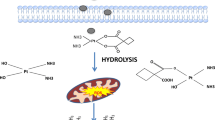
Abstract
Background
Glioblastoma is a severe brain tumor that requires aggressive treatment involving surgery, radiotherapy, and chemotherapy, offering a survival rate of only 15 months. Fortunately, recent nanotechnology progress has enabled novel approaches and, alongside ferrocenes’ unique properties of cytotoxicity, sensitization, and interaction with reactive oxygen species, have brought new possibilities to complement chemotherapy in nanocarrier systems, enhancing treatment results.
Methods
In this work, we developed and characterized a temozolomide-loaded nanoemulsion and evaluated its cytotoxic potential in combination with ferrocene in the temozolomide-resistant T98G and temozolomide-sensitive U87 cell lines. The effects of the treatments were assessed through acute assays of cell viability, cell death, mitochondrial alterations, and a treatment protocol simulation based on different two-cycle regimens.
Results
Temozolomide nanoemulsion showed a z-average diameter of 173.37 ± 0.86 nm and a zeta potential of – 6.53 ± 1.13 mV. Physicochemical characterization revealed that temozolomide is probably associated with nanoemulsion droplets instead of being entrapped within the nanostructure, allowing a rapid drug release. In combination with ferrocene, temozolomide nanoemulsion reduced glioblastoma cell viability in both acute and two-cycle regimen assays. The combined treatment approach also reversed T98G’s temozolomide-resistant profile by altering the mitochondrial membrane potential of the cells, thus increasing reactive oxygen species generation, and ultimately inducing cell death.
Conclusions
Altogether, our results indicate that using nanoemulsion containing temozolomide in combination with ferrocene is an effective approach to improve glioblastoma therapy outcomes.
Graphical abstract








Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- BBB:
-
Blood–brain barrier
- DMEM:
-
Dulbecco’s Modified Eagle Medium
- DDS:
-
Drug delivery system
- EE%:
-
Encapsulation efficiency
- FBS:
-
Fetal bovine serum
- Fc:
-
Ferrocene
- FITC:
-
Fluorescein isothiocyanate
- GB:
-
Glioblastoma
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantification
- MCT:
-
Medium chain triglycerides
- NE:
-
Nanoemulsion
- NRU:
-
Neutral red uptake
- PBS:
-
Phosphate-buffered saline
- PDI:
-
Polydispersity index
- PI:
-
Propidium iodide
- ROS:
-
Reactive oxygen species
- TEM:
-
Transmission electron microscopy
- TMRE:
-
Tetramethylrhodamine ethyl ester
- TMZ:
-
Temozolomide
References
Brodbelt A, Greenberg D, Winters T, Williams M, Vernon S, Collins VP. Glioblastoma in England: 2007–2011. Eur J Cancer. 2015;51(4):533–42.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Guntuku L, Naidu VGM, Ganesh YV. Mitochondrial dysfunction in gliomas: pharmacotherapeutic potential of natural compounds. Curr Neuropharmacol. 2016;14(6):567–83.
Norouzi M, Nazari B, Miller DW. Injectable hydrogel-based drug delivery systems for local cancer therapy. Drug Discov Today. 2016;21(11):1835–49.
Tseng YY, Su CH, Yang ST, Huang YC, Lee WH, Wang YC, et al. Advanced interstitial chemotherapy combined with targeted treatment of malignant glioma in rats by using drug-loaded nanofibrous membranes. Oncotarget. 2016;7(37):59902–16.
Akbar U, Jones T, Winestone J, Michael M, Shukla A, Sun Y, et al. Delivery of temozolomide to the tumor bed via biodegradable gel matrices in a novel model of intracranial glioma with resection. J Neurooncol. 2009;94(2):203–12.
Denny BJ, Wheelhouse RT, Stevens MFG, Tsang LLH, Slack JA. NMR and molecular modeling investigation of the mechanism of activation of the antitumor drug temozolomide and its interaction with DNA. Biochemistry. 1994;33(31):9045–51.
Ohka F, Natsume A, Wakabayashi T. Current trends in targeted therapies for glioblastoma multiforme. Neurol Res Int. 2012;2012:1–13.
Drabløs F, Feyzi E, Aas PA, Vaagbø CB, Kavli B, Bratlie MS, et al. Alkylation damage in DNA and RNA—repair mechanisms and medical significance. DNA Repair (Amst). 2004;3(11):1389–407.
Strobel H, Baisch T, Fitzel R, Schilberg K, Siegelin MD, Karpel-Massler G, et al. Temozolomide and other alkylating agents in glioblastoma therapy. Biomedicines. 2019;7(3):69.
Braga SS, Silva AMS. A new age for iron: antitumoral ferrocenes. Organometallics. 2013;32(20):5626–39.
Ornelas C. Application of ferrocene and its derivatives in cancer research. New J Chem. 2011;35(10):1973–85.
Na Y, Woo J, Choi WI, Sung D. Novel carboxylated ferrocene polymer nanocapsule with high reactive oxygen species sensitivity and on-demand drug release for effective cancer therapy. Colloids Surf B Biointerfaces. 2021;200: 111566.
Qian Y, Zhang J, Xu R, Li Q, Shen Q, Zhu G. Nanoparticles based on polymers modified with pH-sensitive molecular switch and low molecular weight heparin carrying celastrol and ferrocene for breast cancer treatment. Int J Biol Macromol. 2021;183:2215–26.
Idlas P, Lepeltier E, Jaouen G, Passirani C. Ferrocifen loaded lipid nanocapsules: a promising anticancer medication against multidrug resistant tumors. Cancers (Basel). 2021;13(10):2291.
Fan CH, Ting CY, Chang YC, Wei KC, Liu HL, Yeh CK. Drug-loaded bubbles with matched focused ultrasound excitation for concurrent blood-brain barrier opening and brain-tumor drug delivery. Acta Biomater. 2015;15(15):89–101.
Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, et al. Drug resistance in cancer: an overview. Cancers (basel). 2014;6(3):1769–92.
Huang D, Lin C, Wen X, Gu S, Zhao P. A potential nanofiber membrane device for filling surgical residual cavity to prevent glioma recurrence and improve local neural tissue reconstruction. PLoS ONE. 2016;11(8): e0161435.
Sawyer AJ, Piepmeier JM, Saltzman WM. New methods for direct delivery of chemotherapy for treating brain tumors. Yale J Biol Med. 2006;79(3–4):141–52.
Wei X, Chen X, Ying M, Lu W. Brain tumor-targeted drug delivery strategies. Acta Pharm Sin B. 2014;4(3):193–201.
Weller M, Cloughesy T, Perry JR, Wick W. Standards of care for treatment of recurrent glioblastoma-are we there yet? Neuro Oncol. 2013;15:4–27.
Borthakur P, Boruah PK, Sharma B, Das MR. Nanoemulsion: preparation and its application in food industry. In: Grumezescu AM, editor. Emulsions. Academic Press; 2016. p. 153–91.
Heravi Shargh V, Luckett J, Bouzinab K, Paisey S, Turyanska L, Singleton WGB, et al. Chemosensitization of temozolomide-resistant pediatric diffuse midline glioma using potent nanoencapsulated forms of a N(3)-propargyl analogue. ACS Appl Mater Interfaces. 2021;13(30):35266–80.
Chen D, Liu X, Lu X, Tian J. Nanoparticle drug delivery systems for synergistic delivery of tumor therapy. Front Pharmacol. 2023;16:14.
Ganta S, Talekar M, Singh A, Coleman TP, Amiji MM. Nanoemulsions in translational research—opportunities and challenges in targeted cancer therapy. AAPS PharmSciTech. 2014;15(3):694–708.
Jiang Y, Liu C, Zhai W, Zhuang N, Han T, Ding Z. The optimization design of lactoferrin loaded hupa nanoemulsion for targeted drug transport via intranasal route. Int J Nanomed. 2019;14:9217–34.
Sánchez-López E, Guerra M, Dias-Ferreira J, Lopez-Machado A, Ettcheto M, Cano A, et al. Current applications of nanoemulsions in cancer therapeutics. Nanomaterials. 2019;9(6):821.
Venturini CG, Jäger E, Oliveira CP, Bernardi A, Battastini AMO, Guterres SS, et al. Formulation of lipid core nanocapsules. Colloids Surf A Physicochem Eng Asp. 2011;375(1–3):200–8.
Borenfreund E, Puerner JA. toxicity determined in vitro by morphological alterations and neutral red absorption (Surfactants; highest tolerated dose; in vitro alternative; spectrophotometric analysis). Toxicol Lett. 1985;24(2–3):119–24.
Han SJ, Rolston JD, Molinaro AM, Clarke JL, Prados MD, Chang SM, et al. Phase II trial of 7 days on/7 days off temozolmide for recurrent high-grade glioma. Neuro Oncol. 2014;16(9):1255–62.
Wick W, Platten M, Weller M. New (alternative) temozolomide regimens for the treatment of glioma. Neuro Oncol. 2009;11(1):69–79.
Honasoge A, Sontheimer H. Involvement of tumor acidification in brain cancer pathophysiology. Front Physiol. 2013;4:316.
Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–9.
ICH Harmonised Tripartite Guideline (2005) Validation of Analytical Procedures: Text and Methodology Q2 (R1). International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Geneva, 1–17. https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf.
Brasil MS (2017) Resolução da Diretoria Colegiada - RDC no 166. Diário Oficial da República Federativa do Brasil, 2017, 1–22. http://antigo.anvisa.gov.br/documents/10181/2721567/RDC_166_2017_COMP.pdf/d5fb92b3-6c6b-4130-8670-4e3263763401.
Bianchin MD, Külkamp-Guerreiro IC, De Oliveira CP, Contri RV, Guterres SS, Pohlmann AR. Radar charts based on particle sizing as an approach to establish the fingerprints of polymeric nanoparticles in aqueous formulations. J Drug Deliv Sci Technol. 2015;1(30):180–9.
Cardoso AM, de Oliveira EG, Coradini K, Bruinsmann FA, Aguirre T, Lorenzoni R, et al. Chitosan hydrogels containing nanoencapsulated phenytoin for cutaneous use: skin permeation/penetration and efficacy in wound healing. Mater Sci Eng, C. 2019;1(96):205–17.
Metin CO, Lake LW, Miranda CR, Nguyen QP. Stability of aqueous silica nanoparticle dispersions. J Nanopart Res. 2011;13(2):839–50.
Raval N, Maheshwari R, Kalyane D, Youngren-Ortiz SR, Chougule MB, Tekade RK. Importance of physicochemical characterization of nanoparticles in pharmaceutical product development. In: Tekade RK, editor. Basic Fundamentals of Drug Delivery. Academic Press; 2018. p. 369–400.
Giri K, Shameer K, Zimmermann MT, Saha S, Chakraborty PK, Sharma A, et al. Understanding protein-nanoparticle interaction: a new gateway to disease therapeutics. Bioconjug Chem. 2014;25(6):1078–90.
Krai J, Beckenkamp A, Gaelzer MM, Pohlmann AR, Guterres SS, Filippi-Chiela EC, et al. Doxazosin nanoencapsulation improves its in vitro antiproliferative and anticlonogenic effects on breast cancer cells. Biomed Pharmacother. 2017;1(94):10–20.
Sari TP, Mann B, Kumar R, Singh RRB, Sharma R, Bhardwaj M, et al. Preparation and characterization of nanoemulsion encapsulating curcumin. Food Hydrocoll. 2015;1(43):540–6.
Walia N, Zhang S, Wismer W, Chen L. A low energy approach to develop nanoemulsion by combining pea protein and Tween 80 and its application for vitamin D delivery. Food Hydrocoll Health. 2022;1;2:100078.
Ananta JS, Paulmurugan R, Massoud TF. Temozolomide-loaded PLGA nanoparticles to treat glioblastoma cells: a biophysical and cell culture evaluation. Neurol Res. 2016;38(1):51–9.
Ramazani F, Chen W, van Nostrum CF, Storm G, Kiessling F, Lammers T, et al. Strategies for encapsulation of small hydrophilic and amphiphilic drugs in PLGA microspheres: state-of-the-art and challenges. Int J Pharm. 2016;499(1–2):358–67.
Li Q, Li X, Zhao C. Strategies to obtain encapsulation and controlled release of small hydrophilic molecules. Front Bioeng Biotechnol. 2020;8:437.
Maiti D, Saha A, Devi PS. Surface modified multifunctional ZnFe2O4 nanoparticles for hydrophobic and hydrophilic anti-cancer drug molecule loading. Phys Chem Chem Phys. 2016;18(3):1439–50.
Chenthamara D, Subramaniam S, Ramakrishnan SG, Krishnaswamy S, Essa MM, Lin FH, et al. Therapeutic efficacy of nanoparticles and routes of administration. Biomater Res. 2019;23(20):1–29.
Singh S, Pathak K, Bali V. Product development studies on surface-adsorbed nanoemulsion of olmesartan medoxomil as a capsular dosage form. AAPS PharmSciTech. 2012;13(4):1212–21.
Irani M, Sadeghi GMM, Haririan I. The sustained delivery of temozolomide from electrospun PCL-Diol-b-PU/gold nanocompsite nanofibers to treat glioblastoma tumors. Mater Sci Eng, C. 2017;75:165–74.
Reinhardt LS, Morás AM, Henn JG, Arantes PR, Ferro MB, Braganhol E, et al. Nek1-inhibitor and temozolomide-loaded microfibers as a co-therapy strategy for glioblastoma treatment. Int J Pharm. 2022;617: 121584.
Morás AM, Henn JG, Steffens Reinhardt L, Lenz G, Moura DJ. Recent developments in drug delivery strategies for targeting DNA damage response in glioblastoma. Life Sci. 2021;287: 120128.
Grotto HZW. Metabolismo do ferro: uma revisão sobre os principais mecanismos envolvidos em sua homeostase. Rev Bras Hematol Hemoter. 2008;30(5):390–7.
Murillo MI, Gaiddon C, Le Lagadec R. Targeting of the intracellular redox balance by metal complexes towards anticancer therapy. Front Chem. 2022;11:10.
Köpf-Maier P, Köpf H, Neuse EW. Ferricenium complexes: a new type of water-soluble antitumor agent. J Cancer Res Clin Oncol. 1984;108(3):336–40.
Kauffman M, Kauffman M, Traore K, Zhu H, Trush M, Jia Z, et al. MitoSOX-based flow cytometry for detecting mitochondrial ROS. React Oxyg Species. 2016;2(5):361–70.
Perry SW, Norman JP, Barbieri J, Brown EB, Gelbard HA. Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniques. 2011;50:98–115.
Lee SY. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016;3(3):198–210.
Atmaca H, Özkan AN, Zora M. Novel ferrocenyl pyrazoles inhibit breast cancer cell viability via induction of apoptosis and inhibition of PI3K/Akt and ERK1/2 signaling. Chem Biol Interact. 2017;263:28–35.
Tian J, Chen J, Ge C, Liu X, He J, Ni P, et al. Synthesis of PEGylated ferrocene nanoconjugates as the radiosensitizer of cancer cells. Bioconjug Chem. 2016;27(6):1518–24.
Kondratskyi A, Kondratska K, Vanden Abeele F, Gordienko D, Dubois C, Toillon RA, et al. Ferroquine, the next generation antimalarial drug, has antitumor activity. Sci Rep. 2017;7(1):15896.
Podolski-Renić A, Bősze S, Dinić J, Kocsis L, Hudecz F, Csámpai A, et al. Ferrocene–cinchona hybrids with triazolyl-chalcone linkers act as pro-oxidants and sensitize human cancer cell lines to paclitaxel. Metallomics. 2017;9(8):1132–41.
Acknowledgements
This study was supported in parts by grants and/or scholarships from FAPERGS (Fundação de Apoio à Pesquisa do Rio Grande do Sul, Brazil, Grant nº 21/2551-0001965-4), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil). The graphical abstract was created with BioRender.com.
Author information
Authors and Affiliations
Contributions
JGH: conceptualization, data curation, formal analysis, investigation, methodology, project administration, validation, visualization, writing – original draft, and writing – review & editing. MBF, JVRO, BMS, LFS, VRJS, and ACSP: data curation, and investigation. GALA and FPP: data curation, investigation, methodology, and validation. LSR, AMM, and RGR: investigation, methodology, resources, and writing – review & editing. MN: resources, and writing – review & editing. TASA: conceptualization, funding acquisition, methodology, project administration, resources, validation, and writing – review & editing. DJM: conceptualization, funding acquisition, methodology, project administration, resources, supervision, validation, and writing – review & editing. All authors reviewed the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Declaration of generative AI and AI-assisted technologies in the writing process
The authors declare that no Generative AI and AI-assisted technologies were used in the writing process.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
43440_2023_537_MOESM1_ESM.tif
Fig. S1. Schematic representation of ferrocene (A), monopegylated ferrocene (B) and dipegylated ferrocene (C). Supplementary file1 (TIF 32 KB)
43440_2023_537_MOESM2_ESM.tif
Fig. S2. Schematic representation of different treatment protocol simulation based on two-cycle regimens: T98G cells were treated for different days either with 100 µM of Fc and/or TMZ on their own, or TMZ-NE on its own or in combination with Fc, dissolved in DMEM + FBS (5%). In all recovery periods cells were kept in culture media with DMEM + FBS (10 %). All regimens comprised two treatment cycles and in the end cell viability was carried out by NRU assay. Fc ferrocene, TMZ temozolomide, NE nanoemulsion, FBS fetal bovine serum, NRU neutral red uptake. Supplementary file2 (TIF 147 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Henn, J.G., Bernardes Ferro, M., Lopes Alves, G.A. et al. Development and characterization of a temozolomide-loaded nanoemulsion and the effect of ferrocene pre and co-treatments in glioblastoma cell models. Pharmacol. Rep 75, 1597–1609 (2023). https://doi.org/10.1007/s43440-023-00537-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-023-00537-6