Abstract
Background
Morin, a flavonoid extracted from Moraceace family and exhibits several pharmacological activities including anti-cancer activity. Although the anticancer activity of morin in breast cancer was estimated in some investigations, the pharmaceutical mechanism has not been fully elucidated. Therefore, we investigated to unveil the detail signaling pathway in morin-treated in MDA-MB-231 triple-negative breast cancer cells.
Methods
The cytotoxicity of morin in MDA-MB-231 cells was confirmed by sulforhodamine B (SRB) assay and colony formation assay. Flow cytometry was performed to examine the cell cycle and cell death patterns and the protein expression and phosphorylation were detected by western blotting.
Results
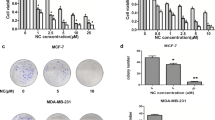
Our results showed that morin inhibited MDA-MB-231 cells proliferation in time and concentration-dependent manner. Morphological changes were observed when treated with various concentration of morin in MDA-MB-231 cells. In regard to protein expression, morin induced the phosphorylation of ERK and p-H2A.X and decreased the level of DNA repair markers, RAD51 and survivin. In addition, flow cytometry showed S and G2/M arrest by morin that was associated with the decrease in the protein expression of cyclin A2 and cyclin B1 and upregulation of p21. Interestingly, annexin V/PI staining result clearly showed that morin induced cell death without apoptosis. Furthermore, attenuated FoxM1 by morin was co-related with cell cycle regulators including p21, cyclin A2 and cyclin B1.
Conclusion
Taken together, our study indicates that morin-induced cell death of MDA-MB-231 is caused by sustained cell cycle arrest via the induction of p21 expression by activation of ERK and repression of FOXM1 signaling pathways.







Similar content being viewed by others
Abbreviations
- AIF:
-
Apoptosis-inducing factor
- BAX:
-
Bcl-2-associated X protein
- Bcl-xL:
-
B-cell lymphoma-extra-large
- ERK:
-
Extracellular signal-regulated kinase ½
- FOXM1:
-
Forkhead Box M1
- H2A.X:
-
H2A histone family member X
- LC3:
-
Microtubule-associated protein 1A/1B-light chain 3
- PARP:
-
Poly (ADP-ribose) polymerase
- RIP3:
-
Receptor-interacting serine/threonine-protein kinase 3
- SRB:
-
Sulforhodamine B
- TNBC:
-
Triple-negative breast cancer
References
Yavari B, Mahjub R, Saidijam M, Raigani M, Soleimani M. The potential use of peptides in cancer treatment. Curr Protein Pept Sci. 2018;19(8):759–70.
Wang LH, Jiang XR, Chen GL, Guo W, Zhang JY, Cui LJ, et al. Anti-tumor activity of SL4 against breast cancer cells: induction of G(2)/M arrest through modulation of the MAPK-dependent p21 signaling pathway. Sci Rep. 2016;6:36486.
Bonsu AB, Ncama BP, Bonsu KO. Breast cancer knowledge, beliefs, attitudes and screening efforts by micro-community of advanced breast cancer patients in Ghana. Int J Africa Nurs Sci. 2019;11:100155.
Wang D, Zhu K, Tian J, Li Z, Du G, Guo Q, et al. Clinicopathological and ultrasonic features of triple-negative breast cancers: a comparison with hormone receptor-positive/human epidermal growth factor receptor-2-negative breast cancers. Ultrasound Med Biol. 2018;44(5):1124–32.
Shi Y, Jin J, Ji W, Guan X. Therapeutic landscape in mutational triple negative breast cancer. Mol cancer. 2018;17(1):99.
Tan Y, Wang Q, Xie Y, Qiao X, Zhang S, Wang Y, et al. Identification of FOXM1 as a specific marker for triple-negative breast cancer. Int J Oncol. 2019;54(1):87–97.
Tian S, Chen Y, Yang B, Lou C, Zhu R, Zhao Y, et al. F1012–2 inhibits the growth of triple negative breast cancer through induction of cell cycle arrest, apoptosis, and autophagy. Phytother Res. 2018;32(5):908–22.
Lee K-S, Lee M-G, Kwon Y-S, Nam K-S. Arctigenin enhances the cytotoxic effect of doxorubicin in MDA-MB-231 breast cancer cells. Int J Mol Sci. 2020;21(8):2997.
Singh SK, Spiegel S. Sphingosine-1-phosphate signaling: a novel target for simultaneous adjuvant treatment of triple negative breast cancer and chemotherapy-induced neuropathic pain. Adv Biol Regul. 2020;75:100670.
Obayemi J, Salifu A, Eluu S, Uzonwanne V, Jusu S, Nwazojie C, et al. LHRH-conjugated drugs as targeted therapeutic agents for the specific targeting and localized treatment of triple negative breast cancer. Sci Rep. 2020;10(1):1–18.
Sauter ER. Cancer prevention and treatment using combination therapy with natural compounds. Expert Rev Clin Pharmacol. 2020;13(3):265–85.
Hazafa A, Rehman K-U, Jahan N, Jabeen Z. The role of polyphenol (Flavonoids) compounds in the treatment of cancer cells. Nutr and cancer. 2020;72(3):386–97.
Sinha K, Ghosh J, Sil PC. Advances in experimental medicine and biology. 2016. Morin and its role in chronic diseases. In: Gupta S, Prasad S, Aggarwal B, editors. Anti-inflammatory nutraceuticals and chronic diseases, vol. 928. Switzerland: Springer Cham; 2016. p. 453–71.
Kapoor R, Kakkar P. Protective role of morin, a flavonoid, against high glucose induced oxidative stress mediated apoptosis in primary rat hepatocytes. PLoS ONE. 2012;7(8):e41663.
Lee YJ, Kim WI, Kim SY, Cho SW, Nam HS, Lee SH, et al. Flavonoid morin inhibits proliferation and induces apoptosis of melanoma cells by regulating reactive oxygen species, Sp1 and Mcl-1. Arch Pharm Res. 2019;42(6):531–42.
Solairaja S, Andrabi MQ, Dunna NR, Venkatabalasubramanian S (2020) Overview of morin and its complementary role as an adjuvant for anticancer agents. Nutr Cancer. 73(6):1–16. https://doi.org/10.1080/01635581.2020.1778747
Zhang Q, Zhang F, Thakur K, Wang J, Wang H, Hu F, et al. Molecular mechanism of anti-cancerous potential of Morin extracted from mulberry in Hela cells. Food Chem Toxicol. 2018;112:466–75.
Kim M-N, Ahn E-Y, Park SE, Hossain MA, Kim MY, Moon J-O, et al. Morin inhibits the growth of murine hepatoma cells via cell cycle arrest and induction of apoptosis. J Cancer Prev. 2010;15(3):190–7.
Hsiang C-Y, Wu S-L, Ho T-Y. Morin inhibits 12-O-tetradecanoylphorbol-13-acetate-induced hepatocellular transformation via activator protein 1 signaling pathway and cell cycle progression. Biochem Pharmacol. 2005;69(11):1603–11.
Jin H, Lee WS, Eun SY, Jung JH, Park H-S, Kim G, et al. Morin, a flavonoid from Moraceae, suppresses growth and invasion of the highly metastatic breast cancer cell line MDA-MB-231 partly through suppression of the Akt pathway. Int J Oncol. 2014;45(4):1629–37.
Clark JA, Black AR, Leontieva OV, Frey MR, Pysz MA, Kunneva L, et al. Involvement of the ERK signaling cascade in protein kinase C-mediated cell cycle arrest in intestinal epithelial cells. J Biol Chem. 2004;279(10):9233–47.
Zhu H, Zhang L, Wu S, Teraishi F, Davis JJ, Jacob D, et al. Induction of S-phase arrest and p21 overexpression by a small molecule 2[[3-(2,3-dichlorophenoxy)propyl] amino]ethanol in correlation with activation of ERK. Oncogene. 2004;23(29):4984–92.
Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med. 2006;12(9):440–50.
Fragkos M, Jurvansuu J, Beard P. H2AX is required for cell cycle arrest via the p53/p21 pathway. Mol Cell Biol. 2009;29(10):2828.
Tang Y, Luo J, Zhang W, Gu W. Tip60-dependent acetylation of p53 modulates the decision between cell-cycle arrest and apoptosis. Mol Cell. 2006;24(6):827–39.
Mgbonyebi OP, Russo J, Russo IH. Roscovitine induces cell death and morphological changes indicative of apoptosis in MDA-MB-231 breast cancer cells. Cancer Res. 1999;59(8):1903.
Ye J, Zhang R, Wu F, Zhai L, Wang K, Xiao M, et al. Non-apoptotic cell death in malignant tumor cells and natural compounds. Cancer Lett. 2018;420:210–27.
Hlavová M, Čížková M, Vítová M, Bišová K, Zachleder V. DNA damage during G2 phase does not affect cell cycle progression of the green alga Scenedesmus quadricauda. PLoS ONE. 2011;6(5):e19626.
Hu B, Mitra J, van den Heuvel S, Enders GH. S and G2 phase roles for Cdk2 revealed by inducible expression of a dominant-negative mutant in human cells. Mol Cell Biol. 2001;21(8):2755–66.
Liao C, Li SQ, Wang X, Muhlrad S, Bjartell A, Wolgemuth DJ. Elevated levels and distinct patterns of expression of A-type cyclins and their associated cyclin-dependent kinases in male germ cell tumors. Int J Cancer. 2004;108(5):654–64.
Yadav V, Sultana S, Yadav J, Saini N. Gatifloxacin induces S and G2-phase cell cycle arrest in pancreatic cancer cells via p21/p27/p53. PLoS ONE. 2012;7(10):e47796.
Choi YH. Molecular mechanism of the G2/M arrest by genistein in human cancer cells. J Cancer Prev. 2002;7(4):254–67.
Badie C, Bourhis J, Sobczak-Thépot J, Haddada H, Chiron M, Janicot M, et al. p53-dependent G2 arrest associated with a decrease in cyclins A2 and B1 levels in a human carcinoma cell line. Br J Cancer. 2000;82(3):642–50.
Kohno M, Pouyssegur J. Targeting the ERK signaling pathway in cancer therapy. Ann Med. 2006;38(3):200–11.
Tang D, Wu D, Hirao A, Lahti JM, Liu L, Mazza B, et al. ERK activation mediates cell cycle arrest and apoptosis after DNA damage independently of p53. J Biol Chem. 2002;277(15):12710–7.
Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9(6):400–14.
Wierstra I, Alves J. FOXM1, a typical proliferation-associated transcription factor. Biol Chem. 2007;388(12):1257–74.
Nakamura S, Hirano I, Okinaka K, Takemura T, Yokota D, Ono T, et al. The FOXM1 transcriptional factor promotes the proliferation of leukemia cells through modulation of cell cycle progression in acute myeloid leukemia. Carcinogenesis. 2010;31(11):2012–21.
Tan Y, Chen Y, Yu L, Zhu H, Meng X, Huang X, et al. Two-fold elevation of expression of FoxM1 transcription factor in mouse embryonic fibroblasts enhances cell cycle checkpoint activity by stimulating p21 and Chk1 transcription. Cell Prolif. 2010;43(5):494–504.
Kim IM, Ackerson T, Ramakrishna S, Tretiakova M, Wang IC, Kalin TV, et al. The Forkhead Box m1 transcription factor stimulates the proliferation of tumor cells during development of lung cancer. Cancer Res. 2006;66(4):2153–61.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Grant no. 2018R1D1A1B07047758).
Author information
Authors and Affiliations
Contributions
K-SN and K-S Lee designed the experiments. K-SN, SM and K-SL analyzed and interpreted data. SM, Y-SK and M-GL performed the experiments and SM and K-SL wrote the manuscript, which was approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Maharjan, S., Kwon, YS., Lee, MG. et al. Cell cycle arrest-mediated cell death by morin in MDA-MB-231 triple-negative breast cancer cells. Pharmacol. Rep 73, 1315–1327 (2021). https://doi.org/10.1007/s43440-021-00272-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-021-00272-w