Abstract
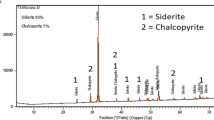
Chalcopyrite is the most abundant copper mineral and its bioleaching has been the subject of several studies due to its refractory nature when submitted to hydrometallurgical processes. Moreover, the growing worldwide demand for copper requires the processing of low-grade ores or mining tailings for which hydrometallurgical processes are a cost-effective alternative. New approaches have shown that the use of chloride ions in chalcopyrite leaching can positively contribute to its dissolution. In this regard, the present work assessed the bioleaching of two chalcopyrite ores—containing 0.34% Cu (copper ore 1) and 1.79% Cu (copper ore 2) by the extreme thermophilic archaea Sulfolobus acidocaldarius. In addition, the effect of different NaCl concentrations (0.25 − 1.0 mol/L) on copper extraction were investigated. In the experiments with the copper ore 1, the copper extractions were higher in the abiotic experiments (from 83 to 90%) than in the biotic experiments (62–80%) for all NaCl concentrations investigated. On the other hand, the experiments with the copper ore 2 showed similar results (around 83% Cu extractions) in all abiotic tests carried out in the presence of chloride and also in the biotic experiments with 0.25 and 0.50 mol/L NaCl concentrations. However, a 97% copper extraction was observed in the biotic experiment carried out with 1.0 mol/L NaCl. Regardless of the type of ore, the lowest copper dissolutions (less than 55%) were obtained in the experiments in the absence of both microorganism and chloride. The XRD analyses of the solid residues of the bioleaching experiments did not reveal the presence of Fe(III)-precipitates such as jarosite.






Similar content being viewed by others
References
Anjum F, Shahid M, Akcil A (2012) Biohydrometallurgy techniques of low grade ores: a review on black shale. Hydrometallurgy 117–118:1–12
Barton IF, Hiskey JB (2022) Chalcopyrite leaching in novel lixiviants. Hydrometallurgy 207:105775
Behrad Vakylabad A, Nazari S, Darezereshki E (2022) Bioleaching of copper from chalcopyrite ore at higher NaCl concentrations. Miner Eng 175:107281
Bevilaqua D, Lahti H, Suegama PH, Garcia O Jr, Benedetti AV, Puhakka JA, Tuovinen OH (2013) Effect of Na–chloride on the bioleaching of a chalcopyrite concentrate in shake flasks and stirred tank bioreactors. Hydrometallurgy 138:1–13
Bobadilla-Fazzini RA, Pérez A, Gautier V, Jordan H, Parada P (2017) Primary copper sulfides bioleaching vs. chloride leaching: advantages and drawbacks. Hydrometallurgy 168:26–31
Carneiro MFC, Leão VA (2007) The role of sodium chloride on surface properties of chalcopyrite leached with ferric sulphate. Hydrometallurgy 87:73–82
Castro C, Donati E (2016) Effects of different energy sources on cell adhesion and bioleaching of a chalcopyrite concentrate by extremophilic archaeon Acidianus copahuensis. Hydrometallurgy 162:49–56
Chang-Li L, Jin-Lan X, Zhen-Yuan N, Yi Y, Chen-Yan M (2012) Effect of sodium chloride on sulfur speciation of chalcopyrite bioleached by the extreme thermophile Acidianus manzaensis. Bioresour Technol 110:462–467
Gericke M, Pinches A, Van Rooyen JV (2001) Bioleaching of a chalcopyrite concentrate using an extremely thermophilic culture. Int J Miner Process 62:243–255
He H, Xia J-L, Hong F-F, Tao X-X, Leng Y-W, Zhao Y-D (2012) Analysis of sulfur speciation on chalcopyrite surface bioleached with Acidithiobacillus ferrooxidans. Miner Eng 27–28:60–64
Konishi Y, Tokushige M, Asai S, Suzuki T (2001) Copper recovery from chalcopyrite concentrate by acidophilic thermophile Acidianus brierleyi in batch and continuous-flow stirred tank reactors. Hydrometallurgy 59:271–282
Lu ZY, Jeffrey MI, Lawson F (2000) The effect of chloride ions on the dissolution of chalcopyrite in acidic solutions. Hydrometallurgy 56:189–202
Martins FL, Leão VA (2023) Chalcopyrite bioleaching in chloride media: a mini-review. Hydrometallurgy 216:105995
Martins FL, Patto GB, Leão VA (2019) Chalcopyrite bioleaching in the presence of high chloride concentrations. J Chem Technol Biotechnol 94:2333–2344
Nicol (2021) The initial stages of the dissolution of chalcopyrite in chloride solutions: validity of mixed potential model and comparison with sulfate solutions. Hydrometallurgy 204:105721
Noguchi H, Okibe N (2020) The role of bioleaching microorganisms in saline water leaching of chalcopyrite concentrate. Hydrometallurgy 195:105397
Parker A, Paul R, Power G (1981) Electrochemical aspects of leaching copper from chalcopyrite in ferric and cupric salt solutions. Aust J Chem 34:13–34
Rubio A, García Frutos FJ (2002) Bioleaching capacity of an extremely thermophilic culture for chalcopyritic materials. Miner Eng 15:689–694
Ruiz MC, Montes KS, Padilla R (2011) Chalcopyrite leaching in sulfate–chloride media at ambient pressure. Hydrometallurgy 109:37–42
Sandström Ã, Shchukarev A, Paul J (2005) XPS characterisation of chalcopyrite chemically and bio-leached at high and low redox potential. Miner Eng 18:505–515
Velásquez-Yévenes L, Nicol M, Miki H (2010) The dissolution of chalcopyrite in chloride solutions: part 1. The effect of solution potential. Hydrometallurgy 103:108–113
Vilcáez J, Suto K, Inoue C (2008) Bioleaching of chalcopyrite with thermophiles: Temperature-pH-ORP dependence. Int J Miner Process 88:37–44
Wang S (2005) Copper leaching from chalcopyrite concentrates. JOM 57:48–51
Wang J, Gan X, Zhao H, Hu M, Li K, Qin W, Qiu G (2016) Dissolution and passivation mechanisms of chalcopyrite during bioleaching: DFT calculation, XPS and electrochemistry analysis. Miner Eng 98:264–278
Wang L, Yin S, Deng B, Wu A (2022) Copper sulfides leaching assisted by acidic seawater-based media: ionic strength and mechanism. Miner Eng 175:107286
Watling HR (2014) Chalcopyrite hydrometallurgy at atmospheric pressure: 2. Review of acidic chloride process options. Hydrometallurgy 146:96–110
Watling HR, Collinson DM, Corbett MK, Shiers DW, Kaksonen AH, Watkin ELJ (2016) Saline-water bioleaching of chalcopyrite with thermophilic, iron(II)-and sulfur–oxidizing microorganisms. Res Microbiol 167:546–554
Xia J-L, Yang Y, He H, Zhao X-J, Liang C-L, Zheng L, MA C-Y, Zhao Y-D, Nie Z-Y, Qiu G-Z (2010) Surface analysis of sulfur speciation on pyrite bioleached by extreme thermophile Acidianus manzaensis using Raman and XANES spectroscopy. Hydrometallurgy 100:129–135
Acknowledgements
This work was supported by Universidade Federal de Ouro Preto. Funding was provided by the agencies Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Financiadora de Estudos e Projetos (FINEP) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). The CNPq scholarship to V. A. Leão is also appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Martins, F.L., Leão, V.A. Bioleaching of two different chalcopyrite ores in chloride media. Braz. J. Chem. Eng. 41, 475–485 (2024). https://doi.org/10.1007/s43153-023-00361-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-023-00361-8