Abstract
Mountain systems around the world represent very important research models because they are hot spots for biological diversity. Understanding how animals communities change across environmental variation (e.g., elevational gradients) is central. Currently, the knowledge of the Mexican avian diversity is incomplete due to the absence of detailed studies and inventories in regions such as the mountainous systems of central Mexico. These surveys represent a simple and effective measure to estimate the diversity and perform as a basis for ecological research, as well as to determine priority areas for biological conservation. Here, we sampled 113 points divided into seven elevational segments ranging from 1000 to 3100 to assess differences along elevation and between seasons. We expected to find a gradual turnover of species, as well as a monotonic decrease in richness with respect to altitude. We obtained a total of 100 bird species representing 23% of the species registered for the state and 30% of the species registered for the Reserva de la Biosfera Sierra Gorda. We observed differences in species composition only in the extremes of the gradient. We recorded highest richness values towards the middle part of the gradient decreasing with elevation in winter. The results of this work contribute to increase the knowledge about bird diversity in the state of Querétaro, and highlights the importance of diversity analysis at different levels, such α and β diversity, through altitudinal clines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In community ecology, mountain systems around the world represent very important research models because they are hot spots for biological diversity (Körner 2004; Fjeldså et al. 2012). For decades, the study of elevational gradients in these systems has focused on documenting ecological aspects such as the change in species richness and abundance within elevational clines, as well as the biotic and abiotic factors that limit the distribution of species through these gradients (e.g., McCain 2009; Quintero and Jetz 2018; Liu et al. 2022).
We acknowledge four main patterns of biological diversity distribution in elevational gradients, among which stands out: maximum values at medium elevations, monotonic decrease with respect to elevation, constant species richness in low-elevation areas with a decrease towards a higher elevation, and, finally, a maximum at mid-elevations with a plateau at low elevations (Grytnes and McCain 2007; McCain and Grytnes 2010). However, according to Sedláček et al. (2023), these patterns can be classified into two categories: the monotonic decrease in the number of species at higher elevations and diversity peaks at intermediate elevations.
Biological community composition is driven by the interaction of abiotic filtering, variation in environmental conditions, and biological interactions (Pavoine and Bonsall 2011; Rodríguez et al. 2019; Montaño-Centellas et al. 2021). Therefore, species composition at a local scale is linked by the interaction of humidity, temperature, types of vegetation, and anthropic factors (McCain 2009; Laiolo et al. 2018; Hunt et al. 2022). In this sense, faunistic diversity analyses across altitudinal gradients are natural experiments useful to test ecological hypotheses (Navas 2003; Guo et al. 2013) and are relevant to establish the ecological/environmental traits that determine species distribution at different spatial and temporal scales (Kraft et al. 2011).
For birds, the maximum of species richness predictably decreases towards higher elevations, but there is no consensus on a pattern of biological diversity distribution, or the factors that shape changes in species composition within mountain systems (McCain 2009; Quintero and Jetz 2018). These systems in central Mexico represent an ideal model for studying species turnover through altitudinal clines due to their high levels of diversity and endemism in different groups of vertebrates including birds (Flores-Villela and Gerez 1994; Navarro-Sigüenza et al. 2014). Its location within the Mexican transition zone of the Nearctic and Neotropical regions (Morrone and Márquez 2003; Escalante et al. 2005), the confluence of three biogeographic regions (Central Plateu, Sierra Madre Oriental, and Trans-Mexican Volcano Belt; Morrone 2014) provides a complex orography that favors a great environmental diversity, microclimates, and vegetation types contained in a relatively small spatial scale (INE-SEMARNAP 1999; Escalante et al. 2005; Medina-Macías et al. 2010).
The knowledge of the Mexican avian diversity is incomplete due to the absence of detailed studies and inventories in regions such as the mountainous systems of central Mexico. These surveys represent a simple and effective measure to estimate the diversity and perform as a basis for ecological research, as well as to determine priority areas for biological conservation (Rosenstock et al. 2002; Watson 2003). In this survey, we set the following objectives: (1) calculate the gradient diversity values (Hill’s numbers) and compare between seasons, (2) determine if there is a gradual species turnover across the gradient and between seasons, and (3) determine the relationship of richness with elevation between seasons. We expect to find differences in diversity values between elevational segments and a gradual turnover of species across the gradient, as well as a monotonic decrease in richness with respect to altitude.
Materials and methods
Study area
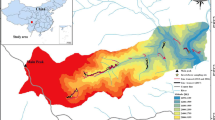
The Sierra Madre Oriental mountain system is in eastern Mexico and extends from Coahuila, Hidalgo, Nuevo León, Puebla, Querétaro, San Luis Potosí, to Veracruz (Morrone 2014). Some of the higher mountains rise abruptly reaching 3600 m above sea level (masl; Goldman and Moore 1945). Municipality of Pinal de Amoles is in the north of the state of Querétaro within the federal protected area Reserva de la Biosfera de la Sierra Gorda, between the parallels 20° 50′ and 21° 45′ north latitude and the meridians 98° 50′ and 100° 10′ west longitude. It is an orographic region characterized by high mountains and wide canyons (INE-SEMARNAP 1999); in addition, it presents an important physiographic complexity with a major altitudinal range from 1000 to 3100 masl. The altitudinal gradient was divided into seven segments separated by approximately 300 m altitude (Fig. 1).
(a) Location of Querétaro within Mexico. (b) Location of the municipality of Pinal de Amoles within Querétaro. (c) Elevational segments analyzed in this work. The intervals of each segment (masl) are indicated in parentheses. The circles correspond to the lower level of the gradient while the triangles correspond to the upper level
The types of climate at elevations above 1800 m above sea level (segments 4, 5, 6 and 7) are temperate sub-humid with summer rains C(w2) and C(w2)(w), with warm summer and little winter precipitation; while in lower parts (segments 1, 2, and 3) they are semi-warm sub-humid (A)C(w0) and (A)C(w1) with summer rains (INEGI 1986). Precipitation ranges from 883 to 313 mm, in sites with high and low elevations, respectively (INE-SEMARNAP 1999). However, the steep slopes and the type of soil do not favor water retention in most of the lower areas of the gradient, except in its final part (segment 1), where the permanent tributary of the Extoraz River is located. Throughout the entire gradient, it is possible to find human settlements and associated agricultural areas, which are almost absent towards the extremes of the gradient (segments 1 and 6).
The vegetation of the study area changes with altitude, with riparian forest in the lower segments, up to a pine-oak forest in its highest portion (Fig. 2). Segment 1 is composed mainly of gallery forest associated to the Extoraz River, and rosetophyllous and crasicaule scrub, composed mainly of Dasylirion sp., Hechtia sp., Stenocereus sp., and Prosopis sp.; it is also possible to find crops on the banks of the stream, together with some human settlements close to the Bucareli community, made up of Mangifera sp., Carica sp., Musa sp., and Pouteria sp. In segment 2, we can find Juniperus forest, with elements of xerophytic scrub being Mimosa sp., Stenocereus sp., Yucca sp., Acacia sp., and Dasylirion sp. In segment 3, we found Juniperus forest and elements of piedmont scrub, as well as xeric scrub, with secondary grasslands, as well as human settlements surrounded by fields of corn cultivation and reforestation with Cupressus trees. In segment 4, there are agriculture fields, with Juniperus forest 4–8-m high and piedmont scrub. In segment 5, it is possible to observe the presence of human settlements and it is made up of pine forest, oak forest, pine-oak forest, and Juniperus forest; along the road there are Cupressus spp., Pinus spp., Eucaliptus spp., Ligustrum spp., and Prunus spp., as well as agriculture fields, the shrub layer is almost zero due to the presence of livestock and consists of Baccharis sp., Buddleia sp., Senecio sp., and Agave sp. with a height of about 1.5 m. In segment 6, it is possible to observe pine-oak forest with some patches of submontane scrub, Juniperus and Quercus, pine-oak forest, pine forest, with a shrub substratum of Cercocarpus sp., Baccharis sp., Buddleia sp., Agave sp., Arbutus sp., and Senecio sp. Segment 7, which corresponds to the highest part of the gradient, consists of a pine-oak forest, made up of a tree layer from 8- to 15-m high, with a shrub sublayer from 0.5 to 2 m, with dominant species such as Cercocarpus. Sp., Arbutus sp., Baccharis sp., Senecio sp., and Buddleia sp.
Field work
The study area is located between the extreme coordinates 21° 9′ 29″–21° 2′ 7″ N and 99° 41′ 21″–99° 37′ 5″ W. We consider two elevation levels of the segments that make up the gradient, the lower level, which includes segments 1, 2, and 3 (1000–1900 masl) and the upper level, which includes segments 4, 5, 6, and 7 (1900–3100 masl). The survey was carried out through direct observation at points with a fixed radius of 25 m and a distance between points of 150–200 m (Bibby et al. 1998), where all birds observed and heard were counted for 10 min. We surveyed 113 points distributed unevenly and according to terrain conditions. Points were sampled on four occasions, two during the rainy season from May to August 2014 (spring–summer), and two in the dry season (winter) from January to February 2015. To avoid biases due to the time or by the observers, we performed the samplings from 7:00 am to 11:00 am, alternating the time and the sampled points with two teams of two people accumulating a total of 544 h/person. We used 10 × 42 mm binoculars and digital cameras (Nikon P600). We determined species using photographs obtained during field outings and based on the identification guides of Howell and Webb (1995) and Sibley (2003). A species list was ordered following the criteria of the AOU (2022).
Data analysis
To determine the inventory completeness, we use the non-parametric estimators Chao1 and ACE because they have high precision regardless of the degree of data aggregation (Hortal et al. 2006). These estimators were calculated with the EstimateS 9.1.0 program (Colwell 2013) by segment and season.
Beta diversity has been a widely used and loosely defined concept encompassing multiple underlying concepts and its exact interpretation and quantification varies substantially across studies (see Tuomisto 2010a,b for a detailed review on this topic). In this work, we estimated Hill’s numbers as a measure of β diversity since it is a simple way to calculate and compare several communities that, in this case, are represented by elevational segments. As the diversity order increases, values become more sensitive to common species. When q = 0, only number of species is evaluated; q = 1, all species abundance is equally weighted (Shannon’s diversity for taxonomic diversity); q = 2, common species get more weight than rare species (inverse Simpson concentration). We calculated diversities 0, 1, and 2 in the iNEXT software (Hsieh et al. 2016).
To determine if the change in species composition was gradual, we performed paired similarity analysis (ANOSIM) using two estimators, Bray–Curtis, which considers the abundance of species, especially those with low abundances (Gauch and Gauch 1982) and Jaccard (Jaccard 1908) which is a presence/absences estimator. We used the Bonferroni correction to determine differences between segments using the software Past 2.17 (Hammer et al. 2001).
To analyze the trend of species richness with respect to altitude, we measured the fit of the data to the trend line, through a linear regression analysis to detect monotonic changes, to the polynomial (order two) or intermediate maximums in the gradient using Excel®. The best fit (R2) between the values of richness and segment was determined.
Results
We recorded a total of 100 bird species belonging to 5 orders and 27 families. From the total of species, 76% are residents (17% winter residents and 5% summer residents), and 2% migrants in transit. Bird diversity reported in this study corresponds to 23% of the total species registered for the state (Pineda-López et al. 2016).
The better represented families in this study were Emberezidae, Fringilidae, and Parulidae, while the least representatives were Accipitridae, Cathartidae, and Peucedramidae. The most abundant species were Aphelocoma wollweberi, Campylorhynchus gularis, and Melozone fusca; in summer, the most abundant species were Melozone fusca, Aphellocoma wollweberi, and Spinus psaltria; in winter, the most abundant species were Spizella passerina, Aphellocoma wollweberi, and Campylorhynchus gularis (Supplementary Material). According to the Chao2 estimator, sampling completeness was lower for the segments during the summer season. We observed overall high sampling completeness (> 80%) for the elevational gradient.
We did not observe significant differences in Hill’s numbers, neither comparing between elevational segments, diversity orders, or season, except for segment 3 (Fig. 3), where Hill’s numbers for q0 and q1 were higher in winter, and in q3 for summer. In winter, we observed significant differences between q0, q1, and q2, while in summer q0 had significant differences with respect to q1 and q2. (Fig. 4).
Through the ANOSIM, we observed overall similarity between segments using both estimators (Bray–Curtis and Jaccard; R < 0.03). We did not observe differences in medium and low elevations of the gradient (1900–2500 masl) for both estimators, especially with the Jaccard index (Table 1) for both seasons. However, we observed differences in community structure in the upper and lower segments which differed drastically from the others.
Regarding the relationship of species richness and elevation, the model that better fitted our data was the polynomial for summer (R = 0.88, P = 0.00) and winter (R = 0.68, P = 0.00), indicating a greater number of species at intermediate elevations (Fig. 4).
Discussion
Contrary to our predictions, we did not observe notorious differences between diversity orders, either between elevational segments or seasons. We did not find gradual changes in terms of species composition or abundances, and we observed that species composition of higher and lower segments differed from the intermediate segments (1900–2500 masl). Regarding the relationship of richness with elevation, we observed higher richness values at intermediate and lower elevations for both seasons, especially during winter.
In the case of diversity orders, we observed differences only in segment 3, obtaining lower diversity values of q1 for both seasons. These differences are related to the few species recorded, dominant species (e.g., Spizella passerina, n = 50), and rare species. If all the species in our sample had equal abundances, we could expect that the diversity values would be identical to q0. On the other hand, if there were less evenness or a greater number of rare species in a sample, the diversity values of positive qn would decrease considerably, approaching to 1 (Pineda-López and Verdú-Franco 2013). However, our data indicate that in terms of net richness (q0), presence of rare species (q1), and abundance of species (q2), the segments behave in a similar way. Hill’s numbers allow us to make inferences related to gain or loss of diversity between communities, even when there is no statistically significant difference (García-Morales et al. 2011). In this case, we observed that, at higher elevations and during winter, 59% of the diversity inhabiting at lower elevations is lost, while in summer only 29% is lost.
In terms of species composition, it is not surprising the distinctiveness in the extremes of the gradient, where it is possible to observe species of Nearctic affinity (e.g., Melospiza lincolnii, Mniotilta varia, Leiothlypis celata) in the upper segments and species of Neotropical affinity in the lower segments (e.g., Euphonia affinis, Leptotila verreauxi, Pitangus sulphuratus). In vertebrates, species turnover at different scales is widely associated with environmental heterogeneity, precipitation, temperature, and soil type (Rodríguez et al. 2019). Similitude in species composition at intermediate elevations (1900–2500 masl) is consistent with the environmental heterogeneity present on segments 3, 4, and 5, where there are many human settlements, cultivated land, slopes, and the transition of vegetation typical of arid areas to the highland’s forests in a reduced area.
In Mexico, there are few works analyzing bird diversity through altitudinal gradients. It has been observed that the dominant pattern is greater richness at low altitudes with a monotonic decrease with respect to altitude (Navarro 1992; Medina-Macías et al. 2010). Similar results have been observed in Sinaloa and Durango (Medina-Macías et al. 2010), Jalisco (Loera-Casillas et al. 2022), and Guerrero (Almazán-Núñez et al. 2020), presenting higher diversity values in intervals of 300–900 masl, 1000–1400 masl, and 1000–1600 masl respectively. On the other hand, there is a tendency to observe lower diversity values at elevations > 1700 masl (e.g., Medina-Macías et al. 2010; Almazán-Núñez et al. 2020). However, and despite these works do not use the diversity metrics presented in this study or were performed far from the study area, they represent the only reference of a similar study in the mountain systems of Mexico. To the knowledge of the authors, this work represents the first analysis of bird diversity in an altitudinal gradient of central Mexico using effective number of species.
It is known that at large scales, β diversity and species richness are higher in mountainous areas, where differences in elevation and temperature occur over small areas (Melo et al. 2009; Graham et al. 2014). Elevation and temperature are not elements that directly determine the diversity but should be considered as a surrogate of heterogeneity and, therefore, the transition between habitats. In addition to the environmental heterogeneity in the study area, the effect of human settlements and activities (e.g., around houses, backyard gardens, and paddocks) can be reflected in bird’s species abundance and composition. Among the common species associated with some levels of anthropization, the following stand out: Columbina inca, Hirundo rustica, Molothurs aeneus, Pyrocephalus rubinus, and Passer domesticus. For example, P. domesticus was the only exotic species found in the gradient and recorded exclusively on segment 1, which is the site with the largest rural settlement; C. inca was found on segments 1 to 5, but its higher abundance was on segment 1; M. aeneus was found only in sites with populated areas, on segments 1 to 4. However, a detailed study involving the effects of human footprint is required.
Determining the differences between communities has become a central issue in ecology and, for this reason, using different approaches to analyze a community considering the abundance, richness, structure, and its association with environmental variables allows us to have a broader view about the factors that may affect bird communities. Currently, a total of 431 bird species are known for the state of Querétaro, of which 347 species are found within the RBSG, which makes it an area of great importance since it is home to more than 80% of the species reported for the state (Pineda-López et al. 2016), in addition to having the largest number of endemic species and species under some category of protection according to Mexican laws (Almazán-Núñez et al. 2013).
References
Almazán-Núñez RC, De Aquino SL, Ríos-Muñoz CA, Navarro-Sigüenza AG (2013) Áreas potenciales de riqueza, endemismo y conservación de las aves del estado de Querétaro. Interciencia 38:26–34
Almazán-Núñez RC, Alvarez-Alvarez, EA, Sierra-Morales P, Rodríguez-Godínez R, Ruíz-Reyes DC, Peñaloza-Montaño M, Salazar-Miranda RI, Morales-Martínez M, López-Flores AI, Gómez-Mendoza JI, Poblete-López DK,Estrada-Ramírez A (2020) Diversidad alfa y beta de la avifauna en bosques tropicales húmedos y semihúmedos de la sierra de Atoyac, una región prioritaria para la conservación del sur de México. Revista mexicana de biodiversidad, 91.
AOU (American Ornithologist’s Union) (2022) Check-list of North American birds. http://www.aou.org/cheklist/north/full.php. Accessed 20 December 2022
Bibby CJ, Marsden S, Jones M (1998) Bird surveys. Expedition Advisory Centre
Colwell RK (2013) EstimateS: statistical estimation of species richness and shared species from samples. Version 9. http:// purl.oclc.org/estimates Accessed 5 May 2016
Escalante T, Rodríguez G, Morrone JJ (2005) Las provincias biogeográficas del componente mexicano de montaña desde la perspectiva de los mamíferos continentales. Rev Mex Biodivers 76:199–205
Fjeldså J, Bowie RC, Rahbek C (2012) The role of mountain ranges in the diversification of birds. Annu Rev Ecol Evol Syst 43:249–265. https://doi.org/10.1146/annurev-ecolsys-102710-145113
Flores-Villela O, Gerez P (1994) Biodiversidad y conservación en México: vertebrados, vegetación y uso del suelo. CONABIO-UNAM, México
García-Morales R, Moreno CE, Bello-Gutiérrez J (2011) Renovando las medidas para evaluar la diversidad en comunidades ecológicas: el número de especies efectivas de murciélagos en el sureste de Tabasco, México. Therya 2:205–215
Gauch HG, Gauch Jr HG (1982) Multivariate analysis in community ecology (No. 1). Cambridge University Press, New York
Goldman EA, Moore RT (1945) The biotic provinces of Mexico. J Mammal 26:347–360
Graham CH, Carnaval AC, Cadena CD, Zamudio KR, Roberts TE, Parra JL, McCain C, Bowie CK, Moritz C, Baines BS, Schneider JC, VanDerWal J, Rhabek C, Kozak KH, Sanders NJ (2014) The origin and maintenance of montane diversity: integrating evolutionary and ecological processes. Ecography 37:711–719. https://doi.org/10.1111/ecog.00578
Grytnes J, McCain CM (2007) Elevational trends in biodiversity. Elsevier, Encyclopedia of biodiversity
Guo Q, Kelt DA, Sun Z, Liu H, Hu L, Ren H, Wen J (2013) Global variation in elevational diversity patterns. Sci Rep 3:3007. https://doi.org/10.1038/srep03007
Hammer O, Harper DAT, Ryan PD (2001) PAST: Paleontological Statistics software package for education and data analysis. Paleontol Electron 4:1–9
Hortal J, Borges PA, Gaspar C (2006) Evaluating the performance of species richness estimators. J Anim Ecol 75:274–287. https://doi.org/10.1111/j.1365-2656.2006.01048.x
Howell SNG, Webb S (1995) A guide to the birds of Mexico and Northern Central America. Oxford University Press, Oxford
Hsieh TC, Ma KH, Chao A (2016) iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers). Meth Ecol Evol 7:1451–1456. https://doi.org/10.1111/2041-210X.12613
Hunt ML, Blackburn GA, Siriwardena GM, Carrasco L, Rowland CS (2022) Using satellite data to assess spatial drivers of bird diversity. Remote Sens Ecol Conserv 9:483–500. https://doi.org/10.1002/rse2.322
INEGI (Instituto Nacional de Estadística, Geografía e Informática) (1986) Síntesis Geográfica, Nomenclator y Anexo Cartográfico del Estado de Querétaro. México
INE-SEMARNAP (Instituto Nacional de Ecología-Secretaría de Medio Ambiente, Recursos Naturales y Pesca) (1999) Programa de manejo, Reserva de la Biosfera Sierra Gorda. INE, Ciudad de México
Jaccard P (1908) Nouvelles recherches sur la distribution florale. BullSoc Vaud Sci Nat 44:223–270
Körner C (2004) Mountain biodiversity, its causes and function. Ambio 33:11–17. https://doi.org/10.1007/0044-7447-33.sp13.11
Kraft NJ, Comita LS, Chase JM, Sanders NJ, Swenson NG, Crist TO, Cornell HV (2011) Disentangling the drivers of β diversity along latitudinal and elevational gradients. Science 333:1755–1758. https://doi.org/10.1126/science.1208584
Laiolo P, Pato J, Obeso JR (2018) Ecological and evolutionary drivers of the elevational gradient of diversity. Ecol Lett 21:1022–1032. https://doi.org/10.1111/ele.12967
Liu W, Yu D, Yuan S, Yi J, Cao Y, Li X, Xu H (2022) Effects of spatial fragmentation on the elevational distribution of bird diversity in a mountain adjacent to urban areas. Ecol Evol 12:e9051. https://doi.org/10.1002/ece3.9051
Loera-Casillas J, Contreras-Martínez S, Favela-García F, Cuevas-Guzmán R (2022) Diversidad de aves en un gradiente altitudinal en la Reserva de la Biosfera Sierra de Manantlán, México. Rev Biol Trop 70:114–131. https://doi.org/10.15517/rev.biol.trop.v70i1.47684
McCain CM (2009) Global analysis of bird elevational diversity. Glob Ecol Biogeogr 18:346–360. https://doi.org/10.1111/j.1466-8238.2008.00443.x
McCain CM, Grytnes JA (2010) Elevational gradients in species richness. Encycl Life Sci. https://doi.org/10.1002/9780470015902.a0022548
Medina-Macías MN, González-Bernal MA, Navarro-Sigüenza AG (2010) Distribución altitudinal de las aves en una zona prioritaria en Sinaloa y Durango, México. Rev Mex Biodivers 81:487–503
Melo AS, Rangel TFLVB, Diniz-Filho JAF (2009) Environmental drivers of beta-diversity patterns in New-World birds and mammals. Ecography 32:226–236. https://doi.org/10.1111/j.1600-0587.2008.05502.x
Montaño‐Centellas FA, Loiselle BA, Tingley MW (2021) Ecological drivers of avian community assembly along a tropical elevation gradient. Ecography 44:574–588. https://doi-org.pbidi.unam.mx:2443/10.1111/ecog.05379
Morrone JJ (2014) Biogeographical regionalization of the Neotropical region. Zootaxa 3782:1–110. https://doi.org/10.11646/zootaxa.3782.1.1
Morrone JJ, Márquez J (2003) Aproximación a un atlas biogeográfico mexicano: componentes bióticos principales y provincias biogeográficas. In: Morrone JJ, Llorente J (eds) Una perspectiva latinoamericana de Biogeografía. Universidad Nacional Autónoma de México, Mexico City, pp 217–220
Navarro AG (1992) Altitudinal distribution of birds in the Sierra Madre del Sur, Guerrero, Mexico. Condor 29–39. https://doi.org/10.2307/1368793
Navarro-Sigüenza AG, Rebón-Gallardo MF, Gordillo-Martínez A, Peterson AT, Berlanga-García H, Sánchez-González LA (2014) Biodiversidad de aves en México. Rev Mex Biodivers 85:476–495
Navas CA (2003) Herpetological diversity along Andean elevational gradients: links with physiological ecology and evolutionary physiology. Comp Biochem Physiol Part A 133:469–485. https://doi.org/10.1016/S1095-6433(02)00207-6
Pavoine S, Bonsall MB (2011) Measuring biodiversity to explain community assembly: a unified approach. Biol Rev 86:792–812. https://doi.org/10.1111/j.1469-185X.2010.00171.x
Pineda-López R, Navarro-Siguenza AG, Arellano-Sanaphre A, Pedraza-Ruiz R (2016) Avifauna de Querétaro. In: Jones RW, Serrano, V (eds) Historia Natural del Estado de Querétaro. Universidad Autónoma de Querétaro, Querétaro
Pineda-López, R, Verdú-Franco, JR. (2013) Cuaderno de prácticas. Medición de la biodiversidad: diversidad alfa, beta y gama. Editorial Universitaria, Querétaro.
Quintero I, Jetz W (2018) Global elevational diversity and diversification of birds. Nature 555:246–250. https://doi.org/10.1038/nature25794
Rodríguez P, Ochoa-Ochoa LM, Munguía M, Sánchez-Cordero V, Navarro-Sigüenza AG, Flores-Villela OA, Nakamura M (2019) Environmental heterogeneity explains coarse–scale β–diversity of terrestrial vertebrates in Mexico. Plos One 14:e0210890. https://doi.org/10.1371/journal.pone.0210890
Rosenstock SS, Anderson DR, Giesen KM, Leukering T, Carter MF (2002) Landbird counting techniques: current practices and an alternative. Auk 119:46–53. https://doi.org/10.1093/auk/119.1.46
Sedláček O, Pernice R, Ferenc M, Mudrová K, Motombi FN, Albrecht T, Hořák D (2023) Abundance variations within feeding guilds reveal ecological mechanisms behind avian species richness pattern along the elevational gradient of Mount Cameroon. Biotropica 55:706–718. https://doi.org/10.1111/btp.13221
Sibley DA (2003) The Sibley field guide to birds of western North America. Chanticleer Press, New York
Tuomisto H (2010) A diversity of beta diversities: straightening up a concept gone awry. Part 1. Defining beta diversity as a function of alpha and gamma diversity. Ecography 33:2–22. https://doi.org/10.1111/j.1600-0587.2009.05880.x
Watson DM (2003) The ‘standardized search’: an improved way to conduct bird surveys. Austral Ecol 28:515–525. https://doi.org/10.1046/j.1442-9993.2003.01308.x
Acknowledgements
We thank the Fund for the Reinforcement of Research (Fondo para el Fortalecimiento de la Investigación) of the Universidad Autónoma de Querétaro for the funding granted for the field work (Project UAQ-FNB201404), and to the Biology, Management, and Conservation of Native Fauna in Anthropic Environments Theme Network (Red Temática Biología Manejo y Conservación de Fauna Nativa en Ambientes Antropizados; REFAMA) and to Consejo Nacional de Ciencia y Tecnología (CONACYT) for the support granted during the writing of the manuscript (Project CONACYT 2718459). We also thank all the kind people of Sierra Gorda Biosphere Reserve for their hospitality and useful comments during our field work.
Funding
The research leading to these results received funding from Universidad Autónoma de Querétaro under Grant Agreement No. UAQ-FNB201404. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by RPL, MT-R, AA-R, AMSC, and AOF. The first draft of the manuscript was written by AA-R and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Marcos Santos
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pineda-López, R., Tepos-Ramírez, M., Acosta-Ramírez, A. et al. Elevational and seasonal changes in a bird assemblage within a mountain system in central Mexico. Ornithol. Res. 31, 274–281 (2023). https://doi.org/10.1007/s43388-023-00151-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43388-023-00151-3