Abstract
Glioblastoma (GBM) is the most aggressive type of central nervous system tumor. Molecular targeting may be important when developing efficient GBM treatment strategies. Sequencing of GBMs revealed that the receptor tyrosine kinase (RTK)/RAS/phosphatidylinositol-3-kinase pathway was altered in 88% of samples. Interestingly, AXL, a member of RTK, was proposed as a promising target in glioma therapy. However, the molecular mechanism of AXL modulation of GBM genesis and proliferation is still unclear. In this study, we investigated the expression and localization of hypoxia-inducible factor-1 alpha (HIF-1α) by AXL in GBM. Both AXL mRNA and protein are overexpressed in GBM. Short-interfering RNA knockdown of AXL in U251-MG cells reduced viability and migration. However, serum withdrawal reduced AXL expression, abolishing the effect on viability. AXL is also involved in hypoxia regulation. In hypoxic conditions, the reduction of AXL decreased the level and nuclear localization of HIF-1α. The co-expression of HIF-1α and AXL was found in human GBM samples but not normal tissue. This finding suggests a mechanism for GBM proliferation and indicates that targeting AXL may be a potential GBM therapeutic.





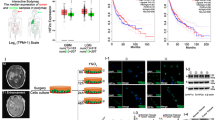
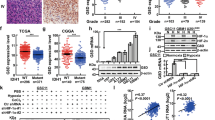
Adapted from cBioPortal: http://www.cbioportal.org/index.do. b Human glioma tissue arrays were immuno-histochemically analyzed in terms of AXL staining. Representative images from samples from two patients are shown. Scale bars: 100 μm. c Human glioma tissue arrays were immunohistochemically analyzed in terms of HIF-1α staining. Representative images from samples from two patients are shown. Scale bars: 100 μm. d the co-expression of AXL mRNA and HIF-1α mRNA from 142 queried glioblastoma (GBM) samples in the TCGA database. e The co-expression of AXL mRNA and HIF-1α mRNA from 182 ovarian serous cystadenocarcinoma queried samples. Adapted from cBioPortal: http://www.cbioportal.org /index.do. f The working model of the role of AXL in GBM survival. Under the control of the tumor microenvironment, such as the nutrient supply and hypoxia conditions, AXL translocates to the nucleus. The inhibition of HIF-1α degradation and the nuclear trans-localization of HIF-1α by hypoxia trigger the expression of AXL. Nuclear HIF-1α promotes the proliferation and survival genes in GBM
Similar content being viewed by others
Data availability
The proper data availability was provided in the figure legends and material and methods section (Fig. 5 legends, M&M).
References
Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, Pekmezci M, Schwartzbaum JA, Turner MC, Walsh KM, Wrensch MR, Barnholtz-Sloan JS (2014) The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol 16:896–913. https://doi.org/10.1093/neuonc/nou087
Ostrom QT, Cote DJ, Ascha M, Kruchko C, Barnholtz-Sloan JS (2018) Adult glioma incidence and survival by race or ethnicity in the united states from 2000 to 2014. JAMA Oncol 4:1254–1262. https://doi.org/10.1001/jamaoncol.2018.1789
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23:1231–1251. https://doi.org/10.1093/neuonc/noab106
Zakharova G, Efimov V, Raevskiy M, Rumiantsev P, Gudkov A, Belogurova-Ovchinnikova O, Sorokin M, Buzdin A (2022) Reclassification of TCGA diffuse glioma profiles linked to transcriptomic, epigenetic, genomic and clinical data, according to the 2021 WHO CNS tumor classification. Int J Mol Sci 24:157. https://doi.org/10.3390/ijms24010157
Mohammed S, Dinesan M, Ajayakumar T (2022) Survival and quality of life analysis in glioblastoma multiforme with adjuvant chemoradiotherapy: a retrospective study. Rep Pract Oncol Radiother 27:1026–1036. https://doi.org/10.5603/RPOR.a2022.0113
Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, Beroukhim R, Bernard B, Wu CJ, Genovese G, Shmulevich I, Barnholtz-Sloan J, Zou L, Vegesna R, Shukla SA, Ciriello G, Yung WK, Zhang W, Sougnez C, Mikkelsen T, Aldape K, Bigner DD, Van Meir EG, Prados M, Sloan A, Black KL, Eschbacher J, Finocchiaro G, Friedman W, Andrews DW, Guha A, Iacocca M, O’Neill BP, Foltz G, Myers J, Weisenberger DJ, Penny R, Kucherlapati R, Perou CM, Hayes DN, Gibbs R, Marra M, Mills GB, Lander E, Spellman P, Wilson R, Sander C, Weinstein J, Meyerson M, Gabriel S, Laird PW, Haussler D, Getz G, Chin L, Network TR (2013) The somatic genomic landscape of glioblastoma. Cell 155:462–477. https://doi.org/10.1016/j.cell.2013.09.034
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, O’Kelly M, Tamayo P, Weir BA, Gabriel S, Winckler W, Gupta S, Jakkula L, Feiler HS, Hodgson JG, James CD, Sarkaria JN, Brennan C, Kahn A, Spellman PT, Wilson RK, Speed TP, Gray JW, Meyerson M, Getz G, Perou CM, Hayes DN, Cancer Genome Atlas Research N (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17:98–110. https://doi.org/10.1016/j.ccr.2009.12.020
Li Y, Ye X, Tan C, Hongo JA, Zha J, Liu J, Kallop D, Ludlam MJ, Pei L (2009) Axl as a potential therapeutic target in cancer: role of Axl in tumor growth, metastasis and angiogenesis. Oncogene 28:3442–3455. https://doi.org/10.1038/onc.2009.212
Hutterer M, Knyazev P, Abate A, Reschke M, Maier H, Stefanova N, Knyazeva T, Barbieri V, Reindl M, Muigg A, Kostron H, Stockhammer G, Ullrich A (2008) Axl and growth arrest-specific gene 6 are frequently overexpressed in human gliomas and predict poor prognosis in patients with glioblastoma multiforme. Clin Cancer Res 14:130–138. https://doi.org/10.1158/1078-0432.CCR-07-0862
Onken J, Torka R, Korsing S, Radke J, Krementeskaia I, Nieminen M, Bai X, Ullrich A, Heppner F, Vajkoczy P (2016) Inhibiting receptor tyrosine kinase AXL with small molecule inhibitor BMS-777607 reduces glioblastoma growth, migration, and invasion in vitro and in vivo. Oncotarget 7:9876–9889. https://doi.org/10.18632/oncotarget.7130
O’Bryan JP, Frye RA, Cogswell PC, Neubauer A, Kitch B, Prokop C, Espinosa R 3rd, Le Beau MM, Earp HS, Liu ET (1991) AXL, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol 11:5016–5031. https://doi.org/10.1128/mcb.11.10.5016-5031.1991
Craven RJ, Xu LH, Weiner TM, Fridell YW, Dent GA, Srivastava S, Varnum B, Liu ET, Cance WG (1995) Receptor tyrosine kinases expressed in metastatic colon cancer. Int J Cancer 60:791–797. https://doi.org/10.1002/ijc.2910600611
Ito T, Ito M, Naito S, Ohtsuru A, Nagayama Y, Kanematsu T, Yamashita S, Sekine I (1999) Expression of the AXL receptor tyrosine kinase in human thyroid carcinoma. Thyroid 9:563–567. https://doi.org/10.1089/thy.1999.9.563
Berclaz G, Altermatt HJ, Rohrbach V, Kieffer I, Dreher E, Andres AC (2001) Estrogen dependent expression of the receptor tyrosine kinase AXL in normal and malignant human breast. Ann Oncol 12:819–824. https://doi.org/10.1023/a:1011126330233
Sun W, Fujimoto J, Tamaya T (2004) Coexpression of Gas6/AXL in human ovarian cancers. Oncology 66:450–457. https://doi.org/10.1159/000079499
Shieh YS, Lai CY, Kao YR, Shiah SG, Chu YW, Lee HS, Wu CW (2005) Expression of AXL in lung adenocarcinoma and correlation with tumor progression. Neoplasia 7:1058–1064. https://doi.org/10.1593/neo.05640
Giles KM, Kalinowski FC, Candy PA, Epis MR, Zhang PM, Redfern AD, Stuart LM, Goodall GJ, Leedman PJ (2013) AXL mediates acquired resistance of head and neck cancer cells to the epidermal growth factor receptor inhibitor erlotinib. Mol Cancer Ther 12:2541–2558. https://doi.org/10.1158/1535-7163.MCT-13-0170
Sainaghi PP, Castello L, Bergamasco L, Galletti M, Bellosta P, Avanzi GC (2005) Gas6 induces proliferation in prostate carcinoma cell lines expressing the AXL receptor. J Cell Physiol 204:36–44. https://doi.org/10.1002/jcp.20265
Falcone I, Conciatori F, Bazzichetto C, Bria E, Carbognin L, Malaguti P, Ferretti G, Cognetti F, Milella M, Ciuffreda L (2020) AXL receptor in breast cancer: molecular involvement and therapeutic limitations. Int J Mol Sci 21. https://doi.org/10.3390/ijms21228419
He L, Lei Y, Hou J, Wu J and Lv G (2020) Implications of the receptor tyrosine kinase Axl in gastric cancer progression. Onco Targets Ther 13:5901–5911. https://doi.org/10.2147/ott.S257606
Chung BI, Malkowicz SB, Nguyen TB, Libertino JA, McGarvey TW (2003) Expression of the proto-oncogene AXL in renal cell carcinoma. DNA Cell Biol 22:533–540. https://doi.org/10.1089/10445490360708946
Mahadevan D, Cooke L, Riley C, Swart R, Simons B, Della Croce K, Wisner L, Iorio M, Shakalya K, Garewal H, Nagle R, Bearss D (2007) A novel tyrosine kinase switch is a mechanism of imatinib resistance in gastrointestinal stromal tumors. Oncogene 26:3909–3919. https://doi.org/10.1038/sj.onc.1210173
Hong CC, Lay JD, Huang JS, Cheng AL, Tang JL, Lin MT, Lai GM, Chuang SE (2008) Receptor tyrosine kinase AXL is induced by chemotherapy drugs and overexpression of AXL confers drug resistance in acute myeloid leukemia. Cancer Lett 268:314–324. https://doi.org/10.1016/j.canlet.2008.04.017
Stone L (2017) Kidney cancer: AXL expression predicts prognosis. Nat Rev Urol 14:700. https://doi.org/10.1038/nrurol.2017.186
Ghiso E, Migliore C, Ciciriello V, Morando E, Petrelli A, Corso S, De Luca E, Gatti G, Volante M, Giordano S (2017) YAP-dependent AXL overexpression mediates resistance to EGFR inhibitors in NSCLC. Neoplasia 19:1012–1021. https://doi.org/10.1016/j.neo.2017.10.003
Kanlikilicer P, Ozpolat B, Aslan B, Bayraktar R, Gurbuz N, Rodriguez-Aguayo C, Bayraktar E, Denizli M, Gonzalez-Villasana V, Ivan C, Lokesh GLR, Amero P, Catuogno S, Haemmerle M, Wu SY, Mitra R, Gorenstein DG, Volk DE, de Franciscis V, Sood AK and Lopez-Berestein G (2017) Therapeutic targeting of AXL receptor tyrosine kinase inhibits tumor growth and intraperitoneal metastasis in ovarian cancer models. Mol Ther Nucleic Acids 9:251–262. https://doi.org/10.1016/j.omtn.2017.06.023
Bao B, Azmi AS, Ali S, Ahmad A, Li Y, Banerjee S, Kong D, Sarkar FH (2012) The biological kinship of hypoxia with CSC and EMT and their relationship with deregulated expression of miRNAs and tumor aggressiveness. Biochim Biophys Acta 1826:272–296. https://doi.org/10.1016/j.bbcan.2012.04.008
Masoud GN, Li W (2015) HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B 5:378–389. https://doi.org/10.1016/j.apsb.2015.05.007
Liu ZJ, Semenza GL, Zhang HF (2015) Hypoxia-inducible factor 1 and breast cancer metastasis. J Zhejiang Univ Sci B 16:32–43. https://doi.org/10.1631/jzus.B1400221
Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL (1996) Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16:4604–4613. https://doi.org/10.1128/MCB.16.9.4604
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70. https://doi.org/10.1016/s0092-8674(00)81683-9
Hanahan D, Folkman J (1996) Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86:353–364. https://doi.org/10.1016/S0092-8674(00)80108-7
Skobe M, Rockwell P, Goldstein N, Vosseler S, Fusenig NE (1997) Halting angiogenesis suppresses carcinoma cell invasion. Nat Med 3:1222–1227. https://doi.org/10.1038/nm1197-1222
Nalwoga H, Ahmed L, Arnes JB, Wabinga H, Akslen LA (2016) Strong expression of hypoxia-inducible factor-1α (HIF-1α) is associated with AXL expression and features of aggressive tumors in African breast cancer. PLoS One 11:e0146823. https://doi.org/10.1371/journal.pone.0146823
Goyette MA, Elkholi IE, Apcher C, Kuasne H, Rothlin CV, Muller WJ, Richard DE, Park M, Gratton JP, Côté JF (2021) Targeting AXL favors an antitumorigenic microenvironment that enhances immunotherapy responses by decreasing Hif-1α levels. Proc Natl Acad Sci USA 118:e2023868118. https://doi.org/10.1073/pnas.2023868118
Raval RR, Lau KW, Tran MG, Sowter HM, Mandriota SJ, Li JL, Pugh CW, Maxwell PH, Harris AL, Ratcliffe PJ (2005) Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol 25:5675–5686. https://doi.org/10.1128/mcb.25.13.5675-5686.2005
Shen C, Beroukhim R, Schumacher SE, Zhou J, Chang M, Signoretti S, Kaelin WG Jr (2011) Genetic and functional studies implicate HIF1α as a 14q kidney cancer suppressor gene. Cancer Discov 1:222–235. https://doi.org/10.1158/2159-8290.cd-11-0098
Mazumder S, Higgins PJ, Samarakoon R (2023) Downstream targets of VHL/HIF-α signaling in renal clear cell carcinoma progression: mechanisms and therapeutic relevance. Cancers (Basel) 15:1316. https://doi.org/10.3390/cancers15041316
Na AY, Yang EJ, Jeon JM, Ki SH, Song KS, Lee S (2018) Protective effect of isoliquiritigenin against ethanol-induced hepatic steatosis by regulating the SIRT1-AMPK pathway. Toxicol Res 34:23–29. https://doi.org/10.5487/TR.2018.34.1.023
Tran Q, Jung JH, Park J, Lee H, Hong Y, Cho H, Kim M, Park S, Kwon SH, Kim SH, Thomas G, Kim KP, Cho MH, Park J (2018) S6 kinase 1 plays a key role in mitochondrial morphology and cellular energy flow. Cell Signal 48:13–24. https://doi.org/10.1016/j.cellsig.2018.04.002
Na CH, Hong JH, Kim WS, Shanta SR, Bang JY, Park D, Kim HK, Kim KP (2015) Identification of protein markers specific for papillary renal cell carcinoma using imaging mass spectrometry. Mol Cells 38:624–629. https://doi.org/10.14348/molcells.2015.0013
Nalwoga H, Ahmed L, Arnes JB, Wabinga H, Akslen LA (2016) Strong expression of hypoxia-inducible factor-1alpha (HIF-1alpha) is associated with AXL expression and features of aggressive tumors in African breast cancer. PLoS One 11:e0146823. https://doi.org/10.1371/journal.pone.0146823
Fernandes G, Fernandes BC, Valente V, Dos Santos JL (2018) Recent advances in the discovery of small molecules targeting glioblastoma. Eur J Med Chem 164:8–26. https://doi.org/10.1016/j.ejmech.2018.12.033
Geraldo LHM, Garcia C, da Fonseca ACC, Dubois LGF, de Sampaio ESTCL, Matias D, de Camargo Magalhaes ES, do Amaral RF, da Rosa BG, Grimaldi I, Leser FS, Janeiro JM, Macharia L, Wanjiru C, Pereira CM, Moura-Neto V, Freitas C, Lima FRS (2019) Glioblastoma therapy in the age of molecular medicine. Trends Cancer 5:46–65. https://doi.org/10.1016/j.trecan.2018.11.002
Brand TM, Iida M, Stein AP, Corrigan KL, Braverman CM, Luthar N, Toulany M, Gill PS, Salgia R, Kimple RJ, Wheeler DL (2014) AXL mediates resistance to cetuximab therapy. Cancer Res 74:5152–5164. https://doi.org/10.1158/0008-5472.Can-14-0294
Zuo Q, Liu J, Huang L, Qin Y, Hawley T, Seo C, Merlino G, Yu Y (2018) AXL/AKT axis mediated-resistance to BRAF inhibitor depends on PTEN status in melanoma. Oncogene 37:3275–3289. https://doi.org/10.1038/s41388-018-0205-4
Mishra A, Wang J, Shiozawa Y, McGee S, Kim J, Jung Y, Joseph J, Berry JE, Havens A, Pienta KJ, Taichman RS (2012) Hypoxia stabilizes GAS6/AXL signaling in metastatic prostate cancer. Mol Cancer Res 10:703–712. https://doi.org/10.1158/1541-7786.MCR-11-0569
Mattila PK, Lappalainen P (2008) Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol 9:446–454. https://doi.org/10.1038/nrm2406
Obacz J, Avril T, Le Reste PJ, Urra H, Quillien V, Hetz C, Chevet E (2017) Endoplasmic reticulum proteostasis in glioblastoma-from molecular mechanisms to therapeutic perspectives. Sci Signal 10:eaal2323. https://doi.org/10.1126/scisignal.aal2323
Acknowledgements
This work was financially supported by a research fund from Chungnam National University (grant to S.H. Kim) and by the Brain Korea 21 PLUS Project for Medical Science, Chungnam National University School of Medicine. This study was also supported by a research fund from Mongolian Foundation for Science and Technology (ShU/x/So-2017/06)
Funding
This work was financially supported by a research fund from Chungnam National University (grant to S.H. Kim) for initial screening and by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MEST) (NRF-2021R1A2C1008492, NRF-2020R1F1A1049801, NRF-2021R1C1C200845611) for the evaluation of AXL function in cell experiments. This study was also supported by a research fund from Mongolian Foundation for Science and Technology (ShU/x/So-2017/06) for bioinformatical analysis.
Author information
Authors and Affiliations
Contributions
Contributes to conception and design: QT, GK, JiP, YH and HL. Acquisition of data, or analysis and interpretation of data: QT, YH, HL, SK, JoP and JiP. Contributions to assist the exam and acquisition of data: CB, DB, CK, SP, SHK and SK. All authors agreed to be accountable for all aspects of the work and all authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vo, TT.T., Tran, Q., Hong, Y. et al. AXL is required for hypoxia-mediated hypoxia-inducible factor-1 alpha function in glioblastoma. Toxicol Res. 39, 669–679 (2023). https://doi.org/10.1007/s43188-023-00195-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43188-023-00195-z