Abstract
Background
Global, randomized clinical trials are extremely complex. Trial start-up is a critical phase and has many opportunities for delay which adversely impact the study timelines and budget. Understanding factors that contribute to delay may help clinical trial managers and other stakeholders to work more efficiently, hastening patient access to potential new therapies.
Methods
We reviewed the available literature related to start-up of global, Phase III clinical trials and then created a fishbone diagram detailing drivers contributing to start-up delays. The issues identified were used to craft a checklist to assist clinical trial managers in more efficient trial start-up.
Results
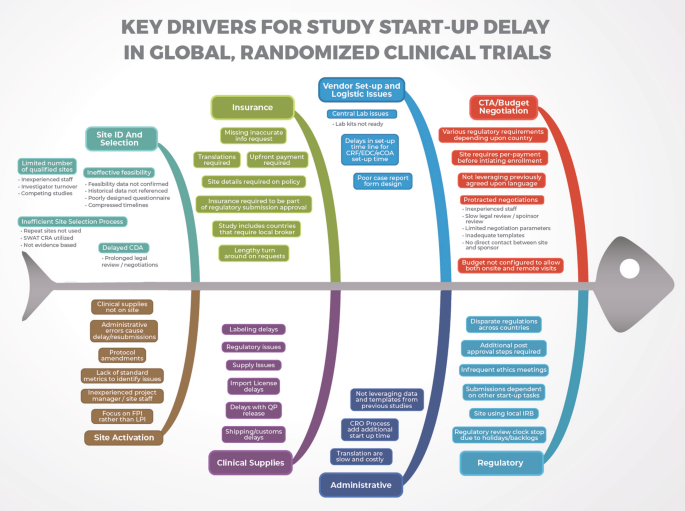
We identified key drivers for start-up delays in the following categories: regulatory, contracts and budgets, insurance, clinical supplies, site identification and selection, site activation, and inefficient processes/pitfalls.
Conclusion
Initiating global randomized clinical trials is a complex endeavor, and reasons for delay are well documented in the literature. By using a checklist, clinical trial managers may mitigate some delays and get clinical studies initiated as soon as possible.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction/Background
This study was conducted prior to the outbreak of the COVID-19 pandemic. After the onset of the pandemic, many planned clinical trials were delayed due to widespread lockdowns and to conserve resources for front-line healthcare workers. Trials in many indications including life-threatening illnesses like cancer and cystic fibrosis have been delayed by the pandemic [1]. Once initiation of delayed studies does resume, it will be essential to conduct start-up activities as efficiently as possible to expeditiously start clinical trials for the benefits of the clinical trial participants.
Randomized, controlled trials (RCT) are considered the gold-standard to assess the safety and efficacy of potential medications/therapies [2, 3]. FDA-regulated trials increasingly engage sites outside the United States, including sites in developing nations, in order to hasten patient enrollment, reduce costs and achieve market expansion by including participants for various geographies [4]. These studies are complex to start-up, especially when they include multinational sites subject to different laws, regulations governing the conduct of research, infrastructure (or lack of infrastructure) and local standards of care [5]. Clinical study start-up is a key determinant of success in a clinical trial, and the time required to activate a trial may be inversely related to its enrollment rate [6, 7]. In order to begin recruitment, sites need to be qualified, gain regulatory approval, including IRB/ethics committee approval, negotiate and execute clinical trial agreements, and receive training and clinical supplies (in addition to many other study level tasks). Delaying start-up often means extending the overall study timelines which can not only incur significant additional cost, but threaten the feasibility of the trial [8,9,10]. Examples of other adverse trial outcomes due to start-up delays include wasted drug or drug shortages due to expiry, loss of clinical sites due to lack of interest or competing studies and loss of ability to enroll clinical trial participants due to a change in the local standard of care rendering the control arm obsolete [11, 12]. Perhaps most importantly, delays in study start-up lead to delay in access to treatment for patients, as well as lost opportunity costs.
While it is important that all clinical trials run efficiently, Phase III trials are typically the largest and most complex studies prior leading to drug approval. A Phase III, randomized, trial may cost anywhere between $11.5 million to $52.9 million depending upon the therapeutic area and complexity of the study [13]. While clinical trial delays are well documented, this study offers a comprehensive start-up checklist as a useful resource for clinical trial managers seeking to improve trial efficiency.
Methods
This qualitative integrative analysis consists of three components: (1) a review of the available literature related to the start-up of clinical trials; (2) a fishbone diagram, created to summarize driving factors of start-up delays in Phase III global clinical trials; and (3) a study start-up checklist that clinical trial managers may use for trial planning. The following areas were considered in-scope for this review: regulatory approvals, site contracts and budgets, insurance, clinical supplies, site identification and selection, site activation, and inefficient processes/pitfalls.
Protection of Human Clinical Trial Participants
This study was exempt from the Medical University of South Carolina (MUSC) institutional review process for protecting human clinical trial participants in research and does not contain any studies with human participants performed by any of the authors.
Results
The literature was reviewed using the phrases “clinical trial start-up and delays” and “study start-up and delays”. The review included 89 peer reviewed journal articles as well as supplemental industry white papers and a book. Various reasons contributing to study start-up delay were well documented and key drivers for delay were detailed in a fishbone diagram (Fig. 1). The major factors identified that contribute to start-up delay in RCTs relate to regulatory approvals, site contracts and budgets, insurance, clinical supplies, site activation, inefficient processes, CROs, and translations. Key findings in each of these areas will be briefly discussed below.
Regulatory
Our literature review identified six key drivers contributing to regulatory delays: disparate regulations, submission delays, additional requirements subsequent to regulatory approval, use of a local ethics committee/IRB, infrequent ethics committee/IRB meetings, and regulatory backlogs/clock-stops.
Disparate regulations and variation in start-up processes across countries have a significant impact on study start-up timelines [5, 14]. Regulatory submission packages are complex and require a great deal of coordination, and when multiple countries are involved, the complexity and level of coordination needed are significantly increased, as the start-up team must carefully track the timeline and requirements of each country [5].
Additionally, regulatory submissions in some countries include difficult-to-obtain documents like executed site contracts or insurance policies, which significantly slow the time to submission of the regulatory package and consequently the approval is delayed. Negotiating and getting a site contract signed can take a considerable amount of time. Once regulatory approval is received from the competent authority, some countries have additional regulatory requirements that must then be initiated before the country has full approval and sites can open for enrollment. For example, before trial medication can be imported, an import license may be required that cannot be submitted until country approval is granted and this may add weeks to study start-up.
Regulatory delays on a site level are dependent on whether a site uses a local Institutional Review Board (IRB)/ethics committee (EC) or is able to utilize a central IRB/EC. Evidence suggests the use of a centralized IRB that governs multiple sites, rather than a local IRB overseeing each site, significantly reduces time to IRB approval [15, 16]. In a retrospective study, central IRBs were associated with significantly shorter cycle times, including conducting protocol review within an average of 7 days as compared to 35 days for local IRBs [15]. Frequency of IRB/EC meetings also has an impact on start-up timelines. Meeting schedules can vary greatly across sites and may occur weekly, monthly, quarterly, or as infrequently as twice per year [17].
Finally, regulatory review timelines may be delayed due to backlogs and clock-stops, at either a country or site level. For example, prior to reforming their regulatory review processes, China had a peak regulatory review backlog of more than 22,000 applications in 2015 [18]. Trial managers working with sites in China during this time would have to plan for very long regulatory review timelines and likely sites in China would join a global study long after other sites in other countries started enrolling participants.
Site Contracts and Budgets
Additional drivers of start-up delay are evident in the process of negotiating site contracts (clinical trial agreements) and investigator grants (the study budget for an investigative site) [19,20,21]. Contract and budget negotiations between clinical sites and sponsors can take months to negotiate and execute. In a global trial conducted at 57 centers in 16 countries, contract executions spanned an average of 7.9 months for US sites (range 2.5–17.2 months) and 8.7 months for sites outside the US (range 2.5–24.9 months) [22]. Contributors to prolonged contract and budget cycle times include inexperienced staff, inadequate budget templates, limited negotiation parameters, and prolonged legal reviews [19]. If a sponsor has worked with a site in the past, leveraging previously negotiated contract and budget terms may significantly reduce cycle times [23].
Start-up activities, including negotiation of clinical trial agreements (CTAs), are often conducted on behalf of sponsor companies by clinical research organizations (CROs) [21]. CRO-managed negotiations require significant sponsor oversight and failure to do so may result in weeks of delay as well as cause damage to the relationship with the study site [19]. It is important to provide sites with a sponsor contact for escalation of negotiation issues as needed [19]. Once contracts are in place, sites may require pre-payments before they will officially initiate the trial and initiate enrollment. Pre-payments can be another source of delay, as it takes time for the payer to set up the site in their systems and generate the payment.
Clinical trials that incorporate decentralized visits (e.g., visits that occur in the patient’s home) also have special contractual considerations. In such studies, it is important that the investigator fee structure allows for both in-office and remote patient visits. If this flexibility is not built in up-front, the contract will likely need to be revised, which is a costly and time-consuming process.
Insurance
Procurement of liability insurance is a complex and critical aspect of clinical trial start-up that may be underestimated by clinical trial managers [24, 25]. It has the potential to add significant cost to the study and delay to the start-up process as proof of insurance is part of the regulatory document submission and approval in some countries [24]. As with other aspects of multinational clinical trials, each country has their own set of rules governing indemnity insurance [5, 26, 27]. Multinational studies include a combination of different policies to mitigate risk to the sponsor in the event a participant is injured and is awarded financial compensation [25]. The sponsor company generally holds a global master liability policy, renewed annually, that is sufficient to cover some countries including the United States, Canada, and New Zealand [5, 25]. Other countries require local policies, issued by a locally licensed insurance company [25]. Local policies have varying requirements and typically cover the duration of the study unless the study runs longer than the initial term covered [25]. Depending upon the information required on the insurance certificate, policies may require update if the number of sites, estimated participants to be screened or randomized in that country changes. A change to an insurance policy can take weeks and hold up start-up in the country so it is important to get this right.
Clinical Supplies
Clinical supplies represent another area with the potential for substantial impediment to initiation of international clinical trials [14, 28]. Preparing and delivering clinical supplies to remote regions around the world is especially challenging as each country has their own particular language and regulatory requirements [29, 30]. Additionally, each country has their own combination of required data elements on the drug label, which must be translated into local language [31]. Smith-Gick et al. documented 19 data elements (e.g., drug name, storage conditions, for “clinical trial use” phrase) that may be required on the label depending upon the country [31]. Packaging and labeling require approximately 30 weeks from design and approval of conventional booklet labels to shipping kits to sites [31]. Incorporating the use of electronic labels (eLabels) presents an opportunity to reduce this timeline to 16 weeks [31].
Multinational studies require the clinical supply manager to keep apprised of local import and export regulations and shipping timelines [30]. Lamberti et al. examined logistics data for 73 clinical trials in a variety of therapeutic areas and across all phases [28]. They found shipping clinical supplies to clinical sites took 3.4 days on average, although there was a wide variation in shipping times depending upon the region and supply strategy (e.g., use of central depot, local or regional depot for distribution) [28]. When trials include therapies that require refrigeration or frozen storage (e.g., biologics), the implementation of a good cold chain strategy is vital. Maintaining the cold chain is further complicated when multiple countries are involved and may include remote sites without ready access to couriers. In order to mitigate drug supply issues, stakeholders must keep apprised of import/export requirements and timelines for shipping and account for ample product overage when calculating drug supply requirements to ensure that local drug depots are well stocked. Finally, stakeholders must thoroughly vet site logistics to understand the flow of clinical supplies from supplier to pharmacy to patient and process temperature excursions quickly.
In addition to managing the investigational agent, many trials use comparator drugs and co-therapies that must be sourced and provided as part of the study. Sourcing and managing these additional drugs are difficult, add significant cost to the study, and often are the source of delay and increased study cycle time [32]. The primary cause for delay is obtaining the requisite paperwork that is needed to support the regulatory submissions and trial operations; these documents include certificates of analysis and stability data to support decisions around temperature excursions [32]. Once comparator products are procured, they may need to be repackaged or relabeled depending upon county-specific regulations [30].
Site Identification and Selection
Increased competition for good, experienced clinical sites is a significant challenge for site selection [33]. In general, the more complex a study, the more difficulty CROs and sponsors have selecting sites [34]. When stakeholders select sites for a clinical study, they carefully evaluate key site qualifications to determine whether the site will be selected to participate in the study. Criteria for assessment include experience with research and the therapeutic area being studied, access to participants that meet eligibility criteria, appropriate staff, facilities, training and equipment, and interest in participating in the study [33, 35].
Generally, Sponsors and CROs reach out to potential sites to determine interest and then require interested sites to sign a confidentiality agreement (CDA). There is opportunity for delay here as legal terms are negotiated between the parties. Once a CDA is in place, a detailed feasibility questionnaire is issued to the site to complete.
Feasibility questionnaires are often designed in a hurry, as sites need to be selected quickly so that regulatory submissions can be prepared and submitted, capturing as many valuable enrollment months as possible. Because of compressed start-up timelines, the time allotted for sites to complete feasibility assessments is often short and as a result questionnaires may yield inaccurate or incomplete information and possibly overly optimistic enrollment projections [36], Sponsors frequently take the site prediction of enrollment and discount the patient numbers that they provide, yet the results rarely align to the site’s actual performance [36]. Often, key documents like the full protocol and budget are not available to sites at the time of feasibility [36]. After sites complete and return the feasibility questionnaire, the data are assessed and a subset of interested and eligible sites is selected to move on to a pre-study visit.
Site infrastructure should be assessed to ensure internet connectivity and ability to meet the technology requirements of the study. Sponsors may need to mitigate technology barriers by providing Internet access or supplying equipment as permitted by local regulations. Selecting “repeat” sites, or sites that have worked with a sponsor or CRO on a previous study, is an opportunity to reduce cycle times [34]. Cycle times for repeat sites were 28% shorter than cycle times for newly selected sites [34]. However, after participating in a clinical study, many sites do no elect to participate in a subsequent one. Key challenges faced by investigators include workload balance, time and financial requirements, complex regulations and contracts, lack of infrastructure, inadequate training, and data collection challenges [37].
Site Activation
Before opening a site to enrollment, sponsors/CROs have a checklist of required documents that must be in place including IRB/EC approval, a signed contract, budget, an FDA 1572 form or equivalent statement of investigator, CVs, medical licenses, and financial disclosure forms from the principal investigator and all subinvestigators. Documents required before the start of a clinical study are detailed in ICH E6 (R2) in Sect. 8.2 [38]. It is imperative that site start-up tasks are completed quickly and correctly, to avoid set-backs and additional cycles of regulatory review [15]. ICH E6 (R2), Sect. 5.14.2 states that the sponsor should not supply a clinical site with study drug until all required documentation is in place including a favorable opinion from the IRB/EC and regulatory authorities [38]. A minor error on a critical document such as an informed consent form, insurance policy or import license can present a significant set-back as the site may not be able to enroll participants until the error is corrected.
Abbott et al. noted that cycle times are not consistently collected across studies/sponsors/CROs and suggested that the industry measure key intervals in the site start-up cycle to assess performance in multisite trials [15]. These include (1) the date the final protocol was sent to a clinical site, (2) the date of IRB decision, (3) the date the contract (initial draft/template) was sent to the site, (4) the date that the site contract was signed, (5) the date the site was activated (open to enrollment), and (6) the date for the first patient’s consent [13]. Employing standard metrics will allow clinical trial managers to identify areas for improvement and assess whether improvement initiatives are working [15].
Discussion
The results of our analysis illustrate the need for increased efficiency in the start-up of global, multicenter randomized clinical trials. These projects are exceedingly complex and any delay in their execution has a significant financial impact and prolongs time to market for potentially life-saving therapies. In order to minimize delays due to all of the identified drivers, the study start-up team should include local experts with a detailed understanding of regulations and requirements in each participating country to accurately predict start-up timelines and help coordinate an efficient submission process. When countries with longer start-up timelines must be used, careful coordination of each step may help to optimize start-up.
One way to increase quality and efficiency in clinical trials is through the incorporation of telemedicine and other technology to facilitate decentralized clinical trials (DCTs) or trials where at least a portion of the activities are conducted at the patient home. Benefits of DCTs include faster recruitment, improved retention of trial participants, increased comfort and convenience for trial participants, and increased access to trials [39]. However, start-up activities in decentralized clinical trials may need additional considerations including establishing new processes and training documents, procuring equipment, and assimilating regulations and legal requirements [40]. Components of DCTs that require special consideration include shipping clinical supplies directly to patients, electronic informed consent (eConsent), home health visits, telemedicine visits, remote site monitoring, and digital data collection tools [40]. To negotiate some of these challenges, study teams should proactively map data flow, data collection, data storage, and study procedures and develop robust training procedures for stakeholders [40].
The most surprising area of potential start-up delay was clinical trial insurance. This is not an area that is widely discussed, but due to varying country requirements and the need to transmit information from the clinical operations team/CRO to an insurance agent who then conveys to a local broker, there is a great deal of potential for delay. This is further complicated by the need for translations and for original documents with signatures in some regions. A simple error on an insurance policy can significantly delay a regulatory submission or prevent a site from being activated when everything else is in place.
The Benjamin Franklin axiom “an ounce of prevention is worth a pound of cure” is relevant to clinical trial start-up in that it is far preferable to prevent start-up delays wherever possible rather than dealing with and resolving delays as they occur [41]. Seemingly small delays across various workstreams can add up significantly and yield substantial delays. While industry practice evolves to incorporate technology and implement evidence-based improvements, our checklist is intended to help clinical trial managers track study start-up activities and manage them as efficiently as possible.
Limitations
The data gathered in support of our fishbone diagram and the resulting checklist (Fig. 2) were primarily obtained through a literature review. We did not consult other clinical trial managers due to limitations in time and the scope of this project; however, this would be a valuable exercise in a future study.
As clinical trials require substantial financial resources to execute, evidence-based methods are needed to improve the efficiency of clinical operations. One means to generate evidence regarding trial efficiency is conducting a study within a trial (SWAT) which examines a specific trial process [42]. Future research should generate evidence that demonstrates which clinical operations methodologies improve efficiency is important to avoid the waste of precious resources. Additionally, DCTs (which became a necessity during the COVID-19 pandemic) are an opportunity to greatly improve efficiency and quality in clinical research [39, 40]. The fishbone diagram and checklist do not detail start-up issues specific to DCTs; however, as they become more common, there is an opportunity to incorporate drivers of start-up delay specific to DCTs.
Conclusion
By following this checklist, clinical trial managers can trim effectively navigate the challenges of clinical trial start-up. With so many activities to coordinate, the start-up process will likely include delays; however, if this can be reduced, it will translate into more time for other high priority activities or contributions.
References
BioWorld. Clinical Trials of Biopharma and Med-tech Products Affected by COVID-19. https://www.bioworld.com/COVID19clinical-affect. Accessed 04 May 2020.
Sullivan GM. Getting off the "gold standard": randomized controlled trials and education research. J Grad Med Educ. 2011;3(3):285–9.
Umscheid C, Margolis D, Grossman C. Key concepts of clinical trials: a narrative review. Postgrad Med. 2011;123(5):194–204.
da Silva RE, Amato AA, Guilhem DB, Novaes MRCG. Globalization of clinical trials: ethical and regulatory implications. Int J Clin Trials. 2016;3:1–8.
Crow RA, Hart KA, McDermott MP, Tawil R, Martens WB, Herr BE, et al. A checklist for clinical trials in rare disease: obstacles and anticipatory actions—lessons learned from the FOR-DMD trial. Trials. 2018;19(1):291.
Huang GD, Bull J, McKee KJ, Mahon E, Harper B, Roberts JN. Clinical trials recruitment planning: a proposed framework from the Clinical Trials Transformation Initiative. Contemp Clin Trials. 2018;66:74–9.
Cheng S, Dietrich M, Dilts D. A sense of urgency: evaluating the link between clinical trial development time and the accrual performance of cancer therapy evaluation program (NCI-CTEP) sponsored studies. Clin Cancer Res. 2010;16(22):5557–63.
Atassi N, Yerramilli-Rao P, Szymonifka J, Yu H, Kearney M, Grasso D, et al. Analysis of start-up, retention, and adherence in ALS clinical trials. Neurology. 2013;81(15):1350–5.
Kantarjian H, Stewart DJ, Zwelling L. Cancer research in the United States: dying by a thousand paper cuts. Cancer. 2013;119(21):3742–5.
Kurzrock R, Pilat S, Bartolazzi M, Sanders D, Van Wart HJ, Tucker SD, et al. Project Zero Delay: a process for accelerating the activation of cancer clinical trials. J Clin Oncol. 2009;27(26):4433–40.
Institute of Medicine (US) Forum on Drug Discovery, Development, and Translation. Transforming Clinical Research in the United States: Challenges and Opportunities: Workshop Summary. Washington (DC): National Academies Press (US); 2010. 3, Challenges in Clinical Research. https://www.ncbi.nlm.nih.gov/books/NBK50888/
Briel M, et al. Comparison of randomized controlled trials discontinued or revised for poor recruitment and completed trials with the same research question: a matched qualitative study. Trials. 2019;20(1):800. https://doi.org/10.1186/s13063-019-3957-4.
Morgan C. Analytics and metrics help pinpoint costs of study start-up. Appl Clin Trials. 2019;28(1/2):10–5.
Rodon J, Soria JC, Berger R, Batist G, Tsimberidou A, Bresson C, et al. Challenges in initiating and conducting personalized cancer therapy trials: perspectives from WINTHER, a Worldwide Innovative Network (WIN) Consortium trial. Ann Oncol. 2015;26(8):1791–8.
Abbott D, Califf R, Morrison BW, Pierre C, Bolte J, Chakraborty S. Cycle time metrics for multisite clinical trials in the United States. Ther Innov Regul Sci. 2013;47(2):152–60.
Krafcik B, Malikova M, Doros G. A single center analysis of factors influencing study start-up timeline in clinical trials. Fut Sci OA. 2017;3(4):FSO223.
Kenyon G, Mendelow A, Gregson B, Rowan E. Obtaining regulatory approval for multicentre randomised controlled trials: experiences in the STICH II trial. Br J Neurosurg. 2011;25(3):352–6.
Xu L, Gao H, Kaitin KI, Shao L. Reforming China’s drug regulatory system. Nat Rev Drug Discov. 2018;17(12):858–9.
Araujo D. The Four Villains of Clinical Trial Agreement Delays and How to Defeat Them: Addressing CTA Delays Comprehensively. United States: WestBow Press; 2018.
Dilts D, Sandler A. Invisible barriers to clinical trials: The impact of structural, infrastructural, and procedural barriers to opening oncology clinical trials. J Clin Oncol. 2006;24(28):4545–52.
Choi YJ, Jeon H, Kim S, In Y, Park SY, Park M, et al. A trial activation initiative to accelerate trial opening in an academic medical center. Ther Innov Regul Sci. 2015;49(2):234–8.
Minisman G, Bhanushali M, Conwit R, Wolfe G, Aban I, Kaminski H, et al. Implementing clinical trials on an international platform: challenges and perspectives. J Neurol Sci. 2012;313(1–2):1–6.
Kiriakis J, Gaich N, Johnston S, Kitterman D, Rosenblum D, Salberg L, et al. Observational study of contracts processing at 29 CTSA sites. Clin Transl Sci. 2013;6(4):279–85.
Brettler D. Insurance & Risk Management for Global Human Clinical Trials [White paper]. Conner Strong & Buckelew. https://www.connerstrong.com/wp-content/uploads/2018/03/1468CSB-LS-Clinical-Human-Trial-Article.pdf. Accessed Jan 2012.
Goudsmit F. Global clinical trial liability insurance. J Clin Res Best Pract. 2013;9(2):1–4.
Chingarande GR, Moodley K. Disparate compensation policies for research related injury in an era of multinational trials: a case study of Brazil, Russia, India, China and South Africa. BMC Med Ethics. 2018;19(1):8.
Tang M, Joensuu H, Simes RJ, Price TJ, Yip S, Hague W, et al. Challenges of international oncology trial collaboration—a call to action. Br J Cancer. 2019;121:515–21.
Lamberti M, Hsia R, Mahon C, Milligan C, Getz K. Assessing global clinical supply logistics. Appl Clin Trials. 2016;25(10/11):26–26.
Bielmeier P, Crauwels G. Managing the extended R&D supply chain. Pharm Eng. 2012;32(4):1–10.
Lamberti M, Costello M, Getz K. Global supply chain management. Appl Clin Trials. 2012;1(9):36–42.
Smith-Gick J, Barnes N, Barone R, Bedford J, James J, Reisner S, et al. The near-term viability and benefits of elabels for patients, clinical sites, and sponsors. Ther Innov Regul Sci. 2018;52(5):537–45.
Lamberti MJ, Walsh T, Getz K. Tracking trial cost drivers: the impact of comparator drugs and co-therapies. Pharm Exec. 2013;33(5):34–7.
Lamberti M, Chakravarthy R, Getz KA. New benchmarks for trial initiation activities. Appl Clin Trials. 2017;25:28–322.
Lamberti MJ, Wilkinson M, Harper B, Morgan C, Getz K. Assessing study start-up practices, performance, and perceptions among sponsors and contract research organizations. Ther Innov Regul Sci. 2018;52(5):572–8.
Hurtado-Chong A, Joeris A, Hess D, Blauth M. Improving site selection in clinical studies: a standardised, objective, multistep method and first experience results. BMJ. 2017;7(7):e014796.
Getz K. Is investigative site feasibility feasible? Appl Clin Trials. 2008;17(7):36–8.
Corneli A, Pierre C, Hinkley T, Lin L, Fordyce CB, Hamre G, et al. One and done: reasons principal investigators conduct only one FDA-regulated drug trial. Contemp Clin Trials Commun. 2017;6:31–8.
Food and Drug Administration (FDA). E6(R2) Good Clinical Practice: Integrated Addendum to ICH E6(R1). https://www.fda.gov/media/93884/download. Accessed Mar 2018.
Clinical Trials Transformation Initiative. Decentralized Clinical Trials. Published September 2018. https://www.ctti-clinicaltrials.org/projects/decentralized-clinical-trials.
Transcelerate BioPharma Inc. Beyond COVID-19: Modernizing Clinical Trial Conduct. Published July 2020. https://transceleratebiopharmainc.com/wp-content/uploads/2020/07/TransCelerate_Beyond-COVID19_Modernizing-Clinical-Trial-Conduct_July-2020.pdf.
Aguinis H, Vandenberg R. An ounce of prevention is worth a pound of cure: improving research quality before data collection. Ann Rev Organ Psychol Organ Behav. 2014;1(1):569–95.
Treweek S, Bevan S, Bower P, Campbell M, Christie J, Clarke M, et al. Trial forge guidance 1: what is a study within a trial (SWAT)? Trials. 2018;19(1):139.
Funding
No financial support of the research, authorship, and/or publication of this article was declared.
Author information
Authors and Affiliations
Contributions
JL was the primary author of the work; this study was conducted for a doctoral project in the DHA program at MUSC. KS, DB, and LF all made revisions and approved the final product.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts were declared.
Rights and permissions
About this article
Cite this article
Lai, J., Forney, L., Brinton, D.L. et al. Drivers of Start-Up Delays in Global Randomized Clinical Trials. Ther Innov Regul Sci 55, 212–227 (2021). https://doi.org/10.1007/s43441-020-00207-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43441-020-00207-2