β enzyme. There is a 180° flip of RSV in the ER-β active site with dihydroxy aryl ring orienting towards the Hie475 residue. This flip has resulted in the loss of H-bonding with Leu339 residue, which was observed in β-estradiol. However, the p-hydroxy OH of RSV displayed H-bonding with Arg346 and Glu305 residues (Fig. 5e). The docking score of RSV was also low with ER-β (−9.59) in comparison to ER-α enzyme (−10.09). The observed differences in the orientation, interaction pattern, and binding affinity of RSV at the active site of ER-α vs. ER-β supports the western blot analysis results.
The molecular docking of 17β-estradiol and resveratrol (1) with ER-α (PDB: 4PP6) and ER-β (5TOA). (a) The 2D-interaction map of 17β-estradiol at the ER-α active site; (b) the 2D-interaction map of RSV at the ER-α active site; (c) the 3D-interaction pattern of RSV at the ER-α active site; (d) the 2D-interaction map of 17β-estradiol at the ER-β active site; (e) the 2D-interaction map of RSV at the ER-β active site; (f) the 3D-interaction pattern of RSV at the ER-β active site [red broken lines, H-bonding; blue double prime–like symbols, π-π stacking interaction]. E, estrogen; ER, estrogen receptor
Estrogen plays an essential role in the development and maintenance of the skeleton via ER-α- and ER-β-mediated pathways. The differential expression of receptor isoforms indicates that ER-α is highly expressed in cortical than in cancellous bone, while ER-β is most evident at cancellous than cortical sites (Bord et al. 2001). It suggests the difference in the osteogenic function of the ligand by activating any of these isoforms. RSV (1) is structurally close to the ER agonist diethylstilbestrol and is known to activate the estrogen receptor. Our present investigation tested the hypothesis that RSV may lead to the transcriptional activation of ER-α or ER-β isoform expression by in silico and in vitro studies. Binding affinities for the receptors are often expressed relative to 17β-estradiol, and the relative binding affinities of RSV have been characterized.
The factors affecting the activations of ER-α and ER-β isoforms by RSV (1) includes the differential tissue distribution of the estrogen receptors, the presence of various transcriptional coactivators present in various tissues, and the ability of RSV to induce conformational changes which may cause tissue- and ligand-specific activation of individual genes. As per the reported literature, RSV performs various physiological actions via activating ER-α isoforms. RSV modulates inflammatory response by suppressing interleukin-6 transcription (Parent et al. 2014) and enhanced muscular glucose uptake via insulin-dependent and insulin-independent pathways (Deng et al. 2008). The reactivation of ER-α expression in ER-α-negative breast cancer cell lines MDA-MB-157 and HCC1806 leads to an effective treatment option in hormonal refractory breast cancer (Kala and Tollefsbol 2016). RSV also plays a significant role via the ER-α-mediated pathway, which involves reducing the progression of restenosis (Khandelwal et al. 2010). As the effect of RSV on the human endometrium is concerned, RSV at higher doses acts as an antiestrogen in Ishikawa cells and decreases epithelial ER-α levels in the endometrium (Bhat and Pezzuto 2001).
Osteogenesis is the process of bone formation and synthesis of bone extracellular proteins governed by the osteoblast. In this study, we have investigated the osteogenic potential of RSV on different phases of osteoblast differentiation. The percentage of cell viability on RSV treatment was at different concentrations, 0.05–2.5 μM range. RSV at 0.05 μM and 0.1 μM showed enhanced cell proliferation. However, the OB differentiation capacity was not affected by increasing RSV concentrations. RSV was found to be non-toxic that covers the wide range, and even at higher concentrations as 2.5 μM, cell viability was found to be 92.02%. It indicates that the RSV is not cytotoxic to the OB cells. In the ALP assay, RSV was tested over a wide range from 1 pM to 1 μM. The ALP activity was not affected by RSV treatment. Therefore, RSV had shown no effect on early osteoblastic differentiation. These results were replicated and consistent with our previous findings in estrogen-negative human fetal osteoblast cells (Shah et al. 2019). hFOB cells express very low levels of the activated estrogen receptor per nucleus. It contains less than about 200 estrogen receptors that are capable of binding 17β-estradiol and translocating to the cell nucleus (Harris et al. 1995; Harris et al. 2002). It demonstrated that RSV did not influence early osteoblastic differentiation in estrogen-dependent and independent cell lines. Therefore, RSV was further evaluated for the mineralization at three different concentrations (0.1, 0.5, and 1 μM). Mineralization studies indicated that RSV stimulated the mineralization phase and subsequently enhanced the calcium deposition at all these concentrations. The quantification results of the mineralization assay showed that RSV at 0.1 μM was more potent and indicated the late phase of OB differentiation. Hence, RSV at 0.1 μM was considered as an effective concentration for in vitro studies and investigated in western blot study to identify ER-α or ER-β isoform specificity. RSV at 0.1-μM concentration treatment increased the ER-α expression in a time-dependent manner and was found to be maximum at 48-h incubation without affecting ER-β isoform expression. In our in silico investigation, RSV interacts with the catalytic amino acid triad of the ER pocket (Hie524, Phe404, and Glu353) with favorable binding energy of interaction with ER-α isoforms. The binding mode of RSV showed a similar pattern to prototype drug 17β-estradiol. Interpretations done by El-Mowafy et al. (2002) supported our findings. This estrogen mimetic potential of RSV revealed a higher affinity for ER-α, which is different from most of the phytoestrogens which are working through the ER-β-mediated pathway. Thus, the study highlighted the effect of RSV (1) on osteoblastic differentiation through the estrogen-dependent pathway via activation of ER-α pathways.
Conclusion
In this work, the osteogenic potential of resveratrol (1) on osteoblast differentiation via an estrogen-dependent pathway was investigated. In vitro studies suggest that RSV is responsible for bone formation by activating ER-α isoform. Molecular docking studies confirmed these findings. Therefore, RSV is a natural phytoestrogens and can be further explored in postmenopausal osteoporosis.
References
Adluri RS, Zhan L, Bagchi M, Maulik N, Maulik G (2010) Comparative effects of a novel plant-based calcium supplement with two common calcium salts on proliferation and mineralization in human osteoblast cells. Mol Cell Biochem 340:73–80. https://doi.org/10.1007/s11010-010-0402-0
Aquino CI, Nori SL (2014) Complementary therapy in polycystic ovary syndrome. Transl Med UniSa 9:56–65. https://doi.org/10.14273/unisa-247
Baile CA, Yang JY, Rayalam S, Hartzell DL, Lai CY, Andersen C, Della-Fera MA (2011) Effect of resveratrol on fat mobilization. Ann N Y Acad Sci 1215:40–47. https://doi.org/10.1111/j.1749-6632.2010.05845.x
Bhat KP, Pezzuto JM (2001) Resveratrol exhibits cytostatic and antiestrogenic properties with human endometrial adenocarcinoma (Ishikawa) cells. Cancer Res 61:6137–6144
Bord S, Horner A, Beavan S, Compston J (2001) Estrogen receptors alpha and beta are differentially expressed in developing human bone. J Clin Endocrinol Metab 86:2309–2314. https://doi.org/10.1210/jcem.86.5.7513
Bowers JL, Tyulmenkov VV, Jernigan SC, Klinge CM (2000) Resveratrol acts as a mixed agonist / antagonist for estrogen receptors alpha and beta. Endocrinology 141:3657–3667. https://doi.org/10.1210/endo.141.10.7721
Cagnacci A, Venier M (2019) The controversial history of hormone replacement therapy. Medicina (B Aires) 55:602. https://doi.org/10.3390/medicina55090602
Cauley JA (2015) Estrogen and bone health in men and women. Steroids 99:11–15. https://doi.org/10.1016/j.steroids.2014.12.010
Chakraborty S, Levenson AS, Biswas PK (2013) Structural insights into resveratrol’s antagonist and partial agonist actions on estrogen receptor alpha. BMC Struct Biol 13:13–27. https://doi.org/10.1186/1472-6807-13-27
Crosignani PG (2003) Breast cancer and hormone-replacement therapy in the Million Women Study. Maturitas 46:91–92. https://doi.org/10.1016/j.maturitas.2003.09.002
Delmas D, Cornebise C, Courtaut F, Xiao J, Aires V (2021) New highlights of resveratrol: a review of properties against ocular diseases. Int J Mol Sci 22:1295. https://doi.org/10.3390/ijms22031295
Deng JY, Hsieh PS, Huang JP, Lu LS, Hung LM (2008) Activation of estrogen receptor is crucial for resveratrol-stimulating muscular glucose uptake via both insulin-dependent and -independent pathways. Diabetes 57:1814–1823. https://doi.org/10.2337/db07-1750
Ding J, Kang Y, Fan Y, Chen Q (2017) Efficacy of resveratrol to supplement oral nifedipine treatment in pregnancy-induced preeclampsia. Endocr Connect 6:595–600. https://doi.org/10.1530/EC-17-0130
El-Mowafy AM, Abou-Zeid LA, Edafiogho I (2002) Recognition of resveratrol by thehuman estrogen receptor-alpha: A molecular modeling approach to understand its biological actions. Med Princ Pract 11:86–92. https://doi.org/10.1159/000058013
García-Zepeda SP, GarcíaVilla E, Díaz-Chávez J, Hernández-Pando R, Gariglio P (2013) Resveratrol induces cell death in cervical cancer cells through apoptosis and autophagy. Eur J Cancer Prev 22:577–584. https://doi.org/10.1097/CEJ.0b013e328360345f
Gehm BD, McAndrews JM, Chien PY, Jameson JL (1997) Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc Natl Acad Sci 94:14138–14143. https://doi.org/10.1073/pnas.94.25.14138
Glazier MG, Bowman MA (2001) A review of the evidence for the use of phytoestrogens as a replacement for traditional estrogen replacement therapy. Arch Intern Med 161:1161–1172. https://doi.org/10.1001/archinte.161.9.1161
Goel A, Raghuvanshi A, Kumar A, Gautam A, Srivastava K, Kureel J, Singh D (2015) 9-Demethoxy-medicarpin promotes peak bone mass achievement and has bone conserving effect in ovariectomized mice: positively regulates osteoblast functions and suppresses osteoclastogenesis. Mol Cell Endocrinol 411:155–166. https://doi.org/10.1016/j.mce.2015.04.023
Gregory CA, Gunn WG, Peister A, Prockop DJ (2004) An alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem 329:77–84. https://doi.org/10.1016/j.ab.2004.02.002
Harris SA, Tau KR, Enger RJ, Toft DO, Riggs BL, Spelsberg TC (1995) Estrogen response in the hFOB 1.19 human fetal osteoblastic cell line stably transfected with the human estrogen receptor gene. J Cell Biochem 59:193–201. https://doi.org/10.1002/jcb.240590209
Harris SA, Rochester MN, STCR (2002) Immortalized human fetal osteoblastic cells (European Patent- EP0743982B1)
He X, Andersson G, Lindgren U, Li Y (2010) Resveratrol prevents RANKL-induced osteoclast differentiation of murine osteoclast progenitor RAW 264.7 cells through inhibition of ROS production. Biochem Biophys Res Commun 401:356–362. https://doi.org/10.1016/j.bbrc.2010.09.053
Kala R, Tollefsbol T (2016) A novel combinatorial epigenetic therapy using resveratrol and pterostilbene for restoring estrogen receptor-α (ERα) expression in ERα-negative breast cancer cells. PLoS One 11:1–17. https://doi.org/10.1371/journal.pone.0155057
Khalid AB, Krum SA (2016) Estrogen receptors alpha and beta in bone. Bone 87:130–135. https://doi.org/10.1016/j.bone.2016.03.016
Khandelwal AR, Hebert VY, Dugas TR (2010) Essential role of ER-α-dependent NO production in resveratrol-mediated inhibition of restenosis. Am J Physiol Circ Physiol 299:H1451–H1458. https://doi.org/10.1152/ajpheart.00369.2010
Kolahdouz Mohammadi R, Arablou T (2017) Resveratrol and endometriosis: in vitro and animal studies and underlying mechanisms (Review). Biomed Pharmacother 91:220–228. https://doi.org/10.1016/j.biopha.2017.04.078
Lee CH, Huang YL, Liao F, Chiou WF (2012) Ugonin K-stimulated osteogenesis involves estrogen receptor-dependent activation of non-classical Src signaling pathway and classical pathway. Eur J Pharmacol 676:26–33. https://doi.org/10.1016/j.ejphar.2011.12.001
Liao MH, Tai YT, Cherng YG, Liu SH, Chang YA, Lin PI, Chen RM (2014) Genistein induces oestrogen receptor-α gene expression in osteoblasts through the activation of mitogen-activated protein kinases/NF-κB/ activator protein-1 and promotes cell mineralisation. Br J Nutr 111:55–63. https://doi.org/10.1017/S0007114513002043
Mobasheri A, Shakibaei M (2013) Osteogenic effects of resveratrol in vitro: potential for the prevention and treatment of osteoporosis. Ann N Y Acad Sci 1290:59–66. https://doi.org/10.1111/nyas.12145
Murgia D, Mauceri R, Campisi G, De Caro V (2019) Advance on resveratrol application in bone regeneration: progress and perspectives for use in oral and maxillofacial surgery. Biomolecules 9:1–17. https://doi.org/10.3390/biom9030094
Nwachukwu JC, Srinivasan S, Bruno NE, Parent AA, Hughes TS, Pollock JA, Gjyshi O, Cavett V, Nowak J, Garcia-Ordonez RD and Houtman R (2014) Resveratrol modulates the inflammatory response via an estrogen receptor-signal integration network. Elife 3:e02057-e02057 PDB. https://doi.org/10.2210/pdb4PP6/pdb. Accessed via: https://www.rcsb.org/structure/4PP6
Ochiai A, Kuroda K (2020) Preconception resveratrol intake against infertility: friend or foe? Reprod Med Biol 19:107–113. https://doi.org/10.1002/rmb2.12303
Pande A, Manchanda M, Bhat HR, Bairy PS, Kumar N, Gahtori P (2021) Molecular insights into a mechanism of resveratrol action using hybrid computational docking/CoMFA and machine learning approach. J Biomol Struct Dyn 0:1–15. https://doi.org/10.1080/07391102.2021.1910572
Parent AA, Nwachukwu JC, Houtman R, Garcia-Ordonez RD, Kojetin DJ, Nettles KW, Griffin PR, Nowak J, Cavett V, Katzenellenbogen JA, Bruno NE, Srinivasan S, Conkright MD, Gjyshi O, Pollock JA, Hughes TS (2014) Resveratrol modulates the inflammatory response via an estrogen receptor-signal integration network. Elife 3:1–30. https://doi.org/10.7554/elife.02057
Rayalam S, Della-Fera MA, Baile CA (2011) Synergism between resveratrol and other phytochemicals: implications for obesity and osteoporosis. Mol Nutr Food Res 55:1177–1185. https://doi.org/10.1002/mnfr.201000616
Sato M, Maulik G, Bagchi D, Das DK (2000) Myocardial protection by Protykin, a novel extract of trans -resveratrol and emodin. Free Radic Res 32:135–144. https://doi.org/10.1080/10715760000300141
Shah AA, Gourishetti K, Nayak Y (2019) Osteogenic activity of resveratrol in human fetal osteoblast cells. Pharmacogn Mag 15:250–255. https://doi.org/10.4103/pm.pm_619_18
Shah AA, Shah A, Lewis S, Ghate V, Saklani R, Narayana Kalkura S, Baby C, Singh PK, Nayak Y, Chourasia MK (2021) Cyclodextrin based bone regenerative inclusion complex for resveratrol in postmenopausal osteoporosis. Eur J Pharm Biopharm 167:127–139. https://doi.org/10.1016/j.ejpb.2021.07.008
Song L, Zhao J, Zhang X, Li H, Zhou Y (2013) Icariin induces osteoblast proliferation, differentiation and mineralization through estrogen receptor-mediated ERK and JNK signal activation. Eur J Pharmacol 714:15–22. https://doi.org/10.1016/j.ejphar.2013.05.039
Souza PCT, Textor LC, Melo DC, Nascimento AS, Skaf MS, Polikarpov I (2017) An alternative conformation of ER beta bound to estradiol reveals H12 in a stable antagonist position. Sci Rep 7:3509–3509 PubMed. https://doi.org/10.1038/s41598-017-03774-x. Accessed via https://www.rcsb.org/structure/5TOA
Su JL, Yang CY, Zhao M, Kuo ML, Yen ML (2007) Forkhead proteins are critical for bone morphogenetic protein-2 regulation and anti-tumor activity of resveratrol. J Biol Chem 282:19385–19398. https://doi.org/10.1074/jbc.M702452200
Subramanian M, Goswami M, Chakraborty S, Jawali N (2014) Resveratrol induced inhibition of Escherichia coli proceeds via membrane oxidation and independent of diffusible reactive oxygen species generation. Redox Biol 2:865–872. https://doi.org/10.1016/j.redox.2014.06.007
Subramanian M, Soundar S, Mangoli S (2016) DNA damage is a late event in resveratrol-mediated inhibition of Escherichia coli. Free Radic Res 50:708–719. https://doi.org/10.3109/10715762.2016.1169404
Sun X, Fu P, Xie L, Chai S, Xu Q, Zeng L, Wang X, Jiang N, Sang M (2020) Resveratrol inhibits the progression of cervical cancer by suppressing the transcription and expression of HPV E6 and E7 genes. Int J Mol Med 47:335–345. https://doi.org/10.3892/ijmm.2020.4789
Tou JC (2014) Resveratrol supplementation affects bone acquisition and osteoporosis: pre-clinical evidence toward translational diet therapy. Biochim Biophys Acta - Mol Basis Dis 1852:1186–1194. https://doi.org/10.1016/j.bbadis.2014.10.003
Wang Y, Wang WL, Xie WL, Li LZ, Sun J, Sun WJ, Gong HY (2013) Puerarin stimulates proliferation and differentiation and protects against cell death in human osteoblastic MG-63 cells via ER-dependent MEK/ERK and PI3K/Akt activation. Phytomedicine 20:787–796. https://doi.org/10.1016/j.phymed.2013.03.005
Xiao HH, Fung CY, Mok SK, Wong KC, Ho MX, Wang XL, Yao XS, Wong MS (2014a) Flavonoids from Herba epimedii selectively activate estrogen receptor alpha (ERα) and stimulate ER-dependent osteoblastic functions in UMR-106 cells. J. Steroid Biochem. Mol. Biol. 143:141–151. https://doi.org/10.1016/j.jsbmb.2014.02.019
Xiao HH, Gao QG, Zhang Y, Wong KC, Dai Y, Yao XS, Wong MS (2014b) Vanillic acid exerts oestrogen-like activities in osteoblast-like UMR 106 cells through MAP kinase (MEK/ERK)-mediated ER signaling pathway. J Steroid Biochem Mol Biol 144:382–391. https://doi.org/10.1016/j.jsbmb.2014.08.002
Zheng S, Feng Q, Cheng J, Zheng J (2018) Maternal resveratrol consumption and its programming effects on metabolic health in offspring mechanisms and potential implications. Biosci Rep 38:2–10. https://doi.org/10.1042/BSR20171741
Acknowledgements
The authors acknowledge Manipal-Schrodinger Centre for Molecular Simulations, Manipal Academy of Higher Education, and Manipal College of Pharmaceutical Sciences for providing necessary supports and facilities to carry out the present research work. The authors are also thankful to the Central Drug Research Institute, Lucknow, to conduct in vitro studies.
Funding
Open access funding provided by Manipal Academy of Higher Education. The Department of Science and Technology, Government of India, financially supported this study under Women Scientist Scheme A (WOS-A # SR/WOS-A/LS-444/2016).
Author information
Authors and Affiliations
Contributions
AAS conceived the study, designed, performed experiments, interpreted results, and wrote the manuscript. AS supported in molecular docking studies with the intellectual input from YN. AK gave an insight on in silico studies with an interpretation of docking data. All in vitro experiments were performed under the guidance of DS along with the kind support of AL. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The studies were performed in compliance with the guidelines set by the Central Drug Research Institute-Institutional Animal Ethics Committee (CDRI-IAEC) with approval number IAEC/2017/290/Renew-0/Dated-31/10/2017.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shah, A.A., Shah, A., Kumar, A. et al. Phytoestrogenic Potential of Resveratrol by Selective Activation of Estrogen Receptor-α in Osteoblast Cells. Rev. Bras. Farmacogn. 32, 248–256 (2022). https://doi.org/10.1007/s43450-022-00239-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43450-022-00239-9