Abstract
Pomegranate, renowned for its delectable taste and remarkable nutritional profile, has witnessed a surge in both production and consumption. However, the by-products generated during industrial processes, such as peels and seeds, have the potential for adverse environmental impacts if not meticulously managed. Similarly, expired fruit juices or spillages that may occur during manufacturing and transportation contribute to agri-food waste. This study focused on the comprehensive assessment of pomegranate by-products and pomegranate juice using ascomycetes and zygomycetes filamentous fungi, namely Aspergillus oryzae, Rhizopus oligosporus, and Neurospora intermedia to obtain mycoprotein for sustainable vegan food production. The findings revealed that pomegranate juice, both fresh and expired commercial, contained essential nutrients for fungal biomass production (up to 0.024 g biomass/mL juice). Nonetheless, fresh juice emerges as a more potent medium in terms of protein production than commercial juice. Cultivating A. oryzae yielded a biomass of 0.39 (g biomass/g peel) from pomegranate peel, while concurrently raising the protein content of raw pomegranate peel from 30.89 g/kg to 85.41 g/kg. Furthermore, incorporating yeast extract into the peel medium not only resulted in an enhanced biomass yield of 0.49 (g biomass/g peel) but also significantly elevated the protein content to 198.63 g/kg. This study provides valuable insights into the potential of pomegranate peel and juice as promising substrate for fungal biomass production, offering opportunities for the development of innovative food and feed products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The global human population has reached 8 billion individuals, and this demographic growth has significant implications for both the economy and the environment. One of the most critical challenges faced by humanity is ensuring sufficient access to food for everyone. As the demand for food increases due to population growth, various economic and environmental issues arise in the effort to meet this demand [1]. The food waste sector contributes substantially to environmental problems. Industries face mounting pressure from the public to adopt environmentally conscious practices and implement sustainable measures. It is crucial to assess and address food waste to minimize its environmental impact and promote a circular economy. Biological conversion of food by-products presents a promising approach to address this issue. The nutrients in food by-products can be converted into a range of valuable products using microorganisms [2]. This method holds significant potential for valorizing food by-products and reducing its environmental waste pollution. Fruit juice industry generates substantial amounts of by-products, particularly seed and peel of fruits [3]. These by-products could serve as a valuable resource for future valorization efforts.
Pomegranate (Punica granatum L.) by-products make up an important part of the industry’s waste due to they being rich in antioxidants such as flavonoids and phenolic components. There are a few cases where pomegranate peel is used in the food industry as a natural preservative [4, 5] or as a source of functional ingredients [6]. The potential health benefits and antioxidant effects of phenolic compounds such as punicalagin and ellagic acid from pomegranate are particularly noteworthy [7]. Moreover, pomegranate by-products (peel and seed) have been proven to have antibacterial, anti-inflammatory, antiviral, and anticarcinogenic properties in both in vivo and in vitro investigations [8, 9]. Their secondary metabolites called antioxidants have a variety of functions, e.g., anthocyanins serve as UV protection components, and osmotic pressure regulators [10, 11]. As a result, several pomegranate fruit components have gained popularity for identifying the health benefits of pomegranate.
The filamentous fungi Aspergillus oryzae, Rhizopus oligosporus, and Neurospora intermedia are considered as generally recognized as safe (GRAS) [12] and also edible. These fungi can grow on a variety of substrates due to their diverse enzymatic mechanisms, which include the generation of cellulase, xylanase, protease, and lipase [9]. As a result of their capacity to generate lipases, which convert fat into short-chain fatty acids and esters with uses in a variety of industrial sectors, they have the potential to valorize a large variety of substrates starting with food waste [13]. Fungi can also grow on several kind of fruit by-products, grape pomace, olive oil mill wastewater, citrus by-products, and carrot pomace [14,15,16,17]. Fungi contribute to fulfilling the requirements of Agenda 2030 by taking part in seven goals set in this agenda [18]. They are widely applied in the creation of human-fermented foods such as red kojic rice, tempeh, soy sauce, rice wine, and Chinese liquor and can open the door to the development of fungal-based products for feed and food applications. The production of fungi using pomegranate by-products could contribute to produce a value-added alternative food.
This study was aimed to investigate the effects of pomegranate by-products, namely aril and peel, and pomegranate juice on fungal biomass production and protein recovery through A. oryzae, N. intermedia, and R. oligosporus cultivations. For this purpose, these feedstocks were compared in detail with different fungi at different initial concentrations with or without supplementation. In addition, the effects of fruit juice that have the potential to be waste (such as expired juice) were also investigated. It was also aimed to investigate the effects of initial pH, dry and wet peel, and different types of nitrogen supplementation on fungal biomass production using pomegranate peels. This study will contribute to the evaluation of feedstocks for resource recovery and protein production through microbial conversion of pomegranate by-products released in the fruit juice industry. It will also minimize the waste generation potential of the use of expired fruit juices and unprocessed fruit by-products.
Materials and methods
Substrate
In this work, pomegranate (Punica granatum L., cv. Hicaznar) was collected from a local market (Orienta, Borås, Sweden). The seed and peel of the pomegranate were manually separated. The seeds were stored at -20 °C. The peels were powdered using a grinder followed by drying at 50 °C for 2 days, and then stored at 4 °C until use.
Fungal strains
Three different edible filamentous fungi strains from the ascomycetes (Aspergillus oryzae var. oryzae CBS 819.72 and Neurospora intermedia CBS 131.92) and zygomycetes (Rhizopus microsporus var. oligosporus CBS 112586) were used in this study.
Potato dextrose agar (PDA; 20 g/L glucose, 15 g/L agar, and 4 g/L potato extract) was used to grow the fungal strains. 100 µL of spore suspension recovered from pre-grown fungal plates treated with 20 mL of distilled water were inoculated into new PDA plates and then spread out using an L-shape spreader. The inoculated PDA plates were incubated at 30 °C for 3 days and then stored at 4 °C until use for a maximum of 1 month [12].
Fungal cultivation
Fungal cultivations were performed in a 250 mL wide-necked Erlenmeyer flask containing 100 mL of medium prepared with pomegranate feedstocks (juice, aril, and peel). To examine the effect of pomegranate juice on fungal biomass production, different levels of fresh fruit juice (0.5–4%) were added to media containing glucose (30 g/L) and yeast extract (5 g/L). Then fresh fruit juice (10, 15, 20% v/v) without supplementation was examined in comparison with commercial fruit juice (10, 15, 20% v/v). Fungal biomass production was comparatively investigated using pomegranate by-products (aril or peel) in media with and without the addition of glucose and yeast extract. In addition, in medium prepared with peel, the effects of peel form (dry versus wet form), initial pH (5.0, 5.5, and 6.0), and the addition of different types of nitrogen sources (yeast extract, urea, sodium nitrate, ammonium sulphate, and ammonium chloride) were also studied.
Each flask was inoculated with 2 mL spore suspension and then incubated at 35 °C at 125 rpm in a water bath (Grant OLS-Aqua pro, Cambridge, UK) for 48 h [19]. At the end of each cultivation, the biomass was harvested using a kitchen sieve (1 mm2 pore size) and washed with distilled water and then dried in an oven at 70 °C for 16 h [16]. All experiments were carried out in duplicate. When aril and peel were used as substrates, the biomass obtained from these media was denoted as harvested solids, since there were particles that remained undissolved in the media.
Analytical methods
The pH of pomegranate by-products and media was measured using a Mettler Toledo pH meter. Total solids (TS), dissolved solids, and ash contents of pomegranate by-products were determined according to Sluiter et al. [20] and Sar et al. [12]. Glucose, other sugars, and ethanol levels were analyzed using an HPLC system (Walters 2695, Walters Corp., Milford, USA) with a hydrogen-based ion-exchange column (Aminex HPX-87H, Bio-Rad, Hercules, USA). Crude protein analysis of biomass and pomegranate by-products were analyzed following the Kjeldahl method, nitrogen-to-protein conversion factors of 6.25 and 5.80 were used for biomass and pomegranate by-products, respectively.
Data analysis
The software Minitab17® was used for the statistical analysis of the obtained results with ANOVA (analysis of variance) tables using general linear models. Pairwise comparisons among groups of data were also carried out using the Tukey test. Significant differences were considered at p value < 0.05 within a 95% confidence interval. All error bars and intervals presented represent two times the standard deviation.
Results and discussion
In this study, the use of pomegranate by-products and pomegranate juice (fresh and commercial) from fruit industry was evaluated through edible filamentous fungi cultivation (A. oryzae, R. oligosporus and N. intermedia). For this, the effects of different concentrations of pomegranate juice, aril, and peel were investigated in detail to determine their potential effects on fungal cultivation. Then the potential uses of the biomass were evaluated by analyzing their protein levels.
Substrate characterization
Pomegranate cultivars exhibit significant variations in terms of weight, size, and the ratio of peel to seed, showcasing distinct characteristics in these aspects [21]. The total weight of the pomegranate variety (Punica granatum L., cv. Hicaznar) used in the study varies from 540 to 700 g which is higher than the weight of different cultivars reported in other studies [22,23,24]. The ratio of peel and seed was determined as 43.99 ± 4.61% and 56.0 ± 4.61%, respectively. These findings align with a study conducted by El Moujahed [22] which reported a range of 28–45% for pomegranate peel content and 48–59% for seed content. The substantial peel content of the fruit presents an opportunity for assessment as a potential substrate in bioproduction processes.
The chemical composition of pomegranate by-products and juice is presented in Table 1. The pH values of pomegranate juice (2.91), pomegranate aril (2.96), and pomegranate peel (3.22) are within the expected range reported in previous studies [24], confirming the acidic nature of these pomegranate by-products. The crude protein levels of pomegranate peel showed significantly higher (30.89 g/kg) than pomegranate aril (19.97 g/kg) and pomegranate juice (3.78 g/kg), which are consistent with previous reports [25, 26]. Among the samples analyzed, dried pomegranate peel exhibited the highest glucose content at 114.57 g/kg, surpassing pomegranate aril (79.21 g/kg) and pomegranate juice (71.38 g/kg). Although the glucose content in pomegranate varies between 6 and 13%, its level may vary depending on the variety and maturity of the fruit [27].
Fungal biomass production
Cultivation in pomegranate juice
Pomegranate products contain various phenolic compounds, mainly ellagic acid, showing potential antimicrobial activities [8, 28]. To assess the effects of pomegranate juice on fungal biomass production, fungal cultivation was conducted in the initial phase by incorporating varying concentrations of pomegranate juice into a nutrient medium containing glucose and yeast extract (Fig. 1).
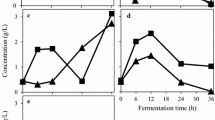
A. oryzae biomass obtained from the media containing different concentrations of pomegranate juice (0.5–4%, v/v) was 10–18% less than the control (p = 0.036; Fig. 1a). However, the highest concentration of pomegranate juice (4%, v/v) resulted in increased biomass production compared to lower juice concentrations. N. intermedia exhibited lower biomass production compared to A. oryzae (Fig. 1b). The highest biomass obtained with N. intermedia in media containing pomegranate juice at 4% juice concentration was 2.55 g/L which was much lower than the control sample (5.63 g/L, p = 0.003). Although biomass production through R. oligosporus (Fig. 1c) was higher at 4% juice concentration, reaching to 4.29 g/L, there is no statistical difference when compared it with control and other juice concentrations (p = 0.213).
It was observed that A. oryzae and N. intermedia completely consumed glucose and other sugars after 48 h (Fig. 2). It was determined that R. oligosporus could not consume other sugars completely. Fungal strains can be sensitive to pomegranate juice, which could be due to the presence of ellagic acid, known to have various attack mechanisms that disrupt membrane stability, prevent ion channels within the membrane, and restrict electron flow in the plasma membrane chain of electron transport needed for ATP synthesis through its electron scavenging attributes [29]. When comparing the ethanol production by A. oryzae, N. intermedia, and R. oligosporus, it is evident that A. oryzae shows the lowest ethanol producer under all conditions. In contrast, N. intermedia exhibits high ethanol producer at 42 h (7.31 g/L) and maintains a relatively higher production at 48 h (6.63 g/L). The reduction in ethanol production may be due to ethanol consumption by the fungus as well as ethanol evaporation [17]. R. oligosporus, known for its ethanol production capability [30], also shows promising results in the presence of pomegranate juice. The ethanol production by R. oligosporus reached to 8.35 g/L (0.27 g ethanol/g initial glucose) after 48 h incubation. These findings are consistent with previous studies by Rhizopus spp., which reported that ethanol production from a variety of substrates such as lignocellulose, thin stillage, and spent sulphite liquor [31,32,33]. Bulkan et al. [30] emphasized that ethanol yields of R. oligosporus ranged from 0.12 to 0.31 g of ethanol per initial glucose. This suggests that substrate composition and fermentation conditions play a crucial role in influencing ethanol production.
When the protein contents of fungal biomass were compared, N. intermedia showed the highest value with 518.45 g/kg, followed by R. oligosporus with 436.72 g/kg and A. oryzae with 414.09 g/kg (Fig. 3a). When these values were compared with the control samples, it was observed that the addition of pomegranate juice (4%) increased the protein content of biomass of A. oryzae and N. intermedia by 13–16%, while there was a slight decrease in the protein content of the R. oligosporus biomass. It was found that the average protein content (ranging from 41 to 51%) of the biomass obtained in the study was higher than typical animal feeds for chicken (25%), pig (13%), shrimp (25–42%), and fish (32–45%) [12]. These results suggest that the biomass produced in this study could potentially serve as a viable alternative to animal feed, given its protein content falls within the range of protein content found in typical animal feed.
In summary, the addition of pomegranate juice to the nutrient medium enhances the biomass production of R. oligosporus and increases the protein content in the biomass of A. oryzae and N. intermedia for fungal biomass and protein production.
Cultivation in commercial pomegranate juice compared to fresh pomegranate juice
Three fungal species were grown in supplement-free media prepared with 4% fresh juice, but the amount of biomass was less than 1 g/L (Fig. 4a), probably due to nutrient deficiency. In addition, R. oligosporus yielded much less biomass than the other two fungal species. Then higher juice concentrations (10, 15, 20%) were used to produce fungal biomass through A. oryzae and N. intermedia. A. oryzae demonstrated higher biomass production compared to N. intermedia, suggesting its better adaptability in utilizing the nutrients present in the juice for efficient growth (Fig. 4). Further research is needed to understand the specific factors contributing to this performance difference, such as the enzymes and metabolic pathways involved.
The levels of fungal biomass (g/L) and biomass yield (g biomass/mL juice) produced a from fresh juice (4%) through A. oryzae, N. intermedia, and R. oligosporus; and b from fresh juice (10, 15, and 20%) and c commercial juice (10, 15, and 20%) through A. oryzae; and from d fresh juice (10, 15, and 20%) and e commercial juice (10, 15, and 20%) through N. intermedia
Both A. oryzae and N. intermedia displayed different biomass production patterns depending on the concentration of fresh pomegranate juice and commercial juice. Specifically, at a 15% concentration of fresh pomegranate juice, both fungal species exhibited higher biomass production compared to other concentrations. A. oryzae achieved a biomass production of 3.33 g/L, while N. intermedia reached 2.45 g/L. On the contrary, it was determined that higher biomass yields (g biomass/mL juice) were obtained with 10% fresh juice concentration. Moreover, both species showed increased biomass production at a 20% concentration of commercial juice.
Regarding the protein contents (Fig. 3b), the results revealed that the protein content of fungal biomass varied between the juice form and fungal species. A. oryzae displayed significantly higher protein content when derived from fresh juice (147.28 g/kg) compared to commercial juice (15.47 g/kg). Similarly, N. intermedia exhibited higher protein content in the biomass derived from fresh juice (128.33 g/kg) compared to commercial juice (19.15 g/kg). These findings suggest that fresh juice provides a more favorable growth media for protein production compared to commercial juice for both A. oryzae and N. intermedia. It is likely that the specific components present in fresh juice play a role in enhancing protein synthesis or utilization by the fungi, leading to the observed higher protein content.
In summary, fresh fruit juice and commercial fruit juice have the potential to be used as feedstock for fungal biomass production; however, the use of fresh fruit juice is more promising for the production of protein-rich biomass.
Cultivation in pomegranate aril
The effects of aril on fungal biomass production were investigated using both supplemented (glucose and yeast extract) and without supplemented media (Fig. 5).
When examining the effects of aril on fungal biomass production using glucose and yeast extract (Fig. 5a), it was observed that R. oligosporus exhibited higher biomass production (4.99 g/L) compared to A. oryzae (3.66 g/L) and N. intermedia (1.65 g/L). However, the solid particles that remained undissolved in the nutrient medium were also recovered and harvested together with the biomass. Therefore, it can be said that lower biomass is obtained compared to juice medium and the presence of aril inhibits fungal biomass production. The protein contents of fungal biomass (A. oryzae, 365.72 g/kg; N. intermedia, 244.35 g/kg; and R. oligosporus, 220.36 g/kg) were also low compared to media containing pomegranate juice.
In the case of pomegranate aril media without supplementation (Fig. 5b), it can be observed that biomass production was comparable for A. oryzae (2.46 g/L) and N. intermedia (2.66 g/L). However, when compared to the media containing aril in supplemented media (Fig. 5a), biomass production remained relatively low. In addition, R. oligosporus, which exhibited higher biomass in the aril experiment with supplemented media, was sensitive in this case, resulting in no biomass production. It is crucial to highlight that obtaining a uniform substrate presented challenges due to the inherent difficulty in thoroughly grinding the seeds.
In conclusion, the investigation into the effects of aril on fungal biomass production revealed some interesting findings. When aril was added to the supplemented media, R. oligosporus showed the highest biomass production, followed by A. oryzae and N. intermedia. However, the overall biomass production was lower compared to the juice experiment, indicating that the presence of aril inhibits fungal biomass production. N. intermedia exhibited the lowest biomass production among the tested strains in this experiment. Furthermore, A. oryzae had the highest protein content, followed by N. intermedia and R. oligosporus. In the unsupplemented media, A. oryzae and N. intermedia demonstrated comparable biomass production, while R. oligosporus did not produce any biomass.
Overall, the findings suggest that the presence of aril has a negative impact on fungal biomass production, regardless of the media composition. Further experiments should be conducted to improve the process and overcome the challenges associated with using aril as a substrate.
Cultivation in pomegranate peel
Pomegranate peel with supplemented media
In the first stage, supplemented media were tested to determine the effects of pomegranate peel on fungal biomass production. It was found that the highest level of harvested solids by A. oryzae was achieved at a 4% peel concentration reaching to 17.03 g/L (Fig. 6). Remarkably, regardless of the peel concentration, glucose was completely consumed, indicating the efficient utilization of this substrate by A. oryzae. In addition, ethanol production showed an increasing trend with the increasing peel concentration, with the highest concentration resulting in the highest ethanol production (Fig. 7). The resistance of A. oryzae to peel can be attributed to its ability to withstand the adverse effects of ellagic acid on the CYP450 enzyme family, as suggested by Bulkan et al. [34].
In the effect of the peel on N. intermedia, the harvested solids obtained reached to 16.5 g/L at 4% peel concentration (Fig. 6b). This finding indicates that incorporating higher concentrations of peel into the cultivation process can lead to a substantial increase in the quantity of harvested solids. Notably, even at a lower concentration of 2% peel, a considerable quantity of solids could still be obtained.
Regarding R. oligosporus, it exhibited the highest harvested solids (18.07 g/L) at the 4% peel condition (Fig. 6c). However, the incomplete consumption of glucose and other sugars could be resulted in an inhibitory effect. Despite this, R. oligosporus exhibited higher ethanol production compared to the two previous fungi; however, the ability to produce ethanol was reduced in the presence of peel (Fig. 7).
When the protein contents of the biomass are examined, it is remarkable that A. oryzae exhibits high protein content of 205.04 g/kg in the biomass obtained from the 4% peel media. On the other hand, R. oligosporus (152.39 g/kg) and N. intermedia (151.93 g/kg) showed slightly lower protein content. These highlight the diversity in protein profiles among different fungal strains and underline the influence of peel as a substrate on the protein composition of biomass. The solids collected were found to be low as they naturally contained pomegranate peel. Similarly, in studies on the production of fungal biomass from fish industry wastes, it was determined that there was a decrease in the protein content of the biomass since it accumulated suspended solid among the filaments [19, 35]. Moreover, it was determined that protein content of fungal biomass was increased to 44.9% from olive oil mill wastewater by removing suspended solids [16]. Similarly, fungal biomass with high protein content can be obtained by recovering sugar and nutrients and removing suspended solids from pomegranate peel.
The role of initial pH without supplementation
The role of the initial pH of the medium in microbial and enzymatic growth, as well as in fungal biomass yield and morphology, has been extensively studied in previous studies [12, 36, 37]. In this study, the effects of initial pH (pH 5.0, 5.5, and 6.0) of the medium using 4% pomegranate peel without any additional supplements on biomass production were investigated (Fig. 8a). A. oryzae and N. intermedia exhibited higher harvested solids at pH 5. Conversely, R. oligosporus showed similar harvested solids across all pH levels, which was also similar to the total amount of harvested solids from the uncultivated peel (Fig. 8b). The same trend was shown in the study conducted by Zhang et al. [36] indicating that biomass production tended to increase under pH 4.5–5 for A. oryzae and not a lot of difference was observed for R. oligosporus in the pH interval of 3.5– 6. However, it is important to note that different fungal strains may have unique adaptation mechanisms and exhibit diverse responses to pH levels. Therefore, when studying the effect of pH on biomass production, it is crucial to consider the specific characteristics of the fungal strain under investigation.
Wet versus dry peel without supplementation
To investigate the effect of wet peel versus dry peel, the fungal cultivation was carried out at pH 5.0 using a concentration of 163.6 g/L of wet peel, equivalent to 40 g/L of dry peel. While the total amount of solids harvested with A. oryzae from medium prepared with dry peel was 15.52 g/L, this amount increased to 19.72 g/L when wet peel was used. There is no statistically significant difference between dry peel and wet peel regarding the harvested solids of N. intermedia (p = 0.668). In R. oligosporus cultivation, the harvested solids were significantly higher when wet peel was used compared to dry peel (p = 0.05). This indicates that R. oligosporus exhibited higher biomass production when wet peel was used compared to dry peel.
The varying responses of A. oryzae, N. intermedia, and R. oligosporus to dry and wet peel highlight the importance of tailoring cultivation conditions based on the specific requirements and preferences of different fungal strains. Further investigation can explore the underlying factors influencing these responses and optimize the use of dry and wet peel substrates to maximize biomass production for each fungal strain. When no fungi were present, the harvested solids obtained solely from the peel were measured at 9.70 g/L. This indicates that the peel itself contributes a certain amount of solid material to the cultivation process, even without the presence of fungal biomass. It is important to consider this baseline measurement when evaluating the impact of fungal growth on harvested solids, as it provides a reference point for assessing the contribution of fungi to overall biomass production.
When comparing the protein content of the biomass obtained, both dry and wet peel showed similar protein content for all three strains (Fig. 9a). These findings demonstrate the differential performance of the fungal strains and highlight the potential of A. oryzae for obtaining higher harvested solids from the wet peel. Five different nitrogen sources were added to media prepared with dry peel to improve both the levels of harvested solids and their protein content.
Nitrogen supplementation
Previous works demonstrated the positive effects of nitrogen supplementation on both enzyme activities and protein contents of biomass of A. oryzae strains cultivated on olive oil mill wastewater [16, 38]. In addition, Zhang et al. [36] reported that nitrogen supplementation to winery wastewater enhanced microbial metabolic efficiency, resulting in increased fungal cell growth and improved utilization of carbon sources. The effects of nitrogen supplementation on protein content (Fig. 9b) and biomass production (Fig. 8c) were investigated using five different nitrogen sources, namely yeast extract, urea, sodium nitrate, ammonium sulphate, and ammonium chloride, through A. oryzae and N. intermedia.
Five different nitrogen additions significantly increased the biomass of A. oryzae to similar levels, with the addition of urea and ammonium sulphate demonstrating the most substantial increase in N. intermedia biomass (Fig. 8c). Among the nitrogen sources tested, yeast extract exhibited the highest increase in both biomass and protein content (198.63 g/kg) of A. oryzae. On the other hand, while urea showed the most significant increase in N. intermedia biomass, yeast extract addition resulted in the highest enhancement of protein content (148.42 g/kg protein). The addition of nitrogen significantly increased the protein content, especially when compared to the protein content of both dry peel (30.89 g/kg) and wet peel (77.44 g/kg) cultivated without nitrogen addition. In summary, the results indicate that different nitrogen sources have significant effects on the protein content and biomass production through A. oryzae and N. intermedia. The choice of nitrogen source is crucial for optimizing the growth and protein production of these fungal strains.
Conclusion
In the fruit processing industry, pomegranate is a potential waste producer due to its significant peel proportion. It can also be an alternative substrate for microbial processes considering the sugar content of the peel. Despite yielding a substantial amount of biomass through cultivation with edible fungi, notably A. oryzae, the peel necessitates nitrogen supplementation to improve its protein content. In addition to peel, other by-products such as pomegranate juice and aril that may occur during the juice processing can also be used for biomass production; however, additional nutrients are needed to obtain biomass from peel. A noteworthy quantity of biomass can also be derived from expired commercial fruit juice; however, the protein content is considerably low compared to fresh pomegranate juice. Furthermore, the biomass derived from pomegranate peel has potential for utilization in food and feed applications, given the edible nature of the peel. For this, product characterization should be performed following scale-up fungal cultivation, and determination of digestibility should also be done.
Data availability
Data supporting the findings of this study are available upon request from the corresponding author.
References
Henchion M, Hayes M, Mullen AM, Fenelon M, Tiwari B. Future protein supply and demand: strategies and factors influencing a sustainable equilibrium. Foods. 2017;6(7):53.
Awasthi MK, Harirchi S, Sar T, Vs V, Rajendran K, Gómez-García R, et al. Myco-biorefinery approaches for food waste valorization: present status and future prospects. Biores Technol. 2022;360: 127592.
Sagar NA, Pareek S, Sharma S, Yahia EM, Lobo MG. Fruit and vegetable waste: bioactive compounds, their extraction, and possible utilization. Compr Rev Food Sci Food Saf. 2018;17(3):512–31.
Kanatt S, Chander R, Sharma A. Antioxidant and antimicrobial activity of pomegranate peel extract improves the shelf life of chicken products. Int J Food Sci Technol. 2010;45:216–22.
Sar T, Kiraz P, Braho V, Harirchi S, Akbas MY. Novel perspectives on food-based natural antimicrobials: a review of recent findings published since 2020. Microorganisms. 2023;11(9):2234.
Çam M, Icyer NC, Fatma E. Pomegranate peel phenolics: microencapsulation, storage stability and potential ingredient for functional food development. LWT-Food Sci Technol. 2014;55(1):117–23.
Panichayupakaranant P, Tewtrakul S, Yuenyongsawad S. Antibacterial, anti-inflammatory and anti-allergic activities of standardised pomegranate rind extract. Food Chem. 2010;123:400–3.
Balaban M, Koc C, Sar T, Akbas MY. Antibiofilm effects of pomegranate peel extracts against B. cereus, B. subtilis, and E. faecalis. Int J Food Sci Technol. 2021;56(10):4915–24.
Lavoro A, Falzone L, Gattuso G, Salemi R, Cultrera G, Leone GM, et al. Pomegranate: a promising avenue against the most common chronic diseases and their associated risk factors. Int J Funct Nutr. 2021;2(2):1–12.
Zarei M, Azizi M, Bashiri-Sadr Z. Studies on physico-chemical properties and bioactive compounds of six pomegranate cultivars grown in Iran. J Food Technol. 2010;8(3):112–7.
Sepúlveda L, Ascacio A, Rodríguez-Herrera R, Aguilera-Carbó A, Aguilar CN. Ellagic acid: biological properties and biotechnological development for production processes. Afr J Biotech. 2011;10(22):4518–23.
Sar T, Larsson K, Fristedt R, Undeland I, Taherzadeh MJ. Demo-scale production of protein-rich fungal biomass from potato protein liquor for use as innovative food and feed products. Food Biosci. 2022;47: 101637.
Andualema B, Gessesse A. Microbial lipases and their industrial applications: review. Biotechnology(Faisalabad). 2012;11:100–18.
Satari B, Karimi K, Taherzadeh MJ, Zamani A. Co-production of fungal biomass derived constituents and ethanol from citrus wastes free sugars without auxiliary nutrients in airlift bioreactor. Int J Mol Sci. 2016;17(3):302.
Papadaki E, Kontogiannopoulos KN, Assimopoulou AN, Mantzouridou FT. Feasibility of multi-hydrolytic enzymes production from optimized grape pomace residues and wheat bran mixture using Aspergillus niger in an integrated citric acid-enzymes production process. Biores Technol. 2020;309: 123317.
Sar T, Ozturk M, Taherzadeh MJ, Ferreira JA. New insights on protein recovery from olive oil mill wastewater through bioconversion with edible filamentous fungi. Processes. 2020;8(10):1210.
Mousavi SN, Parchami M, Ramamoorthy SK, Soufiani AM, Hakkarainen M, Zamani A. Bioconversion of carrot pomace to value-added products: Rhizopus delemar fungal biomass and cellulose. Fermentation. 2023;9(4):374.
Meyer V, Basenko EY, Benz JP, Braus GH, Caddick MX, Csukai M, et al. Growing a circular economy with fungal biotechnology: a white paper. Fungal Biol Biotechnol. 2020;7(1):5.
Sar T, Ferreira JA, Taherzadeh MJ. Conversion of fish processing wastewater into fish feed ingredients through submerged cultivation of Aspergillus oryzae. Syst Microbiol Biomanuf. 2021;1(1):100–10.
Sluiter A, Hames B, Hyman D, Payne C, Ruiz R, Scarlata C et al. Determination of total solids in biomass and total dissolved solids in liquid process samples. National Renewable Energy Laboratory, Golden, CO, USA. NREL Technical Report No.: NREL/TP-510- 42621. 2008.
Chater JM, Merhaut DJ, Jia Z, Mauk PA, Preece JE. Fruit quality traits of ten California-grown pomegranate cultivars harvested over three months. Sci Hortic. 2018;237:11–9.
El Moujahed S, Dinica RM, Cudalbeanu M, Avramescu SM, Msegued Ayam I, Ouazzani Chahdi F, et al. Characterizations of six pomegranate (Punica granatum L.) varieties of global commercial interest in Morocco: pomological, organoleptic, chemical and biochemical studies. Molecules. 2022. https://doi.org/10.3390/molecules27123847.
Martínez JJ, Melgarejo P, Hernández F, Salazar DM, Martínez R. Seed characterisation of five new pomegranate (Punica granatum L.) varieties. Sci Hortic. 2006;110(3):241–6.
Ferrara G, Giancaspro A, Mazzeo A, Giove SL, Matarrese AMS, Pacucci C, et al. Characterization of pomegranate (Punica granatum L.) genotypes collected in Puglia region, Southeastern Italy. Sci Hortic. 2014;178:70–8.
Jalal H, Pal MA, Ahmad SR, Rather M, Andrabi M, Hamdani S. Physico-chemical and functional properties of pomegranate peel and seed powder. Pharma Innov. 2018;7:1127–31.
Rowayshed G, Salama A, Abul-Fadl M, Akila-Hamza S, Emad AM. Nutritional and chemical evaluation for pomegranate (Punica granatum L.) fruit peel and seeds powders by products. Middle East J Appl Sci. 2013;3(4):169–79.
Hasnaoui N, Wathelet B, Jiménez-Araujo A. Valorization of pomegranate peel from 12 cultivars: dietary fibre composition, antioxidant capacity and functional properties. Food Chem. 2014;160:196–203.
Balaban M, Koc C, Sar T, Yesilcimen AM. Screening for bioactive compound rich pomegranate peel extracts and their antimicrobial activities. Johnson Matthey Technol Rev. 2022;66(1):81–9.
Vattem DA, Lin YT, Labbe RG, Shetty K. Antimicrobial activity against select food-borne pathogens by phenolic antioxidants enriched in cranberry pomace by solid-state bioprocessing using the food grade fungus Rhizopus oligosporus. Process Biochem. 2004;39(12):1939–46.
Bulkan G, Yudhanti GT, Sitaresmi S, Millati R, Wikandari R, Taherzadeh MJ. Inhibitory and stimulatory effects of fruit bioactive compounds on edible filamentous fungi: potential for innovative food applications. Fermentation. 2022;8(6):270.
Ferreira JA, Lennartsson PR, Niklasson C, Lundin M, Edebo L, Taherzadeh MJ. Spent sulphite liquor for cultivation of an edible Rhizopus sp. BioResources. 2012;7(1):173–88.
Millati R, Edebo L, Taherzadeh MJ. Performance of Rhizopus, Rhizomucor, and Mucor in ethanol production from glucose, xylose, and wood hydrolyzates. Enzyme Microb Technol. 2005;36(2–3):294–300.
Pietrzak W, Kawa-Rygielska J. Backset valorization in dry-grind ethanol process by co-culture of edible filamentous fungi and fodder yeast. J Clean Prod. 2019;220:376–85.
Bulkan G, Sitaresmi S, Yudhanti GT, Millati R, Wikandari R, Taherzadeh MJ. Enhancing or inhibitory effect of fruit or vegetable bioactive compound on Aspergillus niger and A. oryzae. J Fungi. 2022;8(1):12.
Sar T, Ferreira JA, Taherzadeh MJ. Bioprocessing strategies to increase the protein fraction of Rhizopus oryzae biomass using fish industry sidestreams. Waste Manag. 2020;113:261–9.
Zhang ZY, Jin B, Bai ZH, Wang XY. Production of fungal biomass protein using microfungi from winery wastewater treatment. Biores Technol. 2008;99(9):3871–6.
Nazir MT, Soufiani AM, Ferreira JA, Sar T, Taherzadeh MJ. Production of filamentous fungal biomass with increased oil content using olive oil as a carbon source. J Chem Technol Biotechnol. 2022;97(9):2626–35.
D’Annibale A, Brozzoli V, Crognale S, Gallo AM, Federici F, Petruccioli M. Optimisation by response surface methodology of fungal lipase production on olive mill wastewater. J Chem Technol Biotechnol. 2006;81(9):1586–93.
Acknowledgements
This research was funded by the Swedish Agency for Economic and Regional Growth (20201656) through a European Regional Development Fund.
Funding
Open access funding provided by University of Boras. Open access funding provided by University of Borås. This study was supported by Tillväxtverket, 20201656.
Author information
Authors and Affiliations
Contributions
VB: methodology, formal analysis, investigation, data curation, writing—original draft. TS: conceptualization, investigation, validation, writing—revision and editing, supervision. MJT: conceptualization, writing—revision and editing, project administration, supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Braho, V., Sar, T. & Taherzadeh, M.J. Cultivation of edible filamentous fungi on pomegranate by-products as feedstocks to produce mycoprotein. Syst Microbiol and Biomanuf 4, 675–686 (2024). https://doi.org/10.1007/s43393-023-00212-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43393-023-00212-0