Abstract
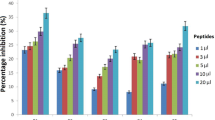
Peptides play important roles in the diagnosis, prognostic predictors, and treatment of various kinds of cancer. Peptides (p.C, p.L and p.14), derived from the phage display peptide libraries, specifically binds to colorectal cancer (CRC) cells in vitro. To allow tumor specificity and selectivity for in vivo diagnosis of CRC, biotinylated p.C, p.L and p.14 were conjugated to AuNPs (14 nm) via the biotin-streptavidin interaction. Male Wistar rats were intravenously injected with a single dose (100 µg/kg body weight) of AuNPs (citrate-AuNPs, PEG-AuNPs, p.C-PEG-, p.L-PEG- and p.14-PEG-AuNPs). Animals were monitored for behavioral changes, and sacrificed either 14 days or 84 days post-injection. Biochemical changes, oxidative stress, and histology of the liver and colon were assessed. No significant changes were noted in the rats injected with all the AuNPs, except p.L-PEG-AuNPs that caused significant toxicity (p < 0.05) 14 days post-exposure when compared to control group, as evidenced by increased relative liver weight, increased malondialdehyde levels and histological changes in the liver. These changes, however, returned to normalcy 84 days post-injection. It can be concluded, based on these findings, that p.L induced a transient toxicity in rats after a single intravenous injection, and can therefore be considered non-toxic long-term after a single exposure.










Similar content being viewed by others
References
Keum N, Giovannucci E (2019) Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol 16:713–732. https://doi.org/10.1038/s41575-019-0189-8
Ferlay J, Soerjomataram I, Dikshit R et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386. https://doi.org/10.1002/ijc.29210
American Cancer Society (2014) Colorectal cancer facts and figures. American Cancer Society, Atlanta
American Cancer Society (2017) Colorectal cancer facts and figures 2017–2019. American Cancer Society, Atlanta
Zauber AG, Winawer SJ, O’Brien MJ et al (2012) Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 366:687–696. https://doi.org/10.1056/NEJMoa1100370
Elia P, Zach R, Hazan S et al (2014) Green synthesis of gold nanoparticles using plant extracts as reducing agents. Int J Nanomedicine 9:4007–4021. https://doi.org/10.2147/IJN.S57343
Thakor AS, Jokerst J, Zavaleta C et al (2011) Gold nanoparticles: a revival in precious metal administration to patients. Nano Lett 11:4029–4036. https://doi.org/10.1021/nl202559p
Alkilany AM, Abulateefeh SR, Mills KK et al (2014) Colloidal stability of citrate and mercaptoacetic acid capped gold nanoparticles upon lyophilization: effect of capping ligand attachment and type of cryoprotectants. Langmuir 30:13799–13808. https://doi.org/10.1021/la504000v
Bohl Kullberg E, Bergstrand N, Carlsson J et al (2002) Development of EGF-conjugated liposomes for targeted delivery of boronated DNA-binding agents. Bioconjug Chem 13:737–743. https://doi.org/10.1021/bc0100713
Goodman CM, McCusker CD, Yilmaz T et al (2004) Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjugate Chem 15:897–900. https://doi.org/10.1021/bc049951i
Arvizo RR, Miranda OR, Moyano DF et al (2011) Modulating Pharmacokinetics, Tumor uptake and biodistribution by engineered nanoparticles. PLoS ONE 6:e24374. https://doi.org/10.1371/journal.pone.0024374
Haggag YA, Matchett KB, Dakir E-H et al (2017) Nano-encapsulation of a novel anti-Ran-GTPase peptide for blockade of regulator of chromosome condensation 1 (RCC1) function in MDA-MB-231 breast cancer cells. Int J Pharm 521:40–53. https://doi.org/10.1016/j.ijpharm.2017.02.006
Comstock SS, Xu D, Hortos K et al (2014) Association of insulin-related serum factors with colorectal polyp number and type in adult males. Cancer Epidemiol Biomark Prev 23:1843–1851. https://doi.org/10.1158/1055-9965.EPI-14-0249-T
Rabinsky EF, Joshi BP, Pant A et al (2016) Overexpressed Claudin-1 can be visualized endoscopically in colonic adenomas in vivo. Cell Mol Gastroenterol Hepatol 2:222–237. https://doi.org/10.1016/j.jcmgh.2015.12.001
Wang W, Guan S, Sun S et al (2014) Detection of circulating antibodies to linear peptide antigens derived from ANXA1 and DDX53 in lung cancer. Tumor Biol 35:4901–4905. https://doi.org/10.1007/s13277-014-1643-4
Zhang M, Li X, Zhang X et al (2014) Association of serum hemoglobin A1c, C-peptide and insulin-like growth factor-1 levels with the occurrence and development of lung cancer. Mol Clin Oncol 2:506–508. https://doi.org/10.3892/mco.2014.289
Roy R, Zurakowski D, Wischhusen J et al (2014) Urinary TIMP-1 and MMP-2 levels detect the presence of pancreatic malignancies. Br J Cancer 111:1772–1779. https://doi.org/10.1038/bjc.2014.462
Han Z, Zhou Z, Shi X et al (2015) EDB fibronectin specific peptide for prostate cancer targeting. Bioconjugate Chem 26:830–838. https://doi.org/10.1021/acs.bioconjchem.5b00178
Michalska M, Florczak A, Dams-Kozlowska H et al (2016) Peptide-functionalized ZCIS QDs as fluorescent nanoprobe for targeted HER2-positive breast cancer cells imaging. Acta Biomater 35:293–304. https://doi.org/10.1016/j.actbio.2016.02.002
Shapira S, Fokra A, Arber N et al (2014) Peptides for diagnosis and treatment of colorectal cancer. Curr Med Chem 21:2410–2416. https://doi.org/10.2174/0929867321666140205134616
Fosgerau K, Hoffmann T (2015) Peptide therapeutics: current status and future directions. Drug Discov Today 20:122–128. https://doi.org/10.1016/j.drudis.2014.10.003
Thundimadathil J (2012) Cancer treatment using peptides: current therapies and future prospects. J Amino Acids 2012:13. https://doi.org/10.1155/2012/967347
Marqus S, Pirogova E, Piva TJ (2017) Evaluation of the use of therapeutic peptides for cancer treatment. J Biomed Sci 24:21. https://doi.org/10.1186/s12929-017-0328-x
Shadidi M, Sioud M (2003) Selective targeting of cancer cells using synthetic peptides. Drug Resist Updat 6:363–371. https://doi.org/10.1016/j.drup.2003.11.002
Mazyambe MK (2013) Evaluating the specificity of cancer cell targeting peptides for applications in cancer diagnostics. University of the Western Cape, Cape Town
Wang J-J, Liu Y, Zheng Y et al (2012) Screening peptides binding specifically to colorectal cancer cells from a phage random peptide library. Asian Pac J Cancer Prev 13:377–381. https://doi.org/10.7314/apjcp.2012.13.1.377
Ferchichi S, Trabelsi H, Azzouz I et al (2016) Evaluation of oxidative response and tissular damage in rat lungs exposed to silica-coated gold nanoparticles under static magnetic fields. Int J Nanomed 11:2711–2719. https://doi.org/10.2147/IJN.S103140
Khan HA, Abdelhalim MAK, Alhomida AS et al (2013) Effects of naked gold nanoparticles on proinflammatory cytokines mRNA expression in rat liver and kidney. BioMed Res Int 2013:590730. https://doi.org/10.1155/2013/590730
Rambanapasi C, Zeevaart JR, Buntting H et al (2016) Bioaccumulation and subchronic toxicity of 14 nm gold nanoparticles in rats. Molecules. https://doi.org/10.3390/molecules21060763
Uchiyama MK, Deda DK, Rodrigues SF et al (2014) In vivo and in vitro toxicity and anti-inflammatory properties of gold nanoparticle bioconjugates to the vascular system. Toxicol Sci 142:497–507. https://doi.org/10.1093/toxsci/kfu202
Yahyaei B, Nouri M, Bakherad S et al (2019) Effects of biologically produced gold nanoparticles: toxicity assessment in different rat organs after intraperitoneal injection. AMB Express 9:38. https://doi.org/10.1186/s13568-019-0762-0
Thovhogi N, Sibuyi N, Meyer M et al (2015) Targeted delivery using peptide-functionalised gold nanoparticles to white adipose tissues of obese rats. J Nanopart Res 17:112. https://doi.org/10.1007/s11051-015-2904-x
Turkevich J, Stevenson PC, Hillier J (1951) A study of the nucleation and growth processes in the synthesis of colloidal gold. Faraday Discuss 11:55–75. https://doi.org/10.1039/DF9511100055
Frens G (1973) Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys Sci 241:20–22. https://doi.org/10.1038/physci241020a0
Sosibo N, Keter F, Skepu A et al (2015) Facile attachment of TAT peptide on gold monolayer protected clusters: synthesis and characterization. Nanomaterials 5:1211–1222. https://doi.org/10.3390/nano5031211
Ghosh S, Patil S, Ahire M et al (2012) Gnidia glauca flower extract mediated synthesis of gold nanoparticles and evaluation of its chemocatalytic potential. J Nanobiotechnol 10:1–9. https://doi.org/10.1186/1477-3155-10-17
Lin Z, Monteiro-Riviere NA, Kannan R et al (2016) A computational framework for interspecies pharmacokinetics, exposure and toxicity assessment of gold nanoparticles. Nanomedicine 11:107–119. https://doi.org/10.2217/nnm.15.177
OECD (2012) Guidance on sample preparation and dosimetry for the safety testing of manufactured nanomaterials. OECD, Paris
Varshney R, Kale RK (1990) Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. Int J Radiat Biol 58:733–743. https://doi.org/10.1080/09553009014552121
Alalaiwe A, Roberts G, Carpinone P et al (2017) Influence of PEG coating on the oral bioavailability of gold nanoparticles in rats. Drug Deliv 24:591–598. https://doi.org/10.1080/10717544.2017.1282554
OECD (2008) Test no. 425: acute oral toxicity: up-and-down procedure. OECD Publishing, Paris
Schmid G, Kreyling WG, Simon U (2017) Toxic effects and biodistribution of ultrasmall gold nanoparticles. Arch Toxicol 91:3011–3037. https://doi.org/10.1007/s00204-017-2016-8
Thi Ha Lien N, Thi Tuyen N, Emmanuel F et al (2012) Capping and in vivo toxicity studies of gold nanoparticles. Adv Nat Sci 3:015002. https://doi.org/10.1088/2043-6262/3/1/015002
Verissimo TV, Santos NT, Silva JR et al (2016) In vitro cytotoxicity and phototoxicity of surface-modified gold nanoparticles associated with neutral red as a potential drug delivery system in phototherapy. Mater Sci Eng C 65:199–204. https://doi.org/10.1016/j.msec.2016.04.030
Kalmodia S, Vandhana S, Tejaswini Rama BR et al (2016) Bio-conjugation of antioxidant peptide on surface-modified gold nanoparticles: a novel approach to enhance the radical scavenging property in cancer cell. Cancer Nanotechnol 7:1. https://doi.org/10.1186/s12645-016-0013-x
Leopold LF, Todor IS, Diaconeasa Z et al (2017) Assessment of PEG and BSA-PEG gold nanoparticles cellular interaction. Colloid Surface A 532:70–76. https://doi.org/10.1016/j.colsurfa.2017.06.061
Jiang W, Hibbert DB, Moran G et al (2013) Characterisation of gold agglomerates: size distribution, shape and optical properties. RSC Adv 3:7367–7374. https://doi.org/10.1039/C3RA22727H
Akrami M, Balalaie S, Hosseinkhani S et al (2016) Tuning the anticancer activity of a novel pro-apoptotic peptide using gold nanoparticle platforms. Sci Rep 6:31030. https://doi.org/10.1038/srep31030
Ghahnavieh MZ, Ajdary M, Ghahnavieh MZ et al (2014) Effects of intraperitoneal injection of gold nanoparticles in male mice. Nanomed J 1:121–127. https://doi.org/10.7508/NMJ.2014.03.001
Adewale OB, Onasanya A, Anadozie SO et al (2016) Evaluation of acute and subacute toxicity of aqueous extract of Crassocephalum rubens leaves in rats. J Ethnopharmacol 188:153–158. https://doi.org/10.1016/j.jep.2016.05.003
Zhang X-D, Wu D, Shen X et al (2011) Size-dependent in vivo toxicity of PEG-coated gold nanoparticles. Int J Nanomed 6:2071–2081. https://doi.org/10.2147/IJN.S21657
Dollah MA, Parhizkar S, Latiff LA et al (2013) Toxicity effect of Nigella sativa on the liver function of rats. Adv Pharm Bull 3:97–102. https://doi.org/10.5681/apb.2013.016
Kwo PY, Cohen SM, Lim JK (2017) ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol 112:18–35. https://doi.org/10.1038/ajg.2016.517
Hanafy A, Aldawsari HM, Badr JM et al (2016) Evaluation of hepatoprotective activity of Adansonia digitata extract on acetaminophen-induced hepatotoxicity in rats. Evid-Based Complement Altern Med 2016:4579149. https://doi.org/10.1155/2016/4579149
Abou Seif HS (2016) Physiological changes due to hepatotoxicity and the protective role of some medicinal plants. Beni-Suef Univ J Basic Appl Sci 5:134–146. https://doi.org/10.1016/j.bjbas.2016.03.004
Ramaiah SK (2007) A toxicologist guide to the diagnostic interpretation of hepatic biochemical parameters. Food Chem Toxicol 45:1551–1557. https://doi.org/10.1016/j.fct.2007.06.007
York MJ (2013) Clinical pathology. In: Faqi AS (ed) A comprehensive guide to toxicology in preclinical drug development, 1st edn. Academic Press, Cambridge, pp 168–206
Okokon JE, Simeon JO, Umoh EE (2017) Hepatoprotective activity of the extract of Homalium letestui stem against paracetamol-induced liver injury. Avicenna J Phytomed 7:27–36. https://doi.org/10.22038/ajp.2016.6950
Kunjiappan S, Bhattacharjee C, Chowdhury R (2015) Hepatoprotective and antioxidant effects of Azolla microphylla based gold nanoparticles against acetaminophen induced toxicity in a fresh water common carp fish (Cyprinus carpio L.). Nanomed J 2:88–110. https://doi.org/10.7508/nmj.2015.02.002
Sefi M, Ben Amara I, Troudi A et al (2014) Effect of selenium on methimazole-induced liver damage and oxidative stress in adult rats and their offspring. Toxicol Ind Health 30:653–669. https://doi.org/10.1177/0748233712462445
Hussein RH, Khalifa FK (2014) The protective role of ellagitannins flavonoids pretreatment against N-nitrosodiethylamine induced-hepatocellular carcinoma. Saudi J Biol Sci 21:589–596. https://doi.org/10.1016/j.sjbs.2014.03.004
Abdelhalim MA, Al-Ayed MS, Moussa SA (2015) The effects of intraperitoneal administration of gold nanoparticles size and exposure duration on oxidative and antioxidants levels in various rat organs. Pak J Pharm Sci 28:705–712
Acknowledgements
This work was supported by the National Research Foundation, South Africa [Grant Number 101132] to Olusola B. Adewale. The authors also acknowledge the technical assistance by Mr. Lawyer Mabulu and Ms. Jabu Madubedube of the Department of Biochemistry and Microbiology, Nelson Mandela University, South Africa, and Prof. M. Meyer of University of the Western Cape, South Africa, for donating the peptides used.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no conflict of interest with this work. This work is contained in the PhD thesis of Olusola B. Adewale at Nelson Mandela University, South Africa. Part of this work has been presented at the 14th International Conference of Nanosciences and Nanotechnologies, 4–7 July 2017, Thessaloniki, Greece, and LAUTECH NANO Conference, 22–24 October 2019, Ogbomoso, Nigeria.
Rights and permissions
About this article
Cite this article
Adewale, O.B., Cairncross, L., Xakaza, H. et al. Short- and long-term effect of colorectal cancer targeting peptides conjugated to gold nanoparticles in rats’ liver and colon after single exposure. Toxicol Res. 38, 259–273 (2022). https://doi.org/10.1007/s43188-021-00108-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43188-021-00108-y