Abstract
Background
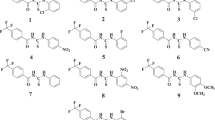
Thiophene(s) are an important group in therapeutic applications, and sulfonamides are the most important class of carbonic anhydrase (CA) inhibitors. In this study, inhibition effects of some thiophene-based sulfonamides on human erythrocytes carbonic anhydrase I and II isoenzymes (hCA-I and hCA-II) were investigated. Thiophene-based sulfonamides used in this study showed potent inhibition effect on both isoenzymes at very small concentrations.
Materials and methods
We report on the purification of the carbonic anhydrase I and II isoenzymes (hCA-I and hCA-II) using affinity chromatography method. The inhibition effect of the thiophene-based sulfonamides was determined by IC50 and Ki parameters. A molecular docking study was performed for each molecule.
Results
Thiophene-based sulfonamides showed IC50 values of in the range of 69 nM to 70 µM against hCA-I, 23.4 nM to 1.405 µM against hCA-II. Ki values were in the range of 66.49 ± 17.15 nM to 234.99 ± 15.44 µM against hCA-I, 74.88 ± 20.65 nM to 38.04 ± 12.97 µM against hCA-II. Thiophene-based sulfonamides studied in this research showed noncompetitive inhibitory properties on both isoenzymes. To elucidate the mechanism of inhibition, a molecular docking study was performed for molecules 1 and 4 exhibiting a strong inhibitory effect on hCA-I and hCA-II. The compounds inhibit the enzymes by interacting out of catalytic active site. The sulfonamide and thiophene moiety played a significant role in the inhibition of the enzymes.
Conclusion
We hope that this study will contribute to the design of novel thiophene-based sulfonamide derived therapeutic agents that may be carbonic anhydrase inhibitors in inhibitor design studies.







Similar content being viewed by others
References
Supuran CT. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin Drug Discov. 2017;12:61–88.
Supuran CT. Special ıssue: sulfonamides. Molecules. 2017;22:1642.
Capasso C, Supuran CT. Sulfa and trimethoprim-like drugs-antimetabolites acting as carbonic anhydrase, dihydropteroate synthase and dihydrofolate reductase inhibitors. J Enzyme Inhib Med Chem. 2014;29:379–87.
Scozzafava A, Owa T, Mastrolorenzo A, Supuran CT. Anticancer and antiviral sulfonamides. Curr Med Chem. 2003;10:925–53.
Monti SM, Supuran CT, De Simone G. Anticancer carbonic anhydrase inhibitors: a patent review (2008–2013). Expert Opin Ther Pat. 2013;23:737–49.
Scozzafava A, Supuran CT, Carta F. Antiobesity carbonic anhydrase inhibitors: a literature and patent review. Expert Opin Ther Pat. 2013;23:725–35.
Loubatières-Mariani MM. The discovery of hypoglycemic sulfonamides. J Soc Biol. 2007;201:121–5.
Carta F, Supuran CT. Diuretics with carbonic anhydrase inhibitory action: a patent and literature review (2005–2013). Expert Opin Ther Pat. 2013;23:681–91.
Carta F, Di Cesare Mannelli L, Pinard M, Ghelardini C, Scozzafava A, McKenna R, Supuran CT. A class of sulfonamide carbonic anhydrase inhibitors with neuropathic pain modulating effects. Bioorg Med Chem. 2015;23:1828–40.
Carta F, Supuran CT, Scozzafava A. Sulfonamides and their isosters as carbonic anhydrase inhibitors. Future Med. Chem. 2014;6:1149–65.
Alterio V, Di Fiore A, D’Ambrosio K, Supuran CT, De Simone G. Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms? Chem Rev. 2012;112:4421–68.
Supuran CT. Carbonic anhydrase inhibitors: an editorial. Expert Opin Ther Pat. 2013;23:677–9.
Scozzafava A, Mastrolorenzo A, Supuran CT. Modulation of carbonic anhydrase activity and its applications in therapy. Expert Opın Ther Pat. 2004;14:667–702.
Supuran CT, Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem. 2007;15:4336–50.
Alım Z, Kilinc N, Sengul B, Beydemir S. Some anti-ınflammatory agents ınhibit esterase activities of human carbonic anhydrase ısoforms i and II: an in vitro study. Chem Biol Drug Des. 2015;86:857–63.
Alım Z. 1H-indazole molecules reduced the activity of human erythrocytes carbonic anhydrase I and II isoenzymes. J Biochem Mol Toxicol. 2018;32:e22194.
Koksal Z, Alım Z, Bayrak S, Gulcin I, Ozdemir H. Investigation of the effects of some sulfonamides on acetylcholinesterase and carbonic anhydrase enzymes. J Biochem Mol Toxicol. 2019;33(5):e22300.
Wilbur KM, Anderson NG. Electrometric and colorimetric determination of carbonic anhydrase. J Biol Chem. 1948;176:147–54.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–54.
Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5.
Verpoorte JA, Mehta S, Edsall JT. Esterase activities of human carbonic anhydrases B and C. J Biol Chem. 1967;242:4221–9.
Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc. 1934;56:658–66.
Protein Preparation Wizard; Epik, Schrödinger, LLC, New York, NY, 2016; Impact, Schrödinger, LLC, New York, NY, 2016; Prime, Schrödinger, LLC, New York, NY. 2019.
Kalin R, Koksal Z, Kalin P, Karaman M, Gulcin I, Ozdemir H. In vitro effects of standard antioxidants on lactoperoxidase enzyme—a molecular docking approach. J Biochem Mol Toxicol. 2020;34:e22421.
Bayrak C, Taslimi P, Karaman HS, Gulcin I, Menzek A. The first synthesis, carbonic anhydrase inhibition and anticholinergic activities of some bromophenol derivatives with S including natural products. Bioorg Chem. 2019;85:128–39.
SiteMap, Schrödinger, LLC, New York, NY. 2019.
Bal S, Kaya R, Gök Y, Taslimi P, Aktaş A, Karaman M, et al. Novel 2-methylimidazolium salts: synthesis, characterization, molecular docking, and carbonic anhydrase and acetylcholinesterase inhibitory properties. Bioorg Chem. 2020;94:103468.
LigPrep, Schrödinger, LLC, New York, NY. 2019.
Taslimi P, Turkan F, Çetin A, Burhan H, Karaman M, Bildirici I, et al. Pyrazole[3,4-d]pyridazine derivatives: molecular docking and explore of acetylcholinesterase and carbonic anhydrase enzymes inhibitors as anticholinergics potentials. Bioorg Chem. 2019;92:103213.
Glide, Schrödinger, LLC, New York, NY. 2019.
Yigit B, Kaya R, Taslimi P, Isık Y, Karaman M, Yigit M, et al. Imidazolinium chloride salts bearing wingtip groups: synthesis, molecular docking and metabolic enzymes inhibition. J Mol Struct. 2019;1179:709–18.
Turkan F, Cetin A, Taslimi P, Karaman HS, Gulcin I. Synthesis, characterization, molecular docking and biological activities of novel pyrazoline derivatives. Arch Pharm. 2019;352:e1800359.
Induced Fit Docking protocol; Glide. Schrödinger, LLC, New York, NY, 2016. Schrödinger, LLC, New York, NY: Prime; 2019.
Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006;440:463–9.
Shah R, Verma PK. Therapeutic importance of synthetic thiophene. Chem Cent J. 2018;12:137.
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26.
Karimov A, Orujova A, Taslimi P, Sadeghian N, Mammadov B, Karaman HS, et al. Novel functionally substituted esters based on sodium diethyldithiocarbamate derivatives: synthesis, characterization, biological activity and molecular docking studies. Bioorg Chem. 2020;99:103762.
Singh P, Swain B, Thacker PS, Sigalapalli DK, Yadav PP, Angeli A, et al. Synthesis and carbonic anhydrase inhibition studies of sulfonamide based indole-1,2,3-triazole chalcone hybrids. Bioorg Chem. 2020;99:103839.
Burmaoglu S, Akin-Kazancioglu E, Kaya R, Kazancioglu M, Karaman M, Algul O, et al. Synthesis of novel organohalogen chalcone derivatives and screening of their molecular docking study and some enzymes inhibition effects. J Mol Struct. 2020;1208:127868.
D’Ambrosio K, Carradori S, Monti SM, Buonanno M, Secci D, Vullo D, et al. Out of the active site binding pocket for carbonic anhydrase inhibitors. Chem Commun. 2015;51:302–5.
Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem. 2016;31:345–60.
Acknowledgements
Thank you to Atatürk University, Faculty of Science, Biochemistry Research Laboratory, which allows us to carry out studies to purify carbonic anhydrase isoenzymes.
Author information
Authors and Affiliations
Contributions
Z. Alim carried out purification and inhibition studies of hCA-I and hCA-II isoenzymes. Analysis of the results was done by Z. Alim and Z. Koksal. Molecular modeling studies were carried out by M. Karaman. The writing and language correction of the article were done by Z. Alim, Z. Koksal and M. Karaman.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no conflicts of interest exist.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alım, Z., Köksal, Z. & Karaman, M. Evaluation of some thiophene-based sulfonamides as potent inhibitors of carbonic anhydrase I and II isoenzymes isolated from human erythrocytes by kinetic and molecular modelling studies. Pharmacol. Rep 72, 1738–1748 (2020). https://doi.org/10.1007/s43440-020-00149-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-020-00149-4