Abstract
The SARS-CoV-2 outbreak has posed a plethora of problems for the global healthcare system and socioeconomic burden. Despite valiant efforts to contain the COVID-19 outbreak, the situation has deteriorated to the point that there are no viable preventive therapies to treat this disease. The case count has skyrocketed globally due to the newly evolved variants. Despite vaccination drives, the re-occurrence of recent pandemic waves has reinforced the importance of innovation/utilization of immune-booster to achieve appropriate long-term vaccine protection. Plant-derived immuno-adjuvants, which have multifaceted functions, can impede infections by boosting the immune system. Many previous studies have shown that formulation of vaccines using plant-derived adjuvant results in long-lasting immunity may overcome the natural tendency of coronavirus immunity to wane quickly. Plant polysaccharides, glycosides, and glycoprotein extracts have reportedly been utilized as enticing adjuvants in experimental vaccines, such as Advax, Matrix-M, and Mistletoe lectin, which have been shown to be highly immunogenic and safe. When employed in vaccine formulation, Advax and Matrix-M generate long-lasting antibodies, a balanced robust Th1/Th2 cytokine profile, and the stimulation of cytotoxic T cells. Thus, the use of adjuvants derived from plants may increase the effectiveness of vaccines, resulting in the proper immunological response required to combat COVID-19. A few have been widely used in epidemic outbreaks, including SARS and H1N1 influenza, and their use could also improve the efficacy of COVID-19 vaccines. In this review, the immunological adjuvant properties of plant compounds as well as their potential application in anti-COVID-19 therapy are thoroughly discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
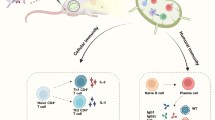
In March 2020, the World Health Organization (WHO) affirmed the COVID-19 outbreak an international public health emergency. However, there is a state of panic in the world as a result of recent COVID-19 outbreaks caused by a new variant of concern. Emergency approval of few vaccines and drugs is permitted by WHO and FDA, USA. The emergence of SARS-CoV-2 variants raised concerns about the efficacy of the approved drugs and vaccines, which are still being investigated. The current standard operating procedures to control the infection include early diagnosis to reduce the chances of secondary infection, followed by isolation of cases, and supportive care using preventive and therapeutic strategies, which include a combination of antiviral drugs, antibiotics, corticosteroids, etc. [1]. In late 2021, India faced massive surge of COVID-19 cases. From the start of the pandemic, many attempts were made to spot the possible drugs against this disease [2,3,4,5]. With the great effort of the scientific community, many FDA/WHO-approved drugs are now available to combat the severity associated with SARS-CoV-2 infection. Many of these are repurposed drugs that have anti COVID-19 activity. Numerous compounds are still being evaluated in many stages of clinical trials. More than 3000 clinical trials on COVID-19 are currently underway as of January 18th, 2022, with new ones being added every day. Undergoing trials may take months or years to develop and hit the market, implying that prompt therapy is urgently required. Efforts have been made globally to find preventive and therapeutic strategies to mitigate the illness of COVID-19 disease. The COVID-19 virus imposes detrimental effects on all groups of people, regardless of their age, gender, race, or physical condition. However, in comparison to immune-competent people, the elderly, immune-compromised, and those with co-morbidities are more likely to acquire infection and endure severe forms of the disease, implying that immunity acts as a weapon against SARS-CoV-2 infection [5]. Despite the fact that the efficacy of vaccinations against SARS-CoV-2 has yet to be proven, numerous predictions have been made around the world about vaccine effectiveness over time. As a result, any clue to improving the efficacy and safety of vaccinations remains an unmet therapeutic need. Traditional medicine, which has a gigantic reservoir of bioactive compounds with broad therapeutic and immune-potentiator properties that have been extensively employed during previous epidemic outbreaks such as SARS and H1N1 influenza, could be a boon in the fight against COVID-19. Natural products are a reservoir of novel remedies; between 1st Jan-1981 and 30th Sep-2019, approximately 441 (23.4%) out of 1881 approved new chemical entities were isolated from natural products of plants and other organisms [6]. Plants have been used as traditional medicines for both communicable and non-communicable diseases for centuries. Since the advent of modern drug discovery techniques, plants with medicinal value have been studied for being novel therapeutic agents [7, 8]. India, China, and South Korea are among the countries that have issued guidelines on traditional regimens for the prevention and management of COVID-19 [9]. Plant products are highly competent due to their pharmacological properties, ease of availability, and safety profile, making them an ideal instant cure for boosting immunity and competing with the new corona virus [10]. Several plants and their phyto-constituents with proven medical value have the capability to work as immune-boosters to alleviate the pandemic situation by preventing viral transmission. Identification of potential adjuvants has been an empirical process for many years. Adjuvants are compounds that enhance the magnitude, breadth, and durability of the vaccine response [11]. Plant-derived adjuvants have two unique characteristics: first, they have no significant side effects and are not toxic, which are the main limitations with using synthetic compounds, and second, they have also been shown to elicit immune response when given orally, making them ideal for developing mucosal vaccines [12]. The protective immunity of COVID-19 involves all the components of the immune system responding collectively to eliminate the virus from the body. Coordination of SARS-CoV-2-specific Th1 and Th2 responses was observed in milder disease, indicating that CD4 + and CD8 + T cells play a collaborative role in COVID-19 protective immunity [14]. Alums are the most commonly used adjuvants in vaccine therapy. However, the response is Th2-biased. Adjuvants capable of inducing both humoral and cell-mediated immune responses could be ideal in the formulation of the COVID-19 vaccine for effectively eliminating the infection. There has been evidence of plant polysaccharides, glycosides, and glycoproteins extracts being used as attractive adjuvants in experimental live vaccines like Advax, Matrix-M. QS and Mistletoe lectin, used in the human viral pandemic, which have been demonstrated to be highly immunogenic, safe and induced balanced humoral and cell-mediated immune response [12,13,14,15,16,17,18,19]. Advax-adjuvant, a plant polysaccharide, and a detla inulin form (β-d- [2-1] poly(fructo-furanosyl) α-d-glucose) found to elicit both humoral and cellular immunity with a wide variety of vaccines against viruses [15, 16, 18]. Matrix-M and QS, plant glycosides generate potent humoral and cellular immunity, when used as adjuvants in vaccine formulations [20,21,22,23]. Immunization experiments with the Matrix-M adjuvant, a Novavx-patent, stimulate an immune response that is unique to an antigen and is characterized by the development of long-lasting antibodies, a balanced Th1/Th2 cytokine profile, and the stimulation of cytotoxic T cells [24, 25], contrast with alum's marked Th2 bias. Mistletoe Lectin, a glycoprotein from mistletoe plant, has also been shown to have strong adjuvant properties in animal models, as evidenced by a strong immune response, when formulated with viral antigens [26, 27]. Moreover, plant-derived adjuvants alone or in combination with synthetic adjuvants have been utilized in the clinical development of several candidate vaccines. Vaccines formulated with the plant-derived adjuvants have an acceptable safety and reactogenicity profile, as well as persuade a high magnitude of long-lasting immunity (Fig. 1). As a result of extensive research on various plant species and their therapeutic properties, plant extracts are currently being revalued globally. Hence, bioprospection and documentation of plant-derived adjuvants having immune-potentiator properties are unmet need for improving current therapies to combat SARS-CoV-2 disease. This review sheds light on the prospective add-on effect of plant-derived immuno-adjuvants in various viral epidemics and their potential application in combating the progression of COVID-19 disease.
COVID-19 vaccines and immune-adjuvants
A range of technologies are being used to produce a possible vaccination for SARS-CoV-2 treatment, including attenuation, DNA, and mRNA-based vaccines. A variety of tactics have been used for the development of a viable vaccine candidate against SARS-CoV-2, including full-length S protein, S1 receptor-binding domain (RBD), DNA or RNA vectors, and production of virus-like proteins (VLP). A number of vaccine candidates have been recommended for clinical trials [1]. Many vaccines have been approved and drive worldwide, all of which are based on the original Wuhan SARS-CoV-2 S protein. Mainly, WHO has authorized ten vaccine candidates for emergency use (Table 1). The three vaccinations, Spikevax, Comirnaty, and Janseen, have also been authorized by the US Food and Drug Administration for COVID-19 disease protection. In the vaccination programme, subunit or inactivated antigens are routinely employed. However, these antigens lack the immunological profiles that mediate the improved adaptive immunity. As a result, adjuvants are required in these vaccinations to stimulate an efficient immune response [28]. Immuno-adjuvants are substances that boost the immune response to antigens that have been inoculated together. Thus, it has the potential to improve the immunogenicity of vaccines [29]. It has been used to improve the efficacy of designed vaccines, earning them the title of “true immune modulators”. It can steer the immune system toward Th1 or Th2 immunity and boost the immune response to an antigen significantly [12, 30]. Adjuvants such as aluminum salts, emulsions, and Toll-like receptor (TLR) agonists have been employed in vaccine formulations for pre-clinical CoV vaccine studies.
So far, many subunit and inactivated COVID-19 vaccines are being clinically trialed, and few have been approved by the World Health Organization for COVID-19 protection. Alum has been demonstrated to be formulated with S protein or RBD to stimulate the production of neutralizing antibodies against CoV, which has been linked with protection against SARS-CoV-2 [31,32,33,34,35]. Alum, on the other hand, is unable to trigger CD4 + and CD8 + T-cell responses, which have been demonstrated to work in concert with antibody responses to provide protection against SARS-CoV-2 [14]. Other adjuvants that have been shown to stimulate both humoral and cellular immune responses could be preferable for formulation. ViroVax LLC of Lawrence, Kansas, developed Alhydroxiquim-II in 2021 with funding from the National Institute of Allergy and Infectious Diseases Adjuvant Development Program in the United States. Covaxin adjuvanted with Alhydroxiquim-II boosts immune response and provides long-lasting immunity against COVID-19 illness [36]. For the successful creation of a safe and effective COVID-19 vaccine, screening of the various combinations of antigens and adjuvants is urgently required. Despite the fact that there are numerous unsolved mysteries in the field, plant-derived adjuvants have high clinical value and a wide range of application possibilities, making them an important tool for the development of new effective medications and improving currently used medications. Identifying ideal adjuvants to elicit appropriate and long-lasting protective immunity via vaccines remains a significant challenge at this time. Plant products have gotten a lot of attention because of their medicinal properties like immunomodulation, anticancer, antiviral, antimicrobial, antidiabetic, anticoagulant, and antitumor exercises [6, 10]. The study of plant-derived immunopotentiators is gaining increasing attention nowadays due to the rapid advancement of science and medicine. Several plant-derived compounds with immuno-adjuvant properties have been used to enhance vaccine efficacy.
Inulin, as an adjuvant
Inulin is isolated from the roots of the Asteraceae (Compositae) family and metabolized into simple sugars in the body by gut microbiota, so there are no safety concerns, and it received safe status by the Food and Drug Administration (FDA), USA, and is assorted with non-toxicity, renewability, and biodegradability compared to other polysaccharides [20]. Onions, garlic, oats, Jerusalem artichokes, and leeks are abundant sources of inulin. The native soluble form of inulin has no immunological activity, but it acquires potent adjuvant activity when crystallized into a stable microcrystalline particulate form (delta inulin) [37]. Several studies demonstrated that the delta form of inulin (semi-crystalline) stimulates strong humoral and cellular immune responses when combined with various antigens, and its immuno-adjuvant properties have been utilized in boosting the immunogenicity of many antigens [38]. Micro-particulate form of inluin acts as a potent humoral and cellular immune adjuvant when formulated with antigens and is non-toxic in nature. It activates alternate complement pathway, and regulates the production of chemokines and cell surface protein expression with formulated antigens [39]. MPI is useful in inducing both Th-1 and Th-2 immune responses, and has no significant local or systemic toxicity [40]. Several semi-crystalline isoforms of inulin, including alpha (AI), beta (BI), gamma (GI), and delta isoforms were found to induce varying degrees of Th1 and Th2 activity. Gamma inulin (GI) has been particularly used as an adjuvant in many vaccines, such as hepatitis, malaria, and influenza vaccines, with little or no side effects. However, the delta isoform of inulin is found to be more thermotolerant than GI and provides stability in body temperature [41, 42]. Advax is the most recent generation of delta inulin-based adjuvants (β-D-(2- > 1)-polyfructofuranosyl-D-glucose) developed by the National Institutes of Health's Adjuvant Development Program [16, 41]. The probable action mechanism of Advax is that it binds to antigen-presenting cells (APCs), such as mononuclear and dendritic cells (DCs), causing antigen presentation to be up-regulated, resulting in B- and T-cell activation [16]. Advax co-administered with vaccines elicits both Th1 (IgG2a) and Th2 (IgG1, IgA) antibody responses, as well as Th1 (IL-2, IFN-) and Th2 (IL-5, IL-6) cytokines, overcoming the limitation of aluminum-based adjuvants. There is also no visible sign of inflammation in the tested subjects, as Advax itself is not capable of stimulating the innate immune response. The safety and tolerability of Advax is superior in comparison to the emulsion-based formulation adjuvants [16, 17]. Adjuvant properties of Advax have been tested in animal models against a variety of pathogens, including malaria, HIV, influenza, tetanus toxoid, hepatitis B virus, diphtheria, respiratory syncytial viruses, E7 protein of HPV, herpes virus 2 glycoprotein D, and others [42,43,44]. Lobigs et al., in 2010, demonstrated that the Advax-adjuvant formulated Vero cell culture vaccine elicited strong Th1/Th2 immune responses and neutralizing antibodies against live Japanese encephalitis virus (JEV) challenged in mouse and horse models in comparison to aluminum adjuvanted vaccine. In addition to this, the Advax-adjuvanted vaccine also targeted Murray Valley encephalitis virus (MVEV), another JEV sero complex virus by elevating Th1/Th2 immune response and neutralizing antibodies. These findings suggest that, when compared to aluminum adjuvants, Advax as an adjuvant improved the efficiency and application range of JEV vaccines [45]. Co-administration of Advax with split virion H5N1 vaccine in ferrets demonstrated significantly improved protection against H5N1 virus by increasing immunogenicity, survival, and ultimately morbidity, in comparison to vaccine alone [46]. The survival of ferrets receiving Advax™ conjugated vaccine was found to be 100%, with survival being only 66% in the case of H5N1 vaccine alone. Immunization of mice with trivalent human influenza vaccine (TIV) with Advax elicited both humoral and cellular responses with a significant antigen-sparing effect in comparison to\influenza antigen alone [16]. Clinical trials studies demonstrated that the formulation of antigens/vaccine with advax adjuvant increase the seroprotection capability of the vaccines (Table 2) [47,48,49,50,51,52]. A study conducted by Gordon et al. in 2012 with 281 adult patients aged 18–70 years depicted that the formulation of the rHA H1N1/2009 vaccine with advax adjuvants resulted in a significant enhancement of seroprotection. Immunization of patients was done at 0 and 3 weeks with 3, 11, or 45 µg recombinant HA. After 3 weeks post 1st dose immunization, clinical outcomes varied according to rHA dosage, presence of adjuvant, subject age, and number of immunizations. The age group 18–49 and the elderly who received rHA 45ug with Advax™ adjuvant were highly responsive, with seroprotection of 80.0%; (95% CI 51.9–95.7), seroconversion 73.3%; (95% CI 44.9–92.2), and GMT increase in fold from baseline of 11.1 (95% CI 4.6–26.4). On the other hand, aged groups over 50 years who received 45ug without adjuvants had the lowest response with a seroprotection rate of 25.0% (95% CI 9.8–46.7); seroconversion 20.8% (7.1–42.2); and a GMT increase in fold from base line of 1.5 (1.1–2.0). Higher antigen doses and adjuvants significantly improved the lowest responders in older subjects after the first immunization. No adverse events were reported in those who received Advax [47]. Gordon et al. in 2016 conducted the first-in-man Phase 1 clinical trial to assess the safety and tolerability of three intramuscular doses of HBsAg formulated with Advax. Patients who received HBsAg formulated with 5 mg and 10 mg Advax showed higher responders after three immunisations. The seroprotection and HBsAb geometric mean titers were found to be 83.3% and 40.7 (95% CI 11.9–139.1) for 5 mg and 80% and 51.6 (95% CI 10.0–266.2) for 10 mg advax formulated groups, respectively, whereas seroprotection with antigen alone was 20% and the GMT titer was 4.1 (95% CI 1.3–12.8). In spite of this, positive CD4 T-cell responses to HBsAg were also higher in the Advax formulated antigen groups, 67% for 5 mg (67%) and 80% for 10 mg Advax, versus HBsAg alone (20%). Overall, the results showed that Advax was safe and well tolerated in adult subjects [49]. Advax enhanced humoral and T-cell responses to recombinant and inactivated whole-virus SARS-CoV antigens, boosting vaccine protection and lowering lung immunopathology risk [16]. On day 3 post-challenge, the presence of adjuvant significantly increased serum neutralizing antibody titers and resulted in decrease lung virus titers. Whereas, on day 6 post-challenge, alum-formulated vaccines significantly increased lung eosinophilic immunopathology, which was absent in the advax formulated vaccine. Vaccine formulation with Advax-1 or -2 significantly enhanced the spike protein-specific immunoglobulin response in comparison to the antigen alone (recombinant spike protein antigen, rSP). At 2 weeks post-boost, Advax-1 significantly stimulated the IgG1 response, whereas Advax-2 significantly increased a wide range of antibody isotypes, including IgG1, IgG2a, IgG2b, and IgG3, which was maintained up to 1 year post-immunization. Advax-adjuvanted groups significantly increase the spike protein-specific IgM responses compared to un-adjuvanted rSP alone up to 1 year post-immunization, indicating that the Advax-immunized groups generate long-lived memory IgM-positive B cells [15]. Li et al. used SARS-CoV-2 spike protein adjuvanted with Advax-SM (AdvaxTM + CpG55.2) to immunize mice and ferrets. The study demonstrated that the immunization induced a strong antibody response, capable of eliminating the original SARS-CoV-2 virus and cross-neutralizing the VOC Alpha (B.1.1.7), resulting in protecting mice and ferrets from SARS-CoV-2 infection [50]. The antibody response to antigen alone was Th-2 biased, while adding adjuvants resulted in a much higher Th-1 biased response. Similarly, adding aluminum hydroxide adjuvant (Alhydrogel) resulted in a more pronounced Th-2 response. Mice immunized with rSp alone or in a formulation with Alhydrogel showed minimal CTL activity against spike-labeled cells, which is consistent with their Th2 immune bias. However, mice immunized with rSp adjuvanted with Advax-SM demonstrated high levels of in vivo cytotoxic T lymphocyte (CTL) killing of spike-labeled target cells. The highest levels of neutralizing antibodies were detected in BL6 mice following immunization with rSp 5 mg + Advax-SM (GMT 3,712), followed by rSp 1 mg + Advax-SM (GMT 1088), and finally rSp alone (GMT 736). The same patterns were observed in BALB/c mice, with rSp 5 mg + Advax-SM producing the largest response (GMT 4,352), followed by rSp 1 mg + Advax-SM (GMT 960), and then rSp alone (GMT 960). (GMT 512) [50]. Li et al. [50] challenged Covax-19-vaccinated hamsters with SARS-CoV-2, and both single- and double-dose adjuvanted vaccines (spike ECD + Advax-CpG55.2) offered strong protection of hamsters against lung infection and pathology, with a strong correlation between serum neutralizing antibody levels, prior to challenge, and lung protection. Interestingly, adjuvanted antigen generates a robust antibody response even after a single dose, but a 2-dose strategy worked better overall and required just half as much antigen. There was a reduced viral load in oropharyngeal swab and nasal turbinate tissue and no live infectious virus in lung tissue in immunized mice post 3 days [51]. The Covax-19 (Vaxine/CinnaGen Co.: SpikoGen) adjuvanted with advax has been referred for clinical trials in Iran and Australia after gaining long-lasting protection with superior safety and tolerability findings from pre-clinical tests on mice, ferrets, and monkeys (Phase I, II) (NCT05005559, NCT05148871, NCT05175625) [51, 53]. Recently, Iran has officially given emergency authorisation for the use of the SpikoGen vaccine in combination with Advax to prevent SARS-CoV-2 infection.
Lectin, as an immune-potentiator/adjuvant
Natural products like plant lectins that specifically target virus-associated glycans work as immune modulators by triggering the production of cytokines and other mediators such as reactive oxygen and nitrogen species and, thereby, improving the immune response against microbes [54,55,56]. The carbohydrate portion of the glycoproteins which play a critical role in the immunity of humans and viral pathology is glycans [57, 58]. SARS-CoV-2 spike proteins are heavily glycosylated by heterogeneous N-linked glycans projecting from the S trimer surface, which act as a glycan shield and play an important role in host immune evasion (modulate accessibility to host proteases and neutralizing antibodies). By dismantling the glycan shields, lectins can make these sugar-coated viruses vulnerable to immune attack [59, 60]. The antiviral activity of the plant-derived lectins has been found to vary depending upon the specificity of the sugar on which the lectins act. In a study, it was demonstrated that out of the 33 plant lectins checked for anti SARS-CoV activity, the strongest were the mannose-binding lectins which interfered in the viral entry and release by binding to the glycans on the spike protein [61]. The blocking of viral entry by lectins can be affected by binding to the glycans of the virus or the host cell [62]. Various enveloped viruses have glycoproteins on their surface, which act as mediators for binding to the receptors of the host cell membrane. The coronavirus has a highly glycosylated envelope which can be used as a promising candidate for therapeutic intervention. The sugar content of the glycoproteins is crucial for the viral infection, replication, and interaction of the virus with the host [63,64,65]. Certain plant lectins are translocated across the gut while interacting with the mucosal epithelium, making them ideal candidates for vaccine formulations by working as adjuvants, inducing the mucosal and systemic immune system [66, 67]. Extensive animal studies have exhibited the safety of the usage of lectins and their derivative biodegradable polymers as immune modulators, such as ScLL, ML, and ArtinM, which are potent adjuvants in immunization protocols. Afonso-Cardoso et al. in 2012 evaluated the adjuvant effect of Synadenium carinatum latex lectin (ScLL) in a murine model of vaccination against American cutaneous leishmaniasis. Immunization of BALB/c mice was done with lectin ScLL (10, 50, 100 µg/animal) individually or in combination with soluble Leishmania amazonensis antigen (SLA). ScLL conferred a significant increase in Th-1 response, higher levels of IgG2a, and a reduction in parasitic load (61%) post 10 weeks of immunization at higher concentrations (50 and 100 µg/animal). The higher ScLL concentration stimulated the delayed-type hypersensitivity (DTH) reaction significantly (P < 0.05), which showed the protective role of this lectin against cutaneous leishmaniasis, when formulated with SLA [68]. Lavelle et al.’s study showed that mistletoe lectin I (ML-I), a strong mucosal adjuvant, stimulated anti-bystander antigen (OVA) antibody titers in sera and secretions merely two-to-fivefold lower as compared to cholera toxin adjuvant [66]. A similar study conducted by Lavelle et al., in 2002, immunized mice with herpes simplex virus glycoprotein D2 antigen adjuvanted with mistletoe lectins, showed its potent adjuvanticity nature. Total three mistletoe lectins from Viscum album (ML-I, ML-II, and ML-III) adjuvants with herpes simplex virus glycoprotein D2 (gD2) and dosed via nasotracheally route, showed significantly higher levels of gD2-specific mucosal immunoglobulin A (IgA) and systemic immunoglobulin G (IgG) antibody than when the antigen was delivered alone [26]. The Korean Mistletoe Lectin C (KML-C) isolated from Viscum album coloratum completely protected mice challenged with H1N1 influenza when the KML-C was intranasally co-administrated with the inactivated virus. There were significantly higher levels of anti-influenza antibodies (IgG and IgA) as well as a noticeably higher level of the population of influenza-specific lymphocytes in spleens and mediastinal lymph nodes in immunized mice. Furthermore, H3N2 challenged mice immunized with KML-C and inactivated H1N1 also showed partial protection [27]. The adjuvant properties of ArtinM and JAC were evaluated in mice immunized against neosporosis (a neuromuscular disease in dogs and reproductive disorders in cattle). Subcutaneous immunization of six C57BL/6 mouse groups was performed thrice at 2-week intervals with Neospora lysate antigen (NLA) alone and co-administered with lectins. Presence of ArtinM lectin resulted in increased NLA immunogenicity, higher levels of specific total IgG and IgG2a/IgG1 ratio, Th-1 cytokine production, enhanced survival, and lowest brain parasite burden, with the highest inflammation scores [69]. ArtinM and Jacalin (JAC) are lectins derived from jackfruit (Artocarpus integrifolia) that have been used as adjuvants in vaccines against protozoan parasites [70,71,72,73]. The interaction of ArtinM with the N-glycans of TLR2 stimulates the macrophages and dendritic cells to produce IL-12, hence inducing a Th1-immune response [74]. Jacalin, the major protein derived from the seeds of Artocarpus integrifolia, works as a potent adjuvant in combination with epimastigotes from Trypanosoma cruzi [71]. Recently, a D-galactose-binding lectin named Synadenium carinatum latex lectin (ScLL) was isolated and characterized, and it exhibited immune stimulatory, immune protective, and adjuvant effects in cerebral neosporosis mouse model when administered along with NLA, further resulting in higher survival rate, increased IgG production, and decreased parasitic burden [75]. Garlic lectin has been shown to have strong immunogenic properties; ASA I, a garlic lectin, has the ability to stimulate splenocytes and thymocytes, resulting in an immune response [76, 77]. The adjuvanticity of mannose-binding lectins from garlic (Allium sativum agglutinins; ASAs) were also evaluated by Padiyappa et al., in 2022. When antigen ovalbumin and garlic lectins (ASA 1 and ASA II, 30 µg each) were administered together to BALB/c mice, the anti-OVA IgG response was noticeably boosted. The lectin ASA 1 immunized groups showed significantly higher level of anti-OVA IgG response on days 35 and 50, in comparison to ASA 2 adjuvanted and OVA antigen alone groups. It was twofold higher antibody response in OVA + ASA I group, showing the potentiality of lectin ASA1 to be used as a formulating agent with weak antigen [77]. Despite research demonstrating that lectins are an excellent choice for adjuvants and can aid in the development of highly effective COVID-19 vaccines and medications, the use of lectins as adjuvants for viral vaccines (particularly those against SARS-CoV-2) remains an option to evaluate their adjuvanticity with COVID-19 vaccines.
Saponins, as an adjuvant
Saponins are antimicrobial and antiviral compounds derived from medicinal plants. It is a naturally occurring sugar-conjugated compound with one or more hydrophilic glycoside moieties together with a lipophilic triterpene derivative in its structure. Saponins have gotten a lot of attention because of their wide range of biological activities, including their ability to stimulate an immune response, making them ideal adjuvant candidate [42, 78]. Saponin-based adjuvants are capable of modulating the cell-mediated and humoral immune systems, and they have the advantage of only requiring a low dose to be effective. Saponins also boost the immune response to mucosal antigens by stimulating cytotoxic CD8 + lymphocyte responses [79, 80]. The saponins isolated from the stem and leaves of Panax ginseng C. A. Meyer (GSLS) led to enhanced vaccination response against bird’s disease. In both normal and immunosuppressive birds, administration of GSLS resulted in better vaccine-induced immune protection against avian influenza, infectious bursal disease, and Newcastle disease [81,82,83]. The use of ginseng saponins as an adjuvant to enhance the immunogenicity of foot and mouth disease (FMD) vaccines is well documented. Use of ginseng saponins resulted in enhanced IgG response against FMD vaccines [84,85,86]. Li et al. in 2016 challenged the mice with foot and mouth disease and evaluated the efficacy of FMD along with orally administered ginseng stem–leaf saponins (GSLS). The mice were immunized twice with the FMD vaccine. Oral administration of GSLS resulted in a significant increase in serum antibody response to FMD vaccine [87]. Wu et al., in 1992, demonstrated the adjuvant activity of QS-21, a saponin isolated from the soap bark tree Quillaja saponaria with recombinant envelope protein antigens (HIV-160D) in murine models. HIV-1 vaccine formulated with QS-21 has shown significantly higher titer of antibodies response and induce group-specific proliferative responses in comparison to alum absorbed HIV vaccines. Additionally, the QS-21 adjuvant responds to weakly immunogenic epitopes of antigen that were not recognized using alum-adsorbed HIV-1 160D [88]. Sasaki et al., 1998 depicted that the formulation of HIV-1 DNA vaccine with QS-21 resulted in enhanced specific IgG2a production, stimulation of delayed-type hypersensitivity reaction (DTH), and cytolytic activity of splenocytes [89]. de Costa et al. in 2014 evaluated the adjuvant properties of aqueous extract (AE), saponin fraction QB-90 from Quillaja brasiliensis and Quill A, a saponin from Quillaja saponaria using poliovirus antigen as a model. Vaccines were formulated with Quil-A (50 µg), AE (400 µg), or QB-90 (50 µg). On day 56, AE, QB-90, and Quil-A significantly increased serum levels of specific IgG, IgG1, and IgG2a compared to the control group. The magnitude of immune response of antigen for QB-90 and Quil-A was statistically equivalent. AE and QB-90 stimulated the generation of Th1 cells against the administered vaccine, and the response was similar to that of Quil-A. However, vaccines adjuvanted with QB-90 improved mucosal immune responses, as evidenced by high specific IgA titers in bile, feces, and vaginal washings in comparison to Quil-A [90]. Studies showed that QB-90 showed lower toxicity when subcutaneously administered to mice than Quil-A and resulted in enhanced immune response to bovine herpes virus type 1 antigen (BoHV-1), suggesting QB-90 to be a safe and effective adjuvant [91]. Bisht et al. in 2005 challenged mice intranasally with SARS-CoV immunized with spike protein antigen co-administered with QS21 and a Ribi (MPL + TDM) adjuvant. The QS-21 adjuvant system induced high titers of antigen-specific serum antibodies. The mean neutralizing antibody titer obtained with QS21 adjuvants was 4.6-fold higher than with MPL + TDM adjuvants. With QS21 adjuvant, virus titers in nasal turbinates were reduced by more than 104-fold, and virus titers were uniformly below detection in the turbinates of mice. However, virus titers were found in the nasal turbinates of four of seven test mice immunized with nS (spike protein antigen) and the MPL + TDM adjuvant [15]. Recently, a saponin-based microemulsion adjuvant has also been investigated for use in the COVID-19 vaccine. Co-administration of saponin microemulsion adjuvant with SARS-CoV-2 S1-Fc vaccine resulted in very high titers specific neutralizing antibodies (titers > 1024 on Day 15) in macaque against live SARS-CoV-2 infection [92]. Based on safety profile and immuno-adjuvant properties to enhance both cell-mediated and humoral responses, QS-21 is an ideal adjuvant candidate for use in humans (Table.3) [93,94,95,96,97,98,99,100,101]. A clinical trial involving QS-21 adjuvant has been registered to evaluate the safety, reactogenicity, and immune response of the SpFN COVID-19 vaccine (NCT04784767, https://clinicaltrials.gov/ct2/show/NCT04784767). The efficacy of NVXCoV2373 vaccine adjuvanted with Matrix-M, a saponin-based vaccine, against COVID-19 has been extensively studied. Titan et al., 2021 immunized mice with the NVXCoV2373 vaccine adjuvanted with Matrix-M, resulting in a strong anti-S response and virus neutralization. There was a higher titer of anti-S IgG, polyfunctional CD4 + and CD8 + T cells, follicular CD4 + Th cells, and antigen-specific germinal center B cells in the spleen of mice. In baboons, a low-dose NVX-CoV2373 vaccine combined with a saponin-based Matrix-M adjuvant results in high titer anti-S IgG, blocking virus attachment to the ACE2 receptor and thus neutralizing the SARS-CoV-2 virus without any signs of vaccine-associated enhanced respiratory disease [102]. On the basis of pre-clinical studies in mice and baboons, which demonstrated high immunogenicity with superior safety and tolerability, Keech et al. [103] evaluated the safety and immunogenicity of NVX-CoV2373, a nanoparticle vaccine for protection against SARS-CoV-2 infection. The vaccine with adjuvant was administered to 83 patients, and 25 patients received the vaccine alone. Patients who received an adjuvant-supplemented vaccine experienced a dose-sparing immune response that induced a Th1 response. The geometric mean of anti-S IgG antibody and neutralization response was found to be 63,160 ELISA units and 3906, respectively, with a double dose of 5 µg NVX-CoV2373 adjuvanted vaccine, which was comparatively higher than the convalescent serum from symptomatic COVID-19 patients, which was 8344 and 983, respectively [103] The phase 1 component of the phase 1 to 2 trial showed that 2-doses of 5 μg and 25 μg rSARS-CoV-2 adjuvanted with 50 μg Matrix-M1 adjuvant were well tolerated and immunogenic in participants 18 to 59 years old [103]. Formica et al. [100] conducted a randomized, placebo-controlled, phase 2 trial to evaluate the efficacy of NVX-CoV2373 vaccine adjuvanted with Matrix-M in two age groups (aged 18 to 59 and 60 to 84 years). A total of 1288 people were randomly assigned to receive either 1 or 2 intramuscular doses of 5 µg or 25 µg NVX-CoV2373 or placebo. The findings showed that the 2-dose regimen of 5 μg NVX-CoV2373 is highly immunogenic and well tolerated in all age groups. Younger people had greater geometric mean titers for anti-spike IgG antibodies than older adults. Seroconversion rates for younger people were also higher (79 and 93% for 5 and 25 µg initial doses, respectively, compared to 43 and 75% for older participants, by dose). In addition, in older adults, the 2-doses of 5 µg vaccine were demonstrated to have adequate immunogenicity, permitting its inclusion in late-phase efficacy studies [100]. In a phase three trial of a matrix-M1 adjuvanted subunit vaccine, the overall efficacy of 96.4% was noted against common SARS-CoV-2 strains, 86.3% efficacy against B.1.1.7 (alpha), and 51% against B.1.351 (beta) variants [101, 104]. Recently, Nuvaxovid and Covavax COVID-19 vaccine formulated with Matrix-M (plant-based adjuvants) have received emergency approval from the World Health Organization for the treatment of COVID-19 disease based on their clinical trial results.
Conclusion
SARS-CoV-2 infections are undeniably a major public health concern that has resulted in significant economic losses around the world. Prophylactic therapies and approved vaccines appear to be the most effective ways to control disease progression. Vaccines are a safer and more sustainable option. However, despite a worldwide vaccination drive, hospitalization cases are still being reported. The emergence of new variants raises concerns about the efficacy of the vaccines approved for COVID-19. Subunit or inactivated antigens are routinely used in vaccination programs. These antigens, however, lack the immunological profiles that mediate the enhanced adaptive immunity. Adjuvants are thus required in these vaccines to stimulate an effective immune response. Adjuvants have unique physicochemical features that can have a big impact on the strength, duration, and types of immune responses, and in the present scenario, proven safe and efficacious adjuvants are urgently required for the SARS-CoV-2 vaccine formulation to obtain rapid authorization from the regulatory agencies. Plant compounds are now receiving attention for their use in vaccine formulation, because they generate robust and balanced Th1 and Th2 responses, which effectively eliminate viral infection. When employed in vaccine formulation, plant-derived adjuvants have shown potent immunogenicity, and are well tolerated across all age groups. They also elicit a strong antiviral immune response from recipients even with low doses of antigens, demonstrating a significant antigen-sparing impact. Delta inulin and saponin were widely used in vaccine formulation during viral epidemics. Advax, a delta inulin formulated COVID-19 vaccine, is under phase-II trials and has demonstrated a robust immune response and virus neutralization effect. QS-21 and Matrix-M (a patented saponin adjuvant from Novavax) are also used in the formulation of COVID-19 vaccines, and a few studies are being under trial to test their use in the formulation of COVID-19 vaccines to combat SARS-CoV-2 infection. Plant lectins have shown strong adjuvanticity in experimental models. However, their usage is limited due to their expensive manufacturing and purification costs. Overall, for the successful development of a safe and effective COVID-19 vaccine, additional extensive mechanistic investigations, including the nature of protective immune responses and screening of alternative combinations of antigens and adjuvants, are urgently required.
Data availability
Not applicable.
Abbreviations
- AE:
-
Aqueous extract
- COVID-19:
-
Coronavirus infectious diseases-19
- ECD:
-
Extra cellular domain
- DCs:
-
Dendritic cells
- FDA:
-
Food and Drug administration
- FMD:
-
Foot and Mouth Disease
- GC:
-
Germinal center
- GI:
-
Gamma inulin
- GMC:
-
Geometric mean concentration
- GMT:
-
Geometric mean titer antibody
- GMFR:
-
Geometric mean fold rise
- GSLS:
-
Ginseng stem-and-leaf saponins
- ACE2:
-
Angiotensin converting enzyme-2
- HBsAg:
-
Hepatitis B antigens
- HIV:
-
Human Immunodeficiency Virus
- HPV:
-
Human papillomavirus
- JEV:
-
Japanese encephalitis virus
- MPI:
-
Micro-particulate Inulin
- MPL:
-
Monophosphoryl lipid A
- NLA:
-
Neospora lysate antigen
- OVA:
-
Anti-bystander antigen
- QB:
-
Quillaja brasiliensis
- QS:
-
Quillaja saponaria
- rS:
-
Recombinant spike
- SARS:
-
Severe acute respiratory syndrome
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- ScLL:
-
Synadenium carinatum latex lectin
- SpFN:
-
SARS-COV-2-Spike-Ferritin-Nanoparticle
- TDM:
-
Trehalose-6,6′-dimycolate
- TIV:
-
Trivalent Inactivated influenza vaccine
- WHO:
-
World Health Organisation
References
Kumar A, Sharma A, Tirpude NV, Thakur S, Kumar S. Combating the progression of novel coronavirus SARS-CoV-2 infectious Disease: current state and future prospects in molecular diagnostic and drug discovery. Curr Mol Med. 2021. https://doi.org/10.2174/1566524021666210803154250.
Giovane RA, Rezai S, Cleland E, Henderson CE. Current pharmacological modalities for management of novel coronavirus disease 2019 (COVID-19) and the rationale for their utilization: a review. Rev Med Virol. 2020;30(5): e2136. https://doi.org/10.1002/rmv.2136.
Valle C, Martin B, Touret F, Shannon A, Canard B, Guillemot J, Coutard B, Decroly E. Drugs against SARS-CoV-2: what do we know about their mode of action? Rev Med Virol. 2020;30(6):1–10. https://doi.org/10.1002/rmv.2143.
Suganya S, Divya S, Parani M. Severe acute respiratory syndrome-coronavirus-2: current advances in therapeutic targets and drug development. Rev Med Virol. 2021;31(3): e2174. https://doi.org/10.1002/rmv.2174.
Kumar A, Sharma A, Tirpude NV, Sharma S, Padwad YS, Kumar S. Pharmaco-immunomodulatory interventions for averting cytokine storm-linked disease severity in SARS-CoV-2 infection. Inflammopharmacology. 2022;30(1):23–49. https://doi.org/10.1007/s10787-021-00903-x.
Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020;83:770–803. https://doi.org/10.1021/acs.jnatprod.9b01285.
Beutler JA. Natural products as tools for discovering new cancer targets. In: Koehn F, editor. Natural products and cancer drug discovery. Cancer drug discovery and development. New York: Springer; 2013. https://doi.org/10.1007/978-1-4614-4654-5_9.
Owen L, Laird K, Shivkumar M. Antiviral plant-derived natural products to combat RNA viruses: targets throughout the viral life cycle. Lett Appl Microbiol. 2021. https://doi.org/10.1111/lam.13637.
Ang L, Lee HW, Choi JY, Zhang J, Lee MS. Herbal medicine and pattern identification for treating COVID-19: a rapid review of guidelines. Integr Med Res. 2020;9: 100407. https://doi.org/10.1016/j.imr.2020.100407.
Khanna K, Kohli SK, Kaur R, Bhardwaj A, Bhardwaj V, Ohri P, et al. Herbal immune-boosters: substantial warriors of pandemic Covid-19 battle. Phytomedicine. 2021;85: 153361. https://doi.org/10.1016/j.phymed.2020.153361.
Pulendran BS, Arunachalam P, O’Hagan DT. Emerging concepts in the science of vaccine adjuvants. Nat Rev Drug Discov. 2021;20:454–75. https://doi.org/10.1038/s41573-021-00163-y.
Sander VA, Corigliano MG, Clemente M. Promising plant-derived adjuvants in the development of coccidial vaccines. Front Vet Sci. 2019;6:20. https://doi.org/10.3389/fvets.2019.00020.
Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–80. https://doi.org/10.1016/j.cell.2021.01.007.
Rydyznski Moderbacher C, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183(4):996-1012.e19. https://doi.org/10.1016/j.cell.2020.09.038.
Bisht H, Roberts A, Vogel L, Subbarao K, Moss B. Neutralizing antibody and protective immunity to SARS coronavirus infection of mice induced by a soluble recombinant polypeptide containing an N-terminal segment of the spike glycoprotein. Virology. 2005;334:160–5. https://doi.org/10.1016/j.virol.2005.01.042.
Honda-Okubo Y, Saade F, Petrovsky N. Advax™, a polysaccharide adjuvant derived from delta inulin, provides improved influenza vaccine protection through broad-based enhancement of adaptive immune responses. Vaccine. 2012;30:5373–81. https://doi.org/10.1016/j.vaccine.2012.06.021.
Honda-Okubo Y, Barnard D, Ong CH, Peng BH, et al. Severe acute respiratory syndrome-associated coronavirus vaccines formulated with delta inulin adjuvants provide enhanced protection while ameliorating lung eosinophilic immunopathology. J Virol. 2015;89:2995–3007. https://doi.org/10.1128/jvi.02980-14.
Petroski N. Advax adjuvant: a potent and safe immunopotentiator composed of delta inulin. In: Immunopotentiators in modern vaccines. 2nd ed. New York: Academic Press; 2017. p. 199–210. https://doi.org/10.1016/B978-0-12-804019-5.00010-4.
Lacaille-Dubois MA. Updated insights into the mechanism of action and clinical profile of the immunoadjuvant QS-21: a review. Phytomedicine. 2019;60: 152905. https://doi.org/10.1016/j.phymed.2019.152905.
Shinde V, Cai R, Plested J, Cho I, Fiske J, Pham X, et al. Induction of cross-reactive hemagglutination inhibiting antibody and polyfunctional CD4+ T-cell responses by a recombinant matrix-M-adjuvanted hemagglutinin nanoparticle influenza vaccine. Clin Infect Dis. 2021;73(11):e4278–87. https://doi.org/10.1093/cid/ciaa1673.
Bengtsson KL, Song H, Stertman L, Liu Y, Flyer DC, Massare MJ, et al. Matrix-M adjuvant enhances antibody, cellular and protective immune responses of a Zaire Ebola/Makona virus glycoprotein (GP) nanoparticle vaccine in mice. Vaccine. 2016;34:1927–35.
Fries L, Cho I, Krähling V, Fehling SK, Strecker T, Becker S, et al. Randomized, blinded, dose-ranging trial of an Ebola virus glycoprotein nanoparticle vaccine with Matrix-M adjuvant in healthy adults. J Infect Dis. 2020;222:572–82.
Shinde V, Cho I, Plested JS, Agrawal S, Fiske J, Cai R, et al. Comparison of the safety and immunogenicity of a novel Matrix-M-adjuvanted nanoparticle influenza vaccine with a quadrivalent seasonal influenza vaccine in older adults: a phase 3 randomised controlled trial. Lancet Infect Dis. 2022;22(1):73–84. https://doi.org/10.1016/S1473-3099(21)00192-4.
Pedersen GK, Sjursen H, Nøstbakken JK, Jul-Larsen Å, Hoschler K, Cox RJ. Matrix M(TM) adjuvanted virosomal H5N1 vaccine induces balanced Th1/Th2 CD4(+) T cell responses in man. Hum Vaccines Immunother. 2014;10:2408–16.
Madhun AS, Haaheim LR, Nilsen MV, Cox RJ. Intramuscular Matrix-M-adjuvanted virosomal H5N1 vaccine induces high frequencies of multifunctional Th1 CD4+ cells and strong antibody responses in mice. Vaccine. 2009;27:7367–76.
Lavelle EC, Grant G, Pusztai A, Pfüller U, Leavy O, McNeela E, et al. Mistletoe lectins enhance immune responses to intranasally co-administered herpes simplex virus glycoprotein D2. Immunology. 2002;107(2):268–74. https://doi.org/10.1046/j.1365-2567.2002.01492.x.
Song SK, Moldoveanu Z, Nguyen HH, Kim EH, Choi KY, Kim JB, Mestecky J. Intranasal immunization with influenza virus and Korean mistletoe lectin C (KML-C) induces heterosubtypic immunity in mice. Vaccine. 2007;25:6359–66.
Liang Z, Zhu H, Wang X, Jing B, Li Z, Xia X, et al. Adjuvants for coronavirus vaccines. Front Immunol. 2020;11: 589833. https://doi.org/10.3389/fimmu.2020.589833.
Vogel FR. Adjuvants in perspective. Dev Biol Stand. 1998;92:241–8.
Afinjuomo F, Barclay TG, Song Y, Parikh A, Petrovsky N, Garg S. Synthesis and characterization of a novel inulin hydrogel crosslinked with pyromellitic dianhydride. React Funct Polym. 2019;134:104–11. https://doi.org/10.1016/j.reactfunctpolym.2018.10.014.
Coleman CM, Liu YV, Mu H, Taylor JK, Massare M, Flyer DC, et al. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine. 2014;32:3169–74. https://doi.org/10.1016/j.vaccine.2014.04.016.
Lan J, Deng Y, Chen H, Lu G, Wang W, Guo X, et al. Tailoring subunit vaccine immunity with adjuvant combinations and delivery routes using the Middle East respiratory coronavirus (MERS-CoV) receptor-binding domain as an antigen. PLoS ONE. 2014;9: e112602. https://doi.org/10.1371/journal.pone.0112602.
Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M, et al. Rapid development of an inactivated vaccine for SARS-CoV-2. Science. 2020;369:77–81. https://doi.org/10.1101/2020.04.17.046375.
Wang H, Zhang Y, Huang B, Deng W, Quan Y, Wang W, et al. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell. 2020;182:713-21.e9. https://doi.org/10.1016/j.cell.2020.06.008.
Yang J, Wang W, Chen Z, Lu S, Yang F, Bi Z, et al. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature. 2020;586:572–7. https://doi.org/10.1038/s41586-020-2599-8.
Adjuvant alhydroxiquim-II to boost immune response of Covaxin. The Hindu 2020. https://www.thehindu.com/business/Industry/adjuvant-alhydroxiquim-ii-to-boost-immune-response-of-covaxin/article32771112.ece
Petrovsky N, Cooper PD. Advax™, a novel microcrystalline polysaccharide particle engineered from delta inulin, provides robust adjuvant potency together with tolerability and safety. Vaccine. 2015;33:5920–6. https://doi.org/10.1016/j.vaccine.2015.09.030.
Wang L, Barclay T, Song Y, Joyce P, Sakala IG, Petrovsky N, Garg S. Investigation of the biodistribution, breakdown and excretion of delta inulin adjuvant. Vaccine. 2017;35:4382–8. https://doi.org/10.1016/j.vaccine.2017.06.045.
Cooper PD. Vaccine adjuvants based on gamma inulin. Pharm Biotechnol. 1995;6:559–80. https://doi.org/10.1007/978-1-4615-1823-5_24.
Silva DG, Cooper PD, Petrovsky N. Inulin-derived adjuvants efficiently promote both Th1 and Th2 immune responses. Immunol Cell Biol. 2004;82:611–6. https://doi.org/10.1111/j.1440-1711.2004.01290.x.
Cooper PD, Petrovsky N. Delta inulin: a novel, immunologically active, stable packing structure comprising -D-[2 -> 1] poly(fructo-furanosyl) -D-glucose polymers. Glycobiology. 2010;21:595–606. https://doi.org/10.1093/glycob/cwq201.
Woods N, Niwasabutra K, Acevedo R, Igoli J, Altwaijry NA, Tusiimire J, et al. Natural vaccine adjuvants and immunopotentiators derived from plants, fungi, marine organisms, and insects. In: Immunopotentiators modern vaccines. 2nd ed. Amsterdam: Elsevier; 2017. p. 211–29. https://doi.org/10.1016/b978-0-12-804019-5.00011-6.
Cooper P, Mccomb C, Stelle E. The adjuvanticity of Algammulin, a new vaccine adjuvant. Vaccine. 1991;9:408–15. https://doi.org/10.1016/0264-410x(91)90127-r.
Cooper PD, Steele EJ. Algammulin, a new vaccine adjuvant comprising gamma inulin particles containing alum: preparation and in vitro properties. Vaccine. 1991;9:351–7. https://doi.org/10.1016/0264-410x(91)90063-c.
Lobigs M, Pavy M, Hall RA, Lobigs P, Cooper P, et al. An inactivated Vero cell-grown Japanese encephalitis vaccine formulated with Advax, a novel inulin-based adjuvant, induces protective neutralizing antibody against homologous and heterologous flaviviruses. J Gen Virol. 2010;91:1407–17. https://doi.org/10.1099/vir.0.019190-0.
Layton RC, Petrovsky N, Gigliotti AP, Pollock Z, Knight J, Donart N, et al. Delta inulin polysaccharide adjuvant enhances the ability of split-virion H5N1 vaccine to protect against lethal challenge in ferrets. Vaccine. 2011;29:6242–51. https://doi.org/10.1016/j.vaccine.2011.06.078.
Gordon DL, Sajkov D, Woodman RJ, Honda-Okubo Y, et al. Randomized clinical trial of immunogenicity and safety of a recombinant H1N1/2009 pandemic influenza vaccine containing Advax™ polysaccharide adjuvant. Vaccine. 2012;30:5407–16. https://doi.org/10.1016/j.vaccine.2012.06.009.
Gordon D, Kelley P, Heinzel S, Cooper P, Petrovsky N. Immunogenicity and safety of Advax™, a novel polysaccharide adjuvant based on delta inulin, when formulated with hepatitis B surface antigen: A randomized controlled Phase 1 study. Vaccine. 2014;32:6469–77. https://doi.org/10.1016/j.vaccine.2014.09.034.
Gordon DL, Sajkov D, Honda-Okubo Y, Wilks SH, Aban M, et al. Human Phase 1 trial of low-dose inactivated seasonal influenza vaccine formulated with Advax™ delta inulin adjuvant. Vaccine. 2016;34:3780–6. https://doi.org/10.1016/j.vaccine.2016.05.071.
Li L, Honda-Okubo Y, Huang Y, Jang H, Carlock MA, et al. Immunisation of ferrets and mice with recombinant SARS-CoV-2 spike protein formulated with Advax-SM adjuvant protects against COVID-19 infection. Vaccine. 2021;39:5940–53.
Li L, Honda-Okubo Y, Baldwin J, Bowen R, Bielefeldt-Ohmann H, Petrovsky N. Covax-19/Spikogen® vaccine based on recombinant spike protein extracellular domain with Advax-CpG55.2 adjuvant provides single dose protection against SARS-CoV-2 infection in hamsters. Vaccine. 2022;40(23):3182–92. https://doi.org/10.1016/j.vaccine.2022.04.041.
Tabarsi P, Anjidani N, Shahpari R, Mardani M, Sabzvari A, Yazdani B, et al. Safety and immunogenicity of SpikoGen®, an Advax-CpG55.2-adjuvanted SARS-CoV-2 spike protein vaccine: a phase 2 randomized placebo-controlled trial in both seropositive and seronegative populations. Clin Microbiol Infect. 2022. https://doi.org/10.1016/j.cmi.2022.04.004.
Chavdaa VP, Vorab LK, Viholc DR. COVAX-19Ⓡ vaccine: completely blocks virus transmission to non-immune individuals. Clin Complement Med Pharmacol. 2021;1(1): 100004.
Souza MA, Carvalho FC, Ruas LP, Ricci-Azevedo R, Roque-Barreira MC. The immunomodulatory effect of plant lectins: a review with emphasis on ArtinM properties. Glycoconj J. 2013;30:641–57. https://doi.org/10.1007/s10719-012-9464-4.
da Silva LCN, Correia MTS. Plant lectins and Toll-like receptors: implications for therapy of microbial infections. Front Microbiol. 2014;5:20. https://doi.org/10.3389/fmicb.2014.00020.
Coelho LC, Silva PM, Lima VL, Pontual EV, Paiva PM, Napoleão TH, et al. Lectins, interconnecting proteins with biotechnological/pharmacological and therapeutic applications. Evid Based Complement Alternat Med. 2017;2017:1594074. https://doi.org/10.1155/2017/1594074.
Van Breedam W, Pöhlmann S, Favoreel HW, de Groot RJ, Nauwynck HJ. Bitter-sweet symphony: glycan–lectin interactions in virus biology. FEMS Microbiol Rev. 2014;38:598–632. https://doi.org/10.1111/1574-6976.12052.
Lardone RD, Garay YC, Parodi P, de la Fuente S, et al. How glycobiology can help us treat and beat the COVID-19 pandemic. J Biol Chem. 2021;296: 100375. https://doi.org/10.1016/j.jbc.2021.100375.
Duan L, Zheng Q, Zhang H, Niu Y, Lou Y, Wang H. The SARS-CoV-2 spike glycoprotein biosynthesis, structure, function, and antigenicity: implications for the design of spike-based vaccine immunogens. Front Immunol. 2020;11: 576622. https://doi.org/10.3389/fimmu.2020.576622.
Saad AAD. Recombinant lectins as pioneering anti-viral agents against COVID-19. Hematol Transfus Int J. 2021;9(4):77–9.
Keyaerts E, Vijgen L, Pannecouque C, Van Damme E, Peumans W, et al. Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antiviral Res. 2007;75:179–87. https://doi.org/10.1016/j.antiviral.2007.03.003.
Carneiro DC, Fernandez LG, Monteiro-Cunha JP, Benevides RG, Cunha Lima ST. A patent review of the antimicrobial applications of lectins: perspectives on therapy of infectious diseases. J Appl Microbiol. 2021;132:841–54. https://doi.org/10.1111/jam.15263.
Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA. Glycosylation and the immune system. Science. 2001;291(5512):2370–6. https://doi.org/10.1126/science.291.5512.2370.
Smith AE, Helenius A. How viruses enter animal cells. Science. 2004;304(5668):237–42. https://doi.org/10.1126/science.1094823.
Rademacher TW, Parekh RB, Dwek RA. Glycobiology. Annu Rev Biochem. 1988;57:785–838.
Lavelle EC, Grant G, Pusztai A, Pfüller U, O’hagan DT. The identification of plant lectins with mucosal adjuvant activity. Immunology. 2001;102:77–86. https://doi.org/10.1046/j.1365-2567.2001.01157.x.
Lavelle EC, Grant G, Pusztai A, Pfüller U, O’Hagan DT. Mucosal immunogenicity of plant lectins in mice. Immunology. 2000;99:30–7. https://doi.org/10.1046/j.1365-2567.2000.00932.x.
Afonso-Cardoso SR, Rodrigues FH, Gomes MAB, Silva AG, Rocha A, GuimarÃes AHB, Candeloro I, Favoreto S, Ferreira MS, de Souza MA. Protective effect of lectin from Synadenium carinatum on Leishmania amazonensis infection in BALB/c mice. Korean J Parasitol. 2007;45:255. https://doi.org/10.3347/kjp.2007.45.4.255.
Cardoso MRD, Mota CM, Ribeiro DP, Noleto PG, Andrade WBF, Souza MA, et al. Adjuvant and immunostimulatory effects of a D-galactose-binding lectin from Synadenium carinatum latex (ScLL) in the mouse model of vaccination against neosporosis. Vet Res. 2012;43(1):76. https://doi.org/10.1186/1297-9716-43-76.
Teixeira CR, Cavassani KA, Gomes RB, Teixeira MJ, Roque-Barreira MC, Cavada BS, et al. Potential of KM+ lectin in immunization against Leishmania amazonensis infection. Vaccine. 2006;24:3001–8. https://doi.org/10.1016/j.vaccine.2005.11.067.
Albuquerque DA, Martins GA, Campos-Neto A, Silva JS. The adjuvant effect of jacalin on the mouse humoral immune response to trinitrophenyl and Trypanosoma cruzi. Immunol Lett. 1999;68:375–81. https://doi.org/10.1016/s0165-2478(99)00079-6.
Unitt J, Hornigold D. Plant lectins are novel Toll-like receptor agonists. Biochem Pharmacol. 2011;81:1324–8. https://doi.org/10.1016/j.bcp.2011.03.010.
Liu Y, Cecílio NT, Carvalho FC, Roque-Barreira MC, Feizi T. Glycan microarray analysis of the carbohydrate-recognition specificity of native and recombinant forms of the lectin ArtinM. Data Br. 2015;5:1035–47. https://doi.org/10.1016/j.dib.2015.11.014.
Panunto-Castelo A, Souza MA, Roque-Barreira MC, Silva JS. KM+, a lectin from Artocarpus integrifolia, induces IL-12 p40 production by macrophages and switches from type 2 to type 1 cell-mediated immunity against Leishmania major antigens, resulting in BALB/c mice resistance to infection. Glycobiology. 2001;11:1035–42. https://doi.org/10.1093/glycob/11.12.1035.
Souza MA, Amâncio-Pereira F, Cardoso CRB, Silva AG, et al. Isolation and partial characterization of a D-galactose-binding lectin from the latex of Synadenium carinatum. Braz Arch Biol Technol. 2005;48:705–16. https://doi.org/10.1590/s1516-89132005000600005.
Dong Q, Sugiura T, Toyohira Y, Yoshida Y, Yanagihara N, Karasaki Y. Stimulation of IFN-γ production by garlic lectin in mouse spleen cells: involvement of IL-12 via activation of p38 MAPK and ERK in macrophages. Phytomedicine. 2011;18(4):309–16. https://doi.org/10.1016/j.phymed.2010.06.008.
Padiyappa SD, Avalappa H, Somegowda M, Sridhara S, et al. Immunoadjuvant and humoral immune responses of garlic (Allium sativum L.) lectins upon systemic and mucosal administration in BALB/c mice. Molecules. 2022;27:1375. https://doi.org/10.3390/molecules27041375.
Moses T, Papadopoulou KK, Osbourn A. Metabolic and functional diversity of saponins, biosynthetic intermediates and semi-synthetic derivatives. Crit Rev Biochem Mol Biol. 2014;49:439–62. https://doi.org/10.3109/10409238.2014.953628.
Kensil CR. Saponins as vaccine adjuvants. Crit Rev Ther Drug Carrier Syst. 1996;13(1–2):1–55.
Oda K, Matsuda H, Murakami T, Katayama S, Ohgitani T, Yoshikawa M. Adjuvant and haemolytic activities of 47 saponins derived from medicinal and food plants. Biol Chem. 2000. https://doi.org/10.1515/bc.2000.009.
Zhai L, Li Y, Wang W, Hu S. Enhancement of humoral immune responses to inactivated Newcastle disease and avian influenza vaccines by oral administration of ginseng stem-and-leaf saponins in chickens. Poult Sci. 2011;90:1955–9. https://doi.org/10.3382/ps.2011-01433.
Zhai L, Li Y, Wang W, Wang Y, Hu S. Effect of oral administration of ginseng stem-and-leaf saponins (GSLS) on the immune responses to Newcastle disease vaccine in chickens. Vaccine. 2011;29:5007–14. https://doi.org/10.1016/j.vaccine.2011.04.097.
Zhai L, Wang Y, Yu J, Hu S. Enhanced immune responses of chickens to oral vaccination against infectious bursal disease by ginseng stem-leaf saponins. Poult Sci. 2014;93:2473–81. https://doi.org/10.3382/ps.2014-04056.
Xiao C, Rajput ZI, Hu S. Improvement of a commercial foot-and-mouth disease vaccine by supplement of Quil A. Vaccine. 2007;25:4795–800. https://doi.org/10.1016/j.vaccine.2007.04.027.
Li Y, Xie F, Chen J, Fan Q, Zhai L, Hu S. Increased humoral immune responses of pigs to foot-and-mouth disease vaccine supplemented with ginseng stem and leaf saponins. Chem Biodivers. 2012;9:2225–35. https://doi.org/10.1002/cbdv.201100377.
Zhang C, Wang Y, Wang M, Su X, Lu Y, Su F, Hu S. Rapeseed oil and ginseng saponins work synergistically to enhance Th1 and Th2 immune responses induced by the foot-and-mouth disease vaccine. Clin Vaccine Immunol. 2014;21:1113–9. https://doi.org/10.1128/cvi.00127-14.
Li R, Ma Y, Zhai L, Lu Y, et al. Enhanced immune response to foot-and-mouth disease vaccine by oral administration of ginseng stem-leaf saponins. Immunopharmacol Immunotoxicol. 2016;38:257–63. https://doi.org/10.1080/08923973.2016.1184680.
Wu JY, Gardner BH, Murphy CI, Seals JR, Kensil CR, Recchia J, et al. Saponin adjuvant enhancement of antigen-specific immune responses to an experimental HIV-1 vaccine. J Immunol. 1992;148(5):1519–25.
Sasaki S, Sumino K, Hamajima K, Fukushima J, Ishii N, Kawamoto S, et al. Induction of systemic and mucosal immune responses to human immunodeficiency virus type 1 by a DNA vaccine formulated with QS-21 saponin adjuvant via intramuscular and intranasal routes. J Virol. 1998;72:4931–9. https://doi.org/10.1128/jvi.72.6.4931-4939.1998.
de Costa F, Yendo ACA, Cibulski SP, Fleck JD, Roehe PM, Spilki FR, et al. Alternative inactivated poliovirus vaccines adjuvanted with Quillaja brasiliensis or Quil-A saponins are equally effective in inducing specific immune responses. PLoS ONE. 2014;9: e105374. https://doi.org/10.1371/journal.pone.0105374.
Silveira F, Cibulski SP, Varela AP, Marqués JM, Chabalgoity A, de Costa F, et al. Quillaja brasiliensis saponins are less toxic than Quil A and have similar properties when used as an adjuvant for a viral antigen preparation. Vaccine. 2011;29:9177–82. https://doi.org/10.1016/j.vaccine.2011.09.137.
Ren W, Sun H, Gao GF, Chen J, Sun S, et al. Recombinant SARS-CoV-2 spike S1-Fc fusion protein induced high levels of neutralizing responses in nonhuman primates. Vaccine. 2020;38:5653–8. https://doi.org/10.1016/j.vaccine.2020.06.066.
Santos WR, de Lima VMF, de Souza EP, Bernardo RR, Palatnik M, de Sousa CBP. Saponins, IL12 and BCG adjuvant in the FML-vaccine formulation against murine visceral leishmaniasis. Vaccine. 2002;21:30–43. https://doi.org/10.1016/s0264-410x(02)00444-9.
Waite DC, Jacobson EW, Ennis FA, Edelman R, White B, et al. Three double-blind, randomized trials evaluating the safety and tolerance of different formulations of the saponin adjuvant QS-21. Vaccine. 2001;19:3957–67. https://doi.org/10.1016/s0264-410x(01)00142-6.
Vandepapelière P, Rehermann B, Koutsoukos M, Moris P, Garçon N, et al. Potent enhancement of cellular and humoral immune responses against recombinant hepatitis B antigens using AS02A adjuvant in healthy adults. Vaccine. 2005;2005(23):2591–601. https://doi.org/10.1016/j.vaccine.2004.11.034.
Leroux-Roels G, Bourguignon P, Willekens J, Janssens M, Clement F, et al. Immunogenicity and safety of a booster dose of an investigational adjuvanted polyprotein HIV-1 vaccine in healthy adults and effect of administration of chloroquine. Clin Vaccine Immunol. 2014;21:302–11. https://doi.org/10.1128/cvi.00617-13.
Leroux-Roels I, Koutsoukos M, Clement F, Steyaert S, et al. Strong and persistent CD4+ T-cell response in healthy adults immunized with a candidate HIV-1 vaccine containing gp120, Nef and Tat antigens formulated in three Adjuvant Systems. Vaccine. 2010;28:7016–24. https://doi.org/10.1016/j.vaccine.2010.08.035.
Van Braeckel E, Bourguignon P, Koutsoukos M, Clement F, et al. An adjuvanted polyprotein HIV-1 vaccine induces polyfunctional cross-reactive CD4+ T cell responses in seronegative volunteers. Clin Infect Dis. 2011;52:522–31. https://doi.org/10.1093/cid/ciq160.
Schwarz TF, Volpe S, Catteau G, Chlibek R, David MP, Richardus JH, Lal H, et al. Persistence of immune response to an adjuvanted varicella-zoster virus subunit vaccine for up to year nine in older adults. Hum Vaccin Immunother. 2018;14:1370–7. https://doi.org/10.1080/21645515.2018.1442162.
Formica N, Mallory R, Albert G, Robinson M, Plested JS, Cho I, et al. Different dose regimens of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373) in younger and older adults: a phase 2 randomized placebo-controlled trial. PLoS Med. 2021;18(10): e1003769. https://doi.org/10.1371/journal.pmed.1003769.
Heath PT, Galiza EP, Baxter DN, et al. Safety and efficacy of NV-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385(13):1172–83.
Tian JH, Patel N, Haupt R, et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat Commun. 2021;12(1):372.
Keech C, Albert G, Cho I, Robertson A, Reed P, Neal S, Plested JS, Zhu M, et al. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383(24):2320–32. https://doi.org/10.1056/NEJMoa2026920.
Bian L, Gao F, Zhang J, He Q, Mao Q, Xu M, Liang Z. Effects of SARS-CoV-2 variants on vaccine efficacy and response strategies. Expert Rev Vaccines. 2021;20(4):365–73. https://doi.org/10.1080/14760584.2021.1903879.
Acknowledgements
Authors dully acknowledge CSIR, New Delhi and Department of Biotechnology, India for providing financial assistance for COVID-19 testing facility [OLP0043]. The authors would like to express their heartfelt gratitude to all COVID warriors around the world for their unwavering support in the fight against this deadly bug.
Funding
The study was funded by CSIR (Grant No. OLP0043).
Author information
Authors and Affiliations
Contributions
ArK: conceptualization, acquisition of data, and wrote manuscript, AaK: wrote manuscript, NVT: analyzing content and manuscript editing, YP: analyzing content, editing, and finalizing the manuscript, VH; critically revised and edit manuscript, and SK; scientific discussions, suggestions, and encourages other authors for opinions.
Corresponding authors
Ethics declarations
Conflict of interest
Authors declare no competing interest.
Ethics approval and consent to participate
Not applicable.
Human and animal rights
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, A., Sharma, A., Tirpude, N.V. et al. Plant-derived immuno-adjuvants in vaccines formulation: a promising avenue for improving vaccines efficacy against SARS-CoV-2 virus. Pharmacol. Rep 74, 1238–1254 (2022). https://doi.org/10.1007/s43440-022-00418-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-022-00418-4