Abstract
Background
Perioperative anesthetic and/or analgesic demand present considerable variation, and part of that variation appears to be genetic in origin. Here we investigate the impact of common polymorphisms in OPRM1, COMT, SLC6A4, ABCB1, and CYP2B6 genes, on the intra-operative consumption of remifentanil and propofol, as well as the postoperative analgesic needs, in patients subjected to thyroidectomy surgery.
Methods
We conducted a prospective cohort study with 90 patients scheduled to undergo elective thyroidectomy, under total intravenous anesthesia achieved by target control infusion (TCI) of propofol and remifentanil. Postoperative analgesics were administered by protocol and on-demand by the individual patient. Genotyping was established by PCR–RFLP methods. Genotyping data, intra-operative hemodynamics, and total consumption of remifentanil and propofol, as well as postoperative analgesic needs and pain perception, were recorded for each individual.
Results
Patients with the ABCB1 3435TT genotype appeared to experience significantly less pain within one hour post-operatively, compared to C carriers [mean VAS (SD) = 0.86 (1.22) vs. 2.42 (1.75); p = 0.017], a finding limited to those seeking rescue analgesic treatment. Intra-operatively, homozygotes patients for the minor allele of OPRM1 A118G and CYP2B6 G516T appeared to consume less remifentanil [mean (SD) = 9.12 (1.01) vs. 13.53 (5.15), for OPRM1 118GG and A carriers] and propofol [median (range) = 14.95 (11.53, 1359.5) vs. 121.4 (1.43, 2349.4), for CYP2B6 516TT and G carriers, respectively] but the difference was not statistically significant in our sample.
Conclusions
The ABCB1 C3435T polymorphism appears to affect the postoperative perception of surgical pain among patients with low pain threshold. The small number of minor allele homozygotes for the OPRM1 A118G and CYP2B6 G516T polymorphisms precludes a definitive conclusion regarding the inclusion of the latter in a TCI-programming algorithm, based on the results of this study.
Clinical trial registration number
ACTRN12616001598471.
Similar content being viewed by others
Introduction
Remifentanil is a synthetic μ-opioid receptor (OPRM1) agonist with rapid onset and short duration of action. It displays fast biotransformation by blood esterases and its context-sensitive half-time (time needed to half its blood concentration following the end of administration) is very short, independent of the duration of infusion, and apparently unaffected by liver or kidney function impairment [1]. As an μ-opioid receptor agonist, remifentanil exerts its antinociceptive effect by activating noradrenergic and serotonergic descending inhibitory pathways and by inhibiting the synaptic activation of secondary afferent spinothalamic pathways by Aδ and C fibers at the dorsal horn of the spinal cord [2]. Thus, remifentanil efficacy depends on its ability to cross the blood–brain barrier and could be modulated by P-glycoprotein (ABCB1) activity, as is the case with most opioids [3].
Propofol is an intravenous anesthetic that acts on GABAA receptors as an agonist. It is extensively metabolized, primarily in the liver, but also in the kidneys and the small intestine, mainly by CYP2B6 and UDP glucuronyl transferases (UDPs) [4]. It displays complex pharmacodynamics and shows a synergistic interaction with remifentanil, with respect to both hypnotic and analgesic endpoints [5].
Remifentanil and propofol are usually administered perioperatively by continuous, commonly target-controlled infusion (TCI), in which the infusion rate needed to achieve a predetermined target concentration is calculated by a computing device based on pharmacokinetic (PK) and pharmacodynamic (PD) modeling and patient anthropometric characteristics [6, 7]. More precise, patient-oriented TCI programming could conceivably benefit from the discovery of genetic biomarkers of remifentanil and propofol drug efficacy and optimize perioperative care. In addition,
In contrast to other commonly used opioid drugs which have had their fair share of pharmacogenetic studies [8, 9], data relevant to remifentanil are scarce. The HTTLPR triallelic polymorphism of the SLC6A4 gene, representing a long/short allele in its promoter region, combined with a MspI restriction site in the extra 43 bp segment of the long allele, was the first polymorphism ever associated with remifentanil efficiency in alleviating experimental thermal pain in healthy volunteers [10], with carriers of the double short allele, or low expressing genotype displaying lower pain perception following bolus intravenous administration of the drug.
The COMT Val158Met polymorphism appears in several opioid pharmacogenetic studies either alone or in combination with other polymorphisms, selected as a candidate gene by virtue of its effect on COMT activity in vitro, associated in turn with the availability of μ opioid receptors in certain areas of the brain (basal ganglia, thalamus) [11]. Work on remifentanil includes studies on the pain sensitivity of healthy volunteers [12], on the efficacy of pain relief of preterm newborns after endotracheal intubation [13], and on the intensity of postoperative acute, chronic, or experimental pain following cardiac surgery, together with the OPRM1 gene (OPRM1) A118G (rs1799971) polymorphism.
The common rs1045642 polymorphism of the P glycoprotein-encoding gene (ABCB1 C3435T) was associated with remifentanil consumption and clinical efficacy during elective surgery [14], and with the stabilizing effect of the drug on the hemodynamics of women undergoing cesarian section, as well as on the adaptation of their newborn babies [15]. In addition, ABCB1 C3435T was recently shown to affect heat-pain threshold in opioid-free adults with chronic pain [16].
CYP2B6 G516T is a component polymorphism of the common CYP2B6*6 haplotype (as well as others, less common ones) [17]. It was linked to aberrant splicing and low expression of CYP2B6 and was associated with suboptimal biotransformation of propofol, among other drugs [18].
The aim of this study was to examine the possible association of the aforementioned polymorphisms with the intra-operative consumption of remifentanil and propofol through a TCI protocol, and the postoperative analgesic needs and pain perception, in a group of successive patients undergoing total thyroidectomy, using a minimally invasive surgical approach.
Materials and methods
Study participants
One hundred and one successively recruited patients (49 from the 3rd Department of Surgery and 40 from the 1st Propedeutic Department of Surgery, AHEPA University Hospital; 12 from the Interbalkan Medical Center, Thessaloniki, Greece) were initially enrolled in this prospective observational study. Participants were all aged 18 years or older, classified according to American Society of Anesthesiology Physical Status (ASA-PS) 1–2, with normal thyroid hormone levels, scheduled to undergo elective thyroidectomy for malignancy, benign disease, or hormonal disease not responsive to medical management. Rules of anonymity and the protection of patients’ personal data were strictly observed throughout the study. Exclusion criteria were age < 18 years, ASA PS ≥ 3, analgesic use up to one week prior to surgery, emergency thyroidectomy, severe thyroid hormone levels disturbance, pregnancy, and diagnosis of a personality disorder. The study protocol was approved by the Scientific Committee of AHEPA University Hospital of Thessaloniki (322/20-5-2016) and the Bioethics Committee of the School of Medicine, Aristotle University of Thessaloniki (316/6-7-2016).
Study protocol
The evening before the scheduled operation, the anxiety level of each participant was rated according to the Hamilton Anxiety Scale [19]. On the day of surgery, the subjects were premedicated with oral diazepam (5−10 mg). On arrival at the operating theatre, routine monitoring involving electrocardiography, pulse oximetry, and non-invasive blood pressure measurements at 5-min intervals were instituted for each patient and baseline recordings were obtained. Thereafter, a peripheral venous catheter was inserted for intravenous (iv) fluid replacement. Additionally, Bispectral Index (BIS) monitoring (Aspect Medical Systems, Natick, MA) was implemented to guide the anesthesia depth. Anesthesia induction was performed by target-controlled infusion (TCI) of propofol (Schneider pharmacokinetic model) with effect-site concentration set at 4–5 μg/mL, while endotracheal intubation with a proper-sized cuffed endotracheal tube was facilitated following a single dose of cisatracurium (0.2 mg/kg).
Anesthesia was maintained with TCI propofol (effect-site concentration of 3 μg/mL) adjusted by increments of 0.5 mg/mL targeting a BIS value between 40 and 60. Intra-operative analgesia was achieved by TCI remifentanil (1.5–3 μg/mL), adjusted to manage hemodynamic changes exceeding 20% of the baseline values. Normothermia, as assessed by the placement of a mid-esophageal temperature probe, was ensured throughout the surgical procedure.
At the start of skin closure, propofol infusion was discontinued, while as soon as skin suturing was completed remifentanil infusion was also terminated. Before awakening, all patients were given paracetamol 1 g iv and ondansetron 4 mg to ensure postoperative analgesia and protection from postoperative nausea and vomiting, respectively. As soon as clinical signs of an adequate level of consciousness and satisfactory breathing efforts were evidenced, the endotracheal tube was removed, and then all patients were transferred to the post-anesthesia care unit (PACU). After at least a one-hour stay in the PACU, they were discharged to the surgical ward.
The severity of pain at the surgical site was assessed by Visual Analogue Scale (VAS) grading as 0 for the absence of pain and 10 for the worst pain imaginable, during PACU stay (15, 30, and 60 min) and at 2 and 6 h after surgery completion. An anesthesiologist—a member of the research team—ensured that a dedicated protocol for postoperative analgesia management was properly implemented. Rescue analgesics involving paracetamol (1–4 g total, iv) supplemented by parecoxib (40–80 mg total, iv) or lornoxicam (8–16 mg total, iv) were prescribed when clinically notable pain perception (VAS ≥ 4) was encountered upon the predefined time-points of assessment. Tramadol boluses (100 mg total, iv) were administered upon demand to control persistent pain. Intra-operative remifentanil and propofol consumption, as well as total postoperative analgesic requirements being transformed to morphine equivalents (mg), were duly recorded [20,21,22,23].
Genotyping
DNA was extracted from peripheral blood with a commercial DNA extraction kit (Quick-DNA Miniprep Kit, Zymo Research, product number: D3025 Irvine, CA, USA). All polymorphisms were genotyped with previously published PCR–RFLP methods (Table 1) [24,25,26,27,28]. Genotyping was validated with negative and positive controls, by omitting DNA in the reaction mix and by using independently genotyped samples from previous studies, respectively.
Statistical analysis
All continuous variables were tested for normality with the Kolmogorov-Smirnoff test. Deviation of genotype distributions from the Hardy–Weinberg equilibrium was tested with the χ2 goodness-of-fit test. The effect of each polymorphism on time- and weight-normalized logarithmically transformed remifentanil consumption was tested with ANCOVA, using age, sex, height, Hamilton anxiety scale, and time- and weight-normalized propofol consumption as covariates. The effect of polymorphisms on time- and weight-normalized propofol consumption was examined with the Jonckheere-Terpstra test, due to the wide deviation of propofol consumption from normality which could not be corrected with logarithmic transformation (Figure S1), initially using all three genotypes of each polymorphism as independent variables, and pairwise comparisons as required. The same type of analysis was applied to test the effect of genotypes on VAS, at different time points, from 15 to 360 min following extubation. Spearman’s correlation tests were used to assess the correlation between intra-operative and postoperative drug consumption with VAS at all time points. IBM SPSS statistics 27 was the latest version used for statistical analyses. Bonferroni correction for five polymorphisms and three dependent variables (remifentanil, propofol, and postoperative demands) returns a significance limit of 0.003.
Results
Patients’ demographic characteristics and genotype distributions for the five polymorphisms are shown in Table 2. For 11 patients, anesthetic/ analgesic drug consumption data were not recorded, and/or DNA was not isolated for various reasons. OPRM1 A118G and CYP2B6 G516T genotyping failed for an additional one and 11 patients, respectively. All genotype distributions were consistent with the Hardy–Weinberg equilibrium.
Intra- and postoperative analgesic/anesthetic drug consumption, as well as VAS pain ratings, are summarized in Table 2. When time-normalized for the duration of anesthesia, drug consumption distributions all deviated from normality, with propofol consumption exhibiting the most skewed distribution, as already mentioned (Figure S1). Post-, but not intra-operative analgesic consumption was positively correlated with Hamilton anxiety scale ratings (p = 0.001; Table S1), and with VAS ratings at all time points (Table S2). VAS distributions also deviated significantly from normality.
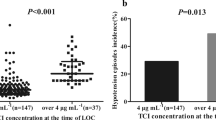
The effect of the five polymorphisms on intra- and postoperative drug consumption is shown in Table 3. No significant differences were observed between patients carrying different genotypes, for any polymorphism. An apparently lower intra-operative remifentanil consumption observed with patients carrying the OPRM1 A118GG genotype in comparison to those carrying the AA and AG genotypes (Fig. 1A; S2A) did not reach statistical significance (p = 0.082 for GG vs. AA and AG combined; t test following logarithmic transformation). Similarly, CYP2B6 516TT genotype carriers appeared to consume less propofol compared to CYP2B6 516GG and GT carriers intra-operatively (Fig. S2B), but, here again, the difference was not significant (p = 0.063 for TT vs. GG and GT combined; Fig. 1B).
Distributions of intra-operative remifentanil and propofol consumptions, stratified according to the genotypes of the OPRM1 A118G (A) and CYP2B6 G516T (B) polymorphisms, respectively. Shaded boxes: Interquartile range; whiskers: 95% confidence interval; horizontal bars: median values; circles: outliers; asterisks: extreme values, defined as values deviating from the limits of the interquartile range by more than 3 times its magnitude (SPSS)
The effect of each polymorphism on VAS ratings obtained at 15, 30, 60, 120 and 360 min after completion of surgical procedures was probed next. Results are displayed in Table 4. While no significant associations were recorded overall, the distribution of VAS ratings at time points t = 15, 30 and 60 min appeared to be inversely related to the presence of the ABCB1 3435 T allele in the patients’ genotype (Fig. S3). As a significant number of patients (n = 38; 42.2%) required rescue analgesic treatment suggesting a lower pain threshold (Table S3), the analysis was repeated following stratification accordingly. As shown in Fig. 2, this apparent effect observed with the entire sample (Fig. S3) reached statistical significance for those patients requiring additional analgesia, but not for those who did not ABCB1. C3435T genotype distributions did not differ between the two subgroups (Table S4). Finally, because anxiety is known to affect the perception of pain and Hamilton rating scale values were positively correlated with postoperative analgetic consumption, we have examined the effect of the ABCB1 C3435T genotype on Hamilton ratings, with negative results (Table S5).
VAS ratings of post-surgical pain, at t = 15, 30, 60, 120, and 360 min. A patients requiring rescue medication (n = 38; CC = 9, CT = 22, TT = 7); B patients not requiring rescue medication (n = 52; CC = 13, CT = 31, TT = 8). Shaded boxes: Interquartile range; whiskers: 95% confidence interval; horizontal bars: median values; circles: outliers; asterisks: extreme values. VAS distributions for each genotype were compared at each time point with the Jonckheere-Terpstra test. p values for three-genotype comparisons are in bold; pairwise comparisons are indicated by two headed arrows (only Bonferroni-adjusted significant p values are shown); hooks indicate use of the recessive model for the T allele (TT vs. CC + CT)
Discussion
While the primary objective of this study was to examine the effect of common polymorphisms previously related to opioid and propofol efficiency on intra-operative remifentanil and propofol requirements (consumption) of by patients who had undertaken elective thyroidectomy, its main—and rather unexpected—finding was a time-dependent effect of ABCB1 C3435T on post-surgical pain perception; patients carrying the TT genotype apparently reported significantly lower pain perception compared to C carriers, at times 15, 30 and 60 min following the end of the surgical procedure, when VAS ratings are highest and most varied. Furthermore, that effect was detected only among those patients requiring rescue analgesic medication, i.e., with a low pain threshold. We argue that the fact that the significance of this association appears to be time-dependent and rather consistent throughout most of the postoperative period argues against it being a chance finding. Since neither VAS ratings nor the ABCB1 C3435T polymorphism is associated with intra-operative consumption of remifentanil or propofol, one should look into postoperative analgesic consumption or the mechanism of pain perception for an explanation. As VAS ratings are consistently higher in patients requiring additional (rescue) postoperative treatment (Table S5), the former must reflect either differences in the interindividual efficiency of standard postoperative paracetamol or in some physiologic parameter causally related to pain perception. Paracetamol is known to induce ABCB1 expression [29] but was not shown to function as its substrate. On the other hand, a recently published report by Hooten et al. [16] suggested that the ABCB1 3435TT genotype is associated with increased threshold to heat pain in opioid-free adults with chronic pain. The authors attributed that apparent effect on alterations in the efflux of endogenous opioid peptides from the brain, previously shown to be mediated by ABCB1 [30]. Even though more needs to be done to corroborate those findings and articulate a more detailed working hypothesis as to a probable underlying mechanism, it is encouraging that in our study the ABCB1 3435TT genotype is also associated with a lower perception of pain and that this effect is made apparent only among those patients predisposed to higher pain sensitivity (i.e., in need of rescue medication). That would be consistent with a situation where the concentration of opioids in the CNS becomes limiting and is thus affected by transport systems in the blood–brain barrier, including ABCB1.
Our findings with respect to the effect of the five gene polymorphisms on the intra-operative remifentanil and propofol consumption were less promising, even though it is perhaps worth mentioning that all four carriers of the OPRM1 118GG genotype consumed low amounts of remifentanil, and so did five out of six patients with the CYP2B6 516TT genotype with respect to propofol. A small number of studies have examined the association of the same OPRM1 polymorphism with intra-operative opioid (fentanyl, sulfentanil, or morphine) requirements, all of them involving obstetrics analgesia [reviewed in [31], and in [32], with inconclusive results, even though carriage of the G allele appeared to somewhat increase opioid efficacy, which is not contradictory to our findings. According to our literature search, no study has examined the effect of OPRM1 A118G on remifentanil requirements thus far, with the exception of a single report highlighting a protective effect of the maternal G allele on neonates whose mothers had received remifentanil in the context of labor anesthesia [15]. Despite propofol being an established substrate for CYP2B6, the effect of CYP2B6 G516T on propofol pharmacokinetics remains controversial, with some evidence supporting the association of the T allele with decreased metabolism and, consequently, lower dose requirements of propofol [33, 34], which is also somewhat consistent to our present findings. The limited number of TT genotypes in this study precludes any further analysis, however, especially since other factors, such as gender and age, are known to also affect CYP2B6 activity [17]. On the other hand, we found no apparent effect of either the OPRM1 or the CYP2B6 polymorphism on postoperative analgesic consumption, which can be explained by the very short context-sensitive half-time of remifentanil on one hand, and the very limited use of opioids and/or CYP2B6 substrates post-operatively.
According to previous studies of the postoperative use of morphine [35, 36] COMT 158MetMet carrier patients should display a lower opioid requirement compared to COMT 158Val carriers. Overall, however, published findings on the effects of COMT Val158Met on opioid (fentanyl, hydromorphone, morphine, butorphanole, or meperidine) requirements or pain threshold, alone or in combination with OPRM1 A118G [13, 37, 38], are characterized by inconsistencies, which our findings are unable to resolve since no significant association was observed with respect to analgesic consumption in our study.
The HTTLPR polymorphism has been examined in association with opioid use in the past, by virtue of 5-HT involvement in the modulation of pain signaling at the spinal level, but no significant differences in daily opioid consumption were found among patients suffering from chronic pain, carrying different genotypes [28]. With respect to non-opioid analgesics, the HTTLPR polymorphism was associated with poorer response to carbamazepine in idiopathic trigeminal neuralgia patients [39], but not with triptan response in patients with cluster headaches [40]. In our study, the HTTLPR polymorphism was not associated with any perioperative related outcome whatsoever, be that remifentanil consumption, propofol consumption, pain perception as assessed by VAS, or postoperative consumption of analgesics in morphine equivalents.
Our rather small sample size and the limited number of OPRM1 A118G and CYP2B6 G516T minor allele homozygotes are obvious limitations of our study, and so could be the over-representation of women, since propofol metabolism is known to be affected by sex, among other things [41]. On the contrary, the single type of elective surgery, with the same standardized surgical and anesthesiologic procedures, and the ethnic homogeneity of the participating patient population have presumably reduced other confounding effects.
In conclusion, we have provided evidence suggestive of an effect of the ABCB1 C3435T polymorphism on the postoperative perception of surgical pain which, combined with recent findings concerning heat pain perception in opioid-free patients with chronic pain, points to a significant contribution of ABCB1 efflux pump to the process of central pain management by the organism. Our data concerning the association of OPRM1 A118G and CYP2B6 G516T with intra-operative remifentanil and propofol consumption does not support their inclusion in a TCI-programming algorithm at this point.
Data availability
The authors have also submitted supplemental data and declare that if needed they can provide all the data concerning the present manuscript.
Abbreviations
- 5-HT:
-
5-Hydroxytryptamine
- ABCB1:
-
ATP binding cassette subfamily B member 1
- ANCOVA:
-
Analysis of covariance
- ANZCTR:
-
Australian New Zealand clinical trials registry
- ASA-PS:
-
American society of anesthesiology physical status
- BIS:
-
Bispectral index
- COMT:
-
Catechol-O-methyltransferase
- CYP:
-
Cytochrome P450
- DNA:
-
Deoxyribonucleic acid
- GABA:
-
Gamma-aminobutyric acid
- HTTLPR:
-
Serotonin-transporter-linked promoter region
- OPRM1:
-
μ-Opioid receptor 1
- PACU:
-
Post anesthesia care unit
- PCR-RFLP:
-
Polymerase chain reaction-restriction fragment length polymorphism
- SD:
-
Standard deviation
- SLC6A4:
-
Solute carrier family 6 member 4
- TCI:
-
Targeted control infusion
- UDP glycuronyl transferases:
-
Uridine 5'-diphospho-glucuronosyltransferase
- VAS:
-
Visual analogue scale
References
Michelsen LG, Hug CC. The pharmacokinetics of remifentanil. J Clin Anesth. 1996;8:679–82.
Corder G, Castro DC, Bruchas MR, Scherrer G. Endogenous and exogenous opioids in pain. Annu Rev Neurosci. 2018;41:453–73.
Chaves C, Remiao F, Cisternino S, Decleves X. Opioids and the blood-brain barrier: a dynamic interaction with consequences on drug disposition in brain. Curr Neuropharmacol. 2017;15(8):1156–73.
Sahinovic MM, Struys MMRF, Absalom AR. Clinical pharmacokinetics and pharmacodynamics of propofol. Clin Pharmacokinet. 2018;57(12):1539–58.
Kern SE, Xie G, White JL, Egan TD. A response surface analysis of propofol-remifentanil pharmacodynamic interaction in volunteers. Anesthesiology. 2004;100(6):1373–81.
Struys MMRF, de Smet T, Glen JB, Vereecke HEM, Absalom AR, Schnider TW. The history of target-controlled infusion. Anesth Analg. 2016;122:56–69.
Eleveld DJ, Colin P, Absalom AR, Struys MMRF. Target-controlled-infusion models for remifentanil dosing consistent with approved recommendations. Br J Anaesth. 2020;125:483–91.
Brandl E, Halford Z, Clark MD, Herndon C. Pharmacogenomics in pain management: a review of relevant gene-drug associations and clinical considerations. Ann Pharmacother. 2021;55:1486–501.
Rodriguez Cairoli F, Appiani F, Sambade JM, Comandé D, Camacho Arteaga L, Ciapponi A. Efficacy and safety of opioid therapy guided by pharmacogenetics: a systematic review. Pharmacogenomics. 2021;22:573–86.
Kosek E, Jensen KB, Lonsdorf TB, Schalling M, Ingvar M. Genetic variation in the serotonin transporter gene (5-HTTLPR, rs25531) influences the analgesic response to the short acting opioid Remifentanil in humans. Mol Pain. 2009;5:1–7.
Kowarik MC, Einhäuser J, Jochim B, Büttner A, Tölle TR, Riemenschneider M, et al. Impact of the COMT Val(108/158)Met polymorphism on the mu-opioid receptor system in the human brain: mu-opioid receptor, met-enkephalin and beta-endorphin expression. Neurosci Lett. 2012;506:214–9.
Jensen KB, Lonsdorf TB, Schalling M, Kosek E, Ingvar M. Increased sensitivity to thermal pain following a single opiate dose is influenced by the COMT val158 met polymorphism. PLoS ONE. 2009;4:2–6.
Elens L, Norman E, Matic M, Rane A, Fellman V, van Schaik RHN. Genetic predisposition to poor opioid response in preterm infants: impact of KCNJ6 and COMT polymorphisms on pain relief after endotracheal intubation. Ther Drug Monit. 2016;38:525–33.
Wang W, Zhou Q, Yuan C. Influence of (ATP)-binding cassette transporter subfamily B member 1 (ABCB1) gene polymorphism on the efficacy of remifentanil. Med Sci Monit. 2019;25:5258–62.
Bakhouche H, Noskova P, Svetlik S, Bartosova O, Ulrichova J, Kubatova J, et al. Maternal and neonatal effects of remifentanil in women undergoing cesarean section in relation to ABCB1 and OPRM1 polymorphisms. Physiol Res. 2015;64:S529-538.
Hooten WM, Hu D, Cunningham JM. Effects of the ABCB1 c.3435C>T (rs1045642) polymorphism on heat pain perception in opioid-free adults with chronic pain. Anesth Analg. 2021;133:1028–35.
Desta Z, El-Boraie A, Gong L, Somogyi AA, Lauschke VM, Dandara C, et al. PharmVar GeneFocus: CYP2B6. Clin Pharmacol Ther. 2021;110:82–97.
Zanger UM, Klein K. Pharmacogenetics of cytochrome P450 2B6 (CYP2B6): advances on polymorphisms, mechanisms, and clinical relevance. Front Genet. 2013;4:24.
Maust D, Cristancho M, Gray L, Rushing S, Tjoa C, Thase ME. Psychiatric rating scales. Handb Clin Neurol. 2012;106:227–37.
Minkowitz H, Salazar H, Leiman D, Solanki D, Lu L, Reines S, et al. Intravenous tramadol is effective in the management of postoperative pain following abdominoplasty: a three-arm randomized placebo- and active-controlled trial. Drugs R D. 2020;20(3):225–36.
Baharuddin KA, Rahman NHN, Wahab SFA, Halim NA, Ahmad R. Intravenous parecoxib sodium as an analgesic alternative to morphine in acute trauma pain in the emergency department. Int J Emerg Med. 2014;7(1):2.
Craig M, Jeavons R, Probert J, Benger J. Randomised comparison of intravenous paracetamol and intravenous morphine for acute traumatic limb pain in the emergency department. Emerg Med J. 2012;29(1):37–9.
Rosenow DE, Albrechtsen M, Stolke D. A comparison of patient-controlled analgesia with lornoxicam versus morphine in patients undergoing lumbar disk surgery. Anesth Analg. 1998;86(5):1045–50.
Gbandi E, Goulas A, Sevastianos V, Hadziyannis S, Panderi A, Koskinas J, et al. Common ABCB1 polymorphisms in Greek patients with chronic hepatitis C infection: a comparison with hyperlipidemic patients and the general population. Pharmacol Rep. 2016;68:476–82.
Kunugi H, Nanko S, Ueki A, Otsuka E, Hattori M, Hoda F, et al. High and low activity alleles of catechol-O-methyltransferase gene: ethnic difference and possible association with Parkinson’s disease. Neurosci Lett. 1997;221:202–4.
Lang T, Klein K, Fischer J, Nüssler AK, Neuhaus P, Hofmann U, et al. Extensive genetic polymorphism in the human CYP2B6 gene with impact on expression and function in human liver. Pharmacogenetics. 2001;11:399–415.
Zhang W, Chang YZ, Kan QC, Zhang LR, Lu H, Chu QJ, et al. Association of human micro-opioid receptor gene polymorphism A118G with fentanyl analgesia consumption in Chinese gynaecological patients. Anaesthesia. 2010;65:130–5.
Hooten WM, Townsend CO, Sletten CD. The triallelic serotonin transporter gene polymorphism is associated with depressive symptoms in adults with chronic pain. J Pain Res. 2017;9:1071–8.
Slosky LM, Thompson BJ, Sanchez-Covarrubias L, Zhang Y, Laracuente M-L, Vanderah TW, et al. Acetaminophen modulates P-glycoprotein functional expression at the blood-brain barrier by a constitutive androstane receptor-dependent mechanism. Mol Pharmacol. 2013;84:774–86.
Oude Elferink RP, Zadina J. MDR1 P-glycoprotein transports endogenous opioid peptides. Peptides. 2001;22(12):2015–20.
Landau R. Genetic contributions to labor pain and progress. Clin Perinat. 2013;40:575–87.
Landau R, Smiley R. Pharmacogenetics in obstetric anesthesia. Best Pract Res Clin Anaesthesiol. 2017;31:23–34.
Mastrogianni O, Gbandi E, Orphanidis A, Raikos N, Goutziomitrou E, Kolibianakis EM, et al. Association of the CYP2B6 c.516G>T polymorphism with high blood propofol concentrations in women from Northern Greece. Drug Metabol Pharmacokinet. 2014;29:215–8.
Mourão AL, de Abreu FG, Fiegenbaum M. Impact of the cytochrome P450 2B6 (CYP2B6) gene polymorphism c.516G>T (rs3745274) on propofol dose variability. Eur J Drug Metabol Pharmacokinet. 2016;41:511–5.
Choi S-W, Lam DMH, Wong SSC, Shiu HHC, Wang AXM, Cheung C-W. Effects of single nucleotide polymorphisms on surgical and postsurgical opioid requirements: a systematic review and meta-analysis. Clin J Pain. 2017;33(12):1117–30.
de Gregori M, Garbin G, de Gregori S, Minella CE, Bugada D, Lisa A, et al. Genetic variability at COMT but not at OPRM1 and UGT2B7 loci modulates morphine analgesic response in acute postoperative pain. Eur J Clin Pharmacol. 2013;69:1651–1618.
Ho KWD, Wallace MR, Staud R, Fillingim RB. OPRM1, OPRK1, and COMT genetic polymorphisms associated with opioid effects on experimental pain: a randomized, double-blind, placebo-controlled study. Pharmacogenomics J. 2020;20(3):471–81.
Khalil H, Sereika SM, Dai F, Alexander S, Conley Y, Gruen G, et al. OPRM1 and COMT gene-gene interaction is associated with postoperative pain and opioid consumption after orthopedic trauma. Biol Res Nurs. 2017;19:170–9.
Cui W, Yu X, Zhang H. The serotonin transporter gene polymorphism is associated with the susceptibility and the pain severity in idiopathic trigeminal neuralgia patients. J Headache Pain. 2014;15:42.
Schürks M, Frahnow A, Diener H-C, Kurth T, Rosskopf D, Grabe H-J. Bi-allelic and tri-allelic 5-HTTLPR polymorphisms and triptan non-response in cluster headache. J Headache Pain. 2014;15:46.
Loryan I, Lindqvist M, Johansson I, Hiratsuka M, van der Heiden I, van Schaik RHN, et al. Influence of sex on propofol metabolism, a pilot study: implications for propofol anesthesia. Eur J Clin Pharmacol. 2012;68(4):397–406.
Funding
Open access funding provided by HEAL-Link Greece. The study was funded in part by the Research Committee of the Aristotle University of Thessaloniki and partly by individual contributions of some of the authors.
Author information
Authors and Affiliations
Contributions
CP: Conception and design of the study, acquisition of data, analysis and interpretation of the data, drafted the manuscript, has provided final approval of the revised version to be published, has agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. IS: helped in the design, data acquisition and analysis, contributed in the writing, and provided part of the funding, has provided final approval of the final version to be published. CN: performed the genotyping and participated in writing the manuscript, has provided final approval of the final version to be published. GT: performed the statistical analyses and participated in the final revision for important intellectual content, has provided final approval of the final version to be published. DG was the anesthesiologist in charge of TCI programming and intra-operative monitoring of the patients, has provided final approval of the final version to be published. ET was in charge of the postoperative protocol and VAS scoring, has provided final approval of the final version to be published. SA: performed and processed the Hamilton anxiety scaling test and provided part of the funding, has provided final approval of the final version to be published. TP was the surgeon in charge of all operations, has provided final approval of the final version to be published. AG: conceived and supervised the project, contributed in the interpretation of the data and has provided final approval of the final version to be published.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Patient consent statement
Informed consent was obtained from the patients before enrolment into the study.
Permission to reproduce material from other sources
The authors declare that there is no reproduced material from other sources in the present manuscript.
Clinical trial registration
The trial was submitted with the acronym “PERRETT”(PErioperative Remifentanyl REquiremenT Thyroidectomy) for inclusion in the Australian New Zealand Clinical Trials Registry (ANZCTR) and has been registered and allocated the ACTRN: ACTRN12616001598471.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Soultati, I., Ntenti, C., Tsaousi, G. et al. Effect of common OPRM1, COMT, SLC6A4, ABCB1, and CYP2B6 polymorphisms on perioperative analgesic and propofol demands on patients subjected to thyroidectomy surgery. Pharmacol. Rep 75, 386–396 (2023). https://doi.org/10.1007/s43440-023-00455-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-023-00455-7