Abstract
Purpose of Review
To highlight the changes in lungs associated with senescent cells and the microbiome that promote a pro-inflammatory milieu and render the aged lungs at risk for debilitating chronic diseases such as cancer, chronic obstructive pulmonary disease, or pulmonary fibrosis.
Recent Findings
Recent studies including “omics” analyses indicate cell type-specific effects of aging and confirm the importance of the inflammation in aged lungs.
Summary
Aging of the lungs is driven by molecular and cellular processes that lead to loss of function and increased risk for diseases. The well-described nine hallmarks of aging are present in the aged lungs. Senescent cells combined with changes in the microbiome create the pro-inflammatory environment previously characterized in aged lungs. The pathobiology of one of the most devastating age-related pulmonary diseases, idiopathic pulmonary fibrosis (IPF), underscores the importance of senescent cells in the aged lungs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging could be described as a deterioration of the function of tissues and organs with an increased risk for diseases. The nine hallmarks of aging, i.e., genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, altered intercellular communication, and stem cell exhaustion, have all been detected in the lungs [1••].
Aging of the lungs is characterized by morphological changes such as airspace enlargement or thickening of the airway walls which directly contribute to the decline of the lung function. Each of the different compartments of the lungs, i.e., airways, parenchyma, and vasculature, shows different aging processes and collectively contributes to their functional decline. In addition, the pulmonary system is one of the few organs with a constant exposure to the outside environment; the impact of the environment on the aging processes is not to be disregarded.
In this review, we will focus on the changes of the microenvironment associated with the presence of senescent cells and of those associated with the lung microbiome, overall creating a more pro-inflammatory environment influencing the behavior of the cells. We will illustrate how these cumulative changes predispose the aged lungs to devastating pulmonary diseases such as pulmonary fibrosis.
Physiological, Age-Related Changes of the Lung
The lung environment is determined by a constant exchange of air and is characterized by a specific architecture, which is essential for the air-blood barrier. The physiologic and cellular composition changes in the aged lungs encompass various compartments that, in the aggregate, tend to lead to an impaired lung function and predisposition to aged-related diseases (Table 1).
The mechanics of the respiratory system supports lung function, mainly characterized by the physiological process of breathing and gas exchange [31]. During inspiration, contraction of the respiratory muscles plays a significant role in inhalation. The strength of the respiratory muscles decreases during aging, and the chest wall compliance decreases [4, 15]. The loss of elasticity leads to heavy breathing with difficulty in expiration [15, 32]. Overall, the physiological changes in function decrease continuously over time and the diverse lung components influence the pulmonary capacity of the aging lung in different ways (Table 1) [4]. In general, the amount of air one can inhale in 1 min (forced expiratory volume in 1 s, FEV1) is impaired during the process of aging as well as the gas exchange determined by the diffusing capacity of carbon monoxide (DLCO) [4].
Two major changes are reported for the airways (trachea, the bronchi, the bronchioles) of the elderly: mucus hypersecretion and decreased ciliary beat frequency (CBF) [5, 9, 33]. In general, ciliary beating and the presence of microvilli protect the deeper lung segments from foreign particles and microorganisms, and a decreased CBF significantly impairs the clearance of the airways, resulting in modification of the microarchitecture of the microtubules.
The pulmonary vasculature (arteries, capillaries, and veins) mainly depends on the hemodynamic conditions of the heart and is thereby affected by the stiffening of the heart and the vessels [10]. During aging, increased rigidity and a decreased vascular compliance are noted [11]. Arterial aging, known as arteriosclerosis, is an aging of the smooth muscle cells in the blood vessels that leads to pulmonary hypertension, resulting in shortness of breath and fatigue [12, 34]. In addition, pulmonary embolisms occur more frequently in the elderly because of the age-related increase in venous thrombosis [35, 36]. The underlying mechanisms of thrombosis involve damaged endothelium and changes in the homeostasis of the blood, which are additionally influenced by high blood pressure [37].
The lung parenchyma consists of thin epithelial cells (alveolar type 1 (AEC1) and type II (AEC2) cells), creating the balloon-shaped alveoli. Interestingly, no destruction of the alveolar wall has been described in the elderly, in contrast to aged mice where the permeability of the blood-air barrier is increased, suggesting a loss of integrity of the alveolar wall [18, 32]. Elevated expression of MMP-14 has been proposed to cause tissue damage and various studies were able to show a negative impact of pro-inflammatory factors on the structure of alveoli [38].
Whether the number of alveoli changes during aging is still unclear. Although findings in mice show a reduction in the number of alveoli during aging, only limited data are currently available for humans. It is unclear whether the number and size of AEC2 are altered [39]. However, recently published transcriptomic data show a decrease in epithelial lung cells, including AEC2, in the aging human lung [40]. Within the alveoli, 95% of the surface is covered with AEC1, which is responsible for the gas exchange. As AEC2 give rise to AEC1 under steady-state conditions, and loss of AEC1 cells is considered a critical factor for aging, this suggests that there is an aged-dependent deficiency in the regenerative potential of AEC2 [17]. The alveolus also consists of an extracellular matrix (ECM) composed of proteoglycans, elastin, and collagen [22,23,24, 41]. The airwall thickness plays a crucial role in the gas exchange. The increase in airway wall thickness with age is associated with the loss of lung function [20]. In addition to the airspace enlargement, the alveolar depth and the alveolar duct radius are enlarged. The alveolar sacs of the elderly show a lower pressure compared to younger alveoli, which impedes mechanical ventilation [19]. However, the decrease in lung volume due to age does not influence the ventilation ratio to perfusion [42].
Cellular Senescence in the Lung
The strict definition of the senescence state for a cell is cell cycle division arrest with a high metabolic activity, leading to the release of multiple soluble and insoluble factors that define the senescent-associated secretory proteome (SASP) in the microenvironment [43, 44]. The composition of the SASP is not only highly variable from one cell type to another but also it changes over time [44]. The SASP is mainly composed of cytokines, growth factors, and extracellular matrix proteins, but other compounds that are not included in these families have also been described [45]. Each of these components has the potential to influence non-senescent cells and thereby promote considerable tissue damage or repair [21, 46]. Markers to identify a senescent cell are not strongly defined or unique [47, 48]. Some of them are more accepted than others, such as p21Waf1, p16INK4a, and Lamin B1. However, classification of senescence requires several characteristics [49]. In aged lungs, the senescence phenotype can stem from DNA damage, reactive oxygen species, mitochondrial dysfunction, or oncogene activation [50].
Although senescent cells have been identified in airways, parenchyma, and vasculature of aging lungs, their relative contribution to the aging phenotype has not been clearly established. However, based on the role of senescent cells in other aged organs or in diseased lungs, it is very likely that autocrine and paracrine effects of neighboring cells could be attributed to senescent cells in aged lungs [50].
In recent years, various animal models have been established to analyze premature senescence. Aged mouse lungs present a similar phenotype (pro-inflammatory milieu and increased extracellular matrix protein expression) as human aged lungs [23, 39]. Unfortunately, neither genetically engineered mouse models where senescent cells can be removed nor progeroid mouse models have revealed mechanistic information on the aging process in the lungs [51•, 52]. Telomerase reverse transcriptase (TERT)- and telomerase RNA component (TERC)-deficient mice demonstrated that telomere function in AEC2 is associated with lung fibrosis [53]. In addition, deletion of the telomere shelterin protein TRF1 in AEC2 mediates age-related lung remodeling and fibrosis [54].
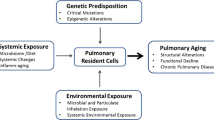
In the following section, we will highlight modifications of the lungs that lead to specific changes in the microenvironment (Fig. 1).
Key differences in the microenvironment of the aging lung. Illustrated are the modifications of the airway, the pulmonary vasculature, the lung parenchyma, and the lung microbiome associated with aging. Original figure created with BioRender.com
The Cells
The lung is composed of a large variety of cells; more than 40 different cell types have been described [55]. An age-associated increase in senescent cells has been recognized in all tissues. As described in several studies, the process of senescence can influence each of these different cells in various ways and most likely affects their specific functions. However, few data on the role of senescent cells in aging lungs are currently available. Assumptions regarding the functional relevance of senescent cells are based on the detection of inflammatory markers present in SASP, such as IL-6 or IL-8 [56]. Senescent cells can also contribute to the diminished tissue-repair capacity of the aged lungs, leading to the development of chronic obstructive pulmonary disease (COPD) or idiopathic pulmonary fibrosis (IPF). As previously mentioned, the lack of specific biomarkers to identify or isolate senescent cells has been a limitation.
In the upper nasopharyngeal part of the lung, the epithelium is composed of columnar epithelia with goblet cells. The bronchi show cuboidal epithelium, which continues to get thinner distally, and the smallest unit, the alveoli, consist of thin AEC1 and AEC2 [57]. Epithelial cells of the lungs are particularly vulnerable to stress-induced cellular senescence [58], resulting in impaired barrier function, decreased ciliary clearance, enhanced mucus production, inflammatory response, and immune cell recruitment. In healthy lungs, AEC2 produce surfactant, but also have stem cell renewal capability that declines with age as they become senescent. Loss of AEC2 and increased low-grade inflammation due to SASP factors, as well as loss of surfactant proteins, could contribute to a more oxidative lung environment and decline in lung function.
The maintenance of local niches (composed of epithelial/mesenchymal cells) that contribute to the integrity of AEC2 is highly dependent on factors secreted by fibroblasts [59, 60]. Especially in chronic lung diseases and aging, the changes in the environment of the niches could inhibit normal tissue regeneration [59]. Associated with senescence, mesenchymal p16INK4a-positive cells were described to stimulate the epithelial progenitor proliferation and act as sentinels for the airway stem cell niche [48]. Driven by cellular senescence, fibroblasts change their morphology to large, flattened, and elongated cells. Senescent fibroblasts secrete ECM, factors that promote ECM production, and ECM-remodeling enzymes, resulting in changes in ECM composition that influence both the structure and the integrity of the aged lungs [21, 43, 61,62,63].
The pulmonary vascular endothelium of the lungs forms a thin layer of squamous cells within the lumen of blood vessels and is therefore essential for the regulation of the blood pressure and the gas exchange. Cellular senescence is described to contribute to the development of vascular diseases such as pulmonary arterial hypertension (PAH) by promoting vascular inflammation with intimal fibrosis and elastin degradation [64]. The subsequent recruitment of immune cells results in further inflammatory processes and hyperproliferation, which together have negative consequences for gas exchange and blood pressure. Recently, Ramadhiana et al. [65] suggested that pathogenesis of PAH involves senescence induced by Notch-mediated juxtacrine signaling.
Alveolar macrophages are being specifically investigated as part of the immunological microenvironment characterization in the lung. The SASP, produced by epithelial/endothelial cells and fibroblasts, modulates the response of immune cells [61]. Within the lungs, stimulation of the resident alveolar macrophages can contribute dramatically to the changes in the microenvironment [66]. Senescent AEC2 induce a profibrotic stimulation of alveolar macrophages [67]. Recently published data highlighted that even the long-lived resident alveolar macrophages are influenced by the microenvironment of the lung, leading to transcriptional changes and substantial macrophage accumulation in IPF [21, 66]. Therefore, a relationship between these processes and the cell-specific effects generated by cellular senescence is conceivable.
The Extracellular Matrix (ECM)
The ECM (laminins, fibronectin, and collagens, etc.) is mainly synthesized by mesenchymal cells such as resident fibroblasts. These proteins are also components of the SASP of many senescent cells [68]. Changes in the ECM can be observed during aging and pathology, in part due to impaired cellular responses [69, 70]. The characteristic excessive ECM deposition observed in aged lungs in mouse models and in elderly human samples has been shown to be a direct consequence of the presence of senescent cells in the lungs [23]. In return, the modified ECM composition contributes to the dysregulated biomechanics of the lungs via the negative impact of senescence on tissue regeneration [71]. Matrix metalloproteinases (MMPs) are relevant SASP components, and they are known to play a crucial role in the degradation of elastin, well described in aged lungs as an emphysema-like loss. Furthermore, other proteases, including a disintegrin and metalloproteinase domain-containing protein 10 (ADAM10), also part of the SASP, are responsible for the cleavage of proteins that form the three-dimensional structure of the ECM [69].
Therefore, senescent cells can influence this sensitive environment by the secretion of a pro-inflammatory SASP as well as ECM proteins. The direct effect of the SASP on the synthesis and degradation of the ECM is supplemented by the indirect effect of the activation of damage-associated molecular patterns (DAMPs). Changes in cell proliferation signaling are mediated by the stimulation of pattern recognition receptors (PRRs), e.g., on fibroblasts [72]. This triggers the stiffening of the tissue associated with the accumulation of ECM.
The Microbiome of the Lung
In recent years, the microbiome has become recognized as an essential factor in aging and longevity [28]. Because the lungs were originally assumed to be sterile, the lung microbiome was neglected entirely for a long time. This assumption has been disproved in the last decade due to increasing evidence that aging has a significant impact on the pulmonary microbiome and, consequently, lung function [73].
The microbiome is defined as all microorganisms in a particular environment (fungi, bacteria, viruses) [74, 75•]. Influenced by various factors such as environment, smoking status, gender, birth, circadian rhythm, and age, the microbiome exhibits great diversity in terms of composition. A high level of biodiversity characterizes a healthy microbiome, and it has been shown that the diversity of the microbiota decreases during aging [27]. This decrease in biodiversity is a known risk factor for the establishment of diseases [27]. The microbiome in aging lungs shows a decrease in anti-inflammatory bacterial genera (e.g., Faecalibacterium, Roseburia), whereas virulent pathogens (e.g., Streptococcaceae, Staphylococcaceae, Enterobacteriaceae) can be detected more frequently [27, 30, 76, 77•]. A substantial amount of data has been published concerning the bacterial microbiota. In contrast, viruses and fungi have not yet been sufficiently investigated [77•].
Interestingly, the gut microbiome is closely related to the lung microbiome, and findings suggest that the gut-lung axis contributes to age-related respiratory diseases [30]. In aging, a dysbiosis of the gut microbiome to obligate and facultative anaerobe bacteria is observed due to nutrition and lifestyle habits [30, 78]. Microbial metabolites of the gut microbiome influence the development of pro-inflammatory immune cells via the bloodstream, which can directly influence infectious diseases in the lungs [74]. In addition to this indirect effect of metabolites, a direct bacterial impact could be observed. Since the barrier function of the intestine and lungs is described as being reduced in the elderly, an easier microbial exchange can result in a loss of compartmentalization [30]. In general, the density of the lung microbiota is considerably lower in the lungs as compared to the gut; however, this density can change due to age-related barrier loss and thus could have a significant impact on the lungs.
In IPF, a coincident gastrointestinal reflux leads to an increase in the bacterial load in the lung. Here, a higher incidence of the pro-inflammatory bacteria Haemophilus, Streptococcus, Neisseria, and Veillonella has been reported [79]. These conditions can lead to an increase in inflammatory lung conditions, contributing to disease progression [80]. Interestingly, Spagnolo et al. suggested that altering the microbiome should be considered a treatment option for IPF patients [81].
Although the majority of microbiome studies have been carried out for the gut, the main findings from these studies on homeostasis, communication, and the interplay between the microbiome and eukaryotic cells may apply to the lung. Although the impact of senescent cells on the lung microbiome has not been analyzed yet, studies conducted on the gut microbiota suggest a potential role of senescence on the microbiome. High mobility group box 1 (HMGB1), a relevant SASP factor, plays a crucial role in protection of the intestine from bacterial infection [82]. In addition, IL-17 mediates communication between the microbiome and the cells in their microenvironment, especially by contributing to cancer pathogenesis [83]. Considerable work has been done to study the crosstalk between the microbiome and the innate immune system. Microbiota promotes the expression of pro- and anti-inflammatory substances, which in turn activate the human innate immune system [84]. For instance, lung resident microbiota such as Pseudomonas and Lactobacillus appears to be responsible for the activation of Th17 response that has been implicated in the susceptibility to develop inflammatory diseases with increasing age [84, 85]. Aged-related factors such as MMP-9, one of the common components of the SASP, have been shown to govern changes in the microbiome in the gut [86].
The influence of the microbiome on the pathogenesis and progression of pulmonary age-related diseases is under investigation. Knowledge of the microbiome composition may eventually provide insights into the course of the disease and/or establish the feasibility of using the microbiome as a biomarker [87].
Idiopathic Pulmonary Fibrosis (IPF) as a Senescence-Associated Disease
Among the age-related diseases in the lungs, IPF is the most devastating and is associated with the worst prognosis, with median survival ranging from 2.5 to 3.5 years [88]. Senescent cells have been one of the newly investigated therapeutic targets to find a cure for IPF, a disease with no treatment other than two FDA-approved drugs that only slow disease progression [89]. The detection of senescent cells (epithelial and mesenchymal cells) has been reported for over a decade, but their function is still elusive [90]. The general agreement is that elements of the SASP contribute to the pathology of IPF, but knowledge of the mechanisms is wanted. Interestingly, the p53 signaling cascade was identified as the most significant pathway contributing to IPF, with this pathway being closely related to AEC senescence [91]. Recently, the secretion by senescent IPF mesenchymal cells of leukotrienes, known eicosanoid inflammatory mediators that promote the activation of mesenchymal cells and the production of collagen, has been reported [21]. In addition, a new subpopulation of basal epithelial cells expressing markers of cellular senescence has been identified by transcriptome analyses [92, 93]. These cells are localized in the honeycombing structure of the lungs, suggesting that they are part of the pathobiology. In IPF, senescent cells have been identified in all cell types (epithelial and mesenchymal cells) by transcriptome analysis, but it is also clear that there is little overlap in the transcriptional phenotype of each cell type [92]. Interest in senescent cells in IPF emerged due to the improvement of fibrosis after removal of senescent cells in mouse models of pulmonary fibrosis [94,95,96,97]. Following successful ablation of senescent cells using the senolytic drugs dasatinib plus quercetin (DQ) in bleomycin-administered mice, DQ was given to fourteen IPF patients in a small clinical trial; although pulmonary function was not altered, physical attributes such as walking and standing were improved, providing initial evidence that senolytics may be clinically useful in IPF [98]. However, the question remains open on the beneficial effect of senolytics (small molecules that selectively induce death of senescent cells) versus senomorphics (small molecules that selectively modulate the phenotypes of senescent cells).
Conclusions
Aging in the lungs is a complex phenomenon, as its impact varies in the different compartments of the lungs. Overall, the pro-inflammatory milieu characteristic of aging organs is documented in the lungs as well. In this review, we have described that senescent cells increase in the aging lung. Furthermore, these cells can influence their environment through release of SASP factors and can provoke changes in the microbiome. Although studies related to the microbiome in aging lungs are very limited, the association of the microbiome with development of age-related diseases in other organs suggests that it likely impacts the lung.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Meiners S, Eickelberg O, Königshoff M. Hallmarks of the ageing lung. Eur Respir J. 2015;45(3):807. https://doi.org/10.1183/09031936.00186914. This paper lays out all the hallmarks of aging accepted by researchers involved in this field.
Pride NB. Ageing and changes in lung mechanics. Eur Respir J. 2005;26(4):563–5. https://doi.org/10.1183/09031936.05.00079805.
Bowdish DME. The aging lung: is lung health good health for older adults? Chest. 2019;155(2):391–400. https://doi.org/10.1016/j.chest.2018.09.003.
Sharma G, Goodwin J. Effect of aging on respiratory system physiology and immunology. Clin Interv Aging. 2006;1(3):253–60. https://doi.org/10.2147/ciia.2006.1.3.253.
Pistelli R, Lange P, Miller DL. Determinants of prognosis of COPD in the elderly: mucus hypersecretion, infections, cardiovascular comorbidity. Eur Respir J. 2003;21(40 suppl):10s. https://doi.org/10.1183/09031936.03.00403403.
Allinson JP, Hardy R, Donaldson GC, Shaheen SO, Kuh D, Wedzicha JA. The presence of chronic mucus hypersecretion across adult life in relation to chronic obstructive pulmonary disease development. Am J Respir Crit Care Med. 2016;193(6):662–72. https://doi.org/10.1164/rccm.201511-2210OC.
Walski M, Pokorski M, Antosiewicz J, Rekawek A, Frontczak-Baniewicz M, Jernajczyk U, et al. Pulmonary surfactant: ultrastructural features and putative mechanisms of aging. J Physiol Pharmacol. 2009;60(Suppl 5):121–5.
Adivitiya KMS, Chakraborty S, Veleri S, Kateriya S. Mucociliary respiratory epithelium integrity in molecular defense and susceptibility to pulmonary viral infections. Biology (Basel). 2021;10(2):95. https://doi.org/10.3390/biology10020095.
Bailey KL, Bonasera SJ, Wilderdyke M, Hanisch BW, Pavlik JA, DeVasure J, Robinson JE, Sisson JH, Wyatt TA. Aging causes a slowing in ciliary beat frequency, mediated by PKCε. Am J Phys Lung Cell Mol Phys. 2014;306(6):L584–L9. https://doi.org/10.1152/ajplung.00175.2013.
Poor H. Pulmonary vascular diseases in the elderly. Clin Geriatr Med. 2017;33(4):553–62. https://doi.org/10.1016/j.cger.2017.06.007.
Miller AP, Huff CM, Roubin GS. Vascular disease in the older adult. J Geriatr Cardiol. 2016;13(9):727–32. https://doi.org/10.11909/j.issn.1671-5411.2016.09.011.
O'Rourke MF. Arterial aging: pathophysiological principles. Vasc Med. 2007;12(4):329–41. https://doi.org/10.1177/1358863x07083392.
Madhuri V, Chandra S, Jabbar A. Age associated increase in intima media thickness in adults. Indian J Physiol Pharmacol. 2010;54(4):371–5.
Janssens JP, Pache JC, Nicod LP. Physiological changes in respiratory function associated with ageing. Eur Respir J. 1999;13(1):197–205. https://doi.org/10.1034/j.1399-3003.1999.13a36.x.
Brandsma C-A, de Vries M, Costa R, Woldhuis RR, Königshoff M, Timens W. Lung ageing and COPD: is there a role for ageing in abnormal tissue repair? Eur Respir Rev. 2017;26(146):170073. https://doi.org/10.1183/16000617.0073-2017.
Suki B, Stamenović D, Hubmayr R. Lung parenchymal mechanics. Compr Physiol. 2011;1(3):1317–51. https://doi.org/10.1002/cphy.c100033.
Navarro S, Driscoll B. Regeneration of the aging lung: a mini-review. Gerontology. 2017;63(3):270–80. https://doi.org/10.1159/000451081.
Parrish AR. The impact of aging on epithelial barriers. Tissue Barriers. 2017;5(4):e1343172. https://doi.org/10.1080/21688370.2017.1343172.
Aghasafari P, Heise RL, Reynolds A, Pidaparti RM. Aging effects on alveolar sacs under mechanical ventilation. J Gerontol: Ser A. 2018;74(2):139–46. https://doi.org/10.1093/gerona/gly097.
Telenga ED, Oudkerk M, van Ooijen PMA, Vliegenthart R, ten Hacken NHT, Postma DS, van den Berge M. Airway wall thickness on HRCT scans decreases with age and increases with smoking. BMC Pulm Med. 2017;17(1):27. https://doi.org/10.1186/s12890-017-0363-0.
Wiley CD, Brumwell AN, Davis SS, Jackson JR, Valdovinos A, Calhoun C, et al. Secretion of leukotrienes by senescent lung fibroblasts promotes pulmonary fibrosis. JCI Insight. 2019;4(24). https://doi.org/10.1172/jci.insight.130056.
Burgstaller G, Oehrle B, Gerckens M, White ES, Schiller HB, Eickelberg O. The instructive extracellular matrix of the lung: basic composition and alterations in chronic lung disease. Eur Respir J. 2017;50(1):1601805. https://doi.org/10.1183/13993003.01805-2016.
Calhoun C, Shivshankar P, Saker M, Sloane LB, Livi CB, Sharp ZD, Orihuela CJ, Adnot S, White ES, Richardson A, Jourdan le Saux C. Senescent cells contribute to the physiological remodeling of aged lungs. J Gerontol A Biol Sci Med Sci. 2016;71(2):153–60. https://doi.org/10.1093/gerona/glu241.
Sherratt MJ. Tissue elasticity and the ageing elastic fibre. Age (Dordr). 2009;31(4):305–25. https://doi.org/10.1007/s11357-009-9103-6.
Wong CK, Smith CA, Sakamoto K, Kaminski N, Koff JL, Goldstein DR. Aging impairs alveolar macrophage phagocytosis and increases influenza-induced mortality in mice. J Immunol (Baltimore, Md : 1950). 2017;199(3):1060–8. https://doi.org/10.4049/jimmunol.1700397.
Wu Y, Zhu B, Zhang R, Goplen NPJ, Wang Z, Li Y, et al. Zom-biecoming: single-cell RNA-sequencing reveals senescence-like features of alveolar macrophages during aging. J Immunol. 2020;204(1 Supplement):235.13.
Santoro A, Zhao J, Wu L, Carru C, Biagi E, Franceschi C. Microbiomes other than the gut: inflammaging and age-related diseases. Semin Immunopathol. 2020;42(5):589–605. https://doi.org/10.1007/s00281-020-00814-z.
Kim M, Benayoun BA. The microbiome: an emerging key player in aging and longevity. Transl Med Aging. 2020;4:103–16. https://doi.org/10.1016/j.tma.2020.07.004.
Lee SY, Mac Aogáin M, Fam KD, Chia KL, Binte Mohamed Ali NA, Yap MMC, et al. Airway microbiome composition correlates with lung function and arterial stiffness in an age-dependent manner. PLoS One. 2019;14(11):e0225636. https://doi.org/10.1371/journal.pone.0225636.
Saint-Criq V, Lugo-Villarino G, Thomas M. Dysbiosis, malnutrition and enhanced gut-lung axis contribute to age-related respiratory diseases. Ageing Res Rev. 2021;66:101235. https://doi.org/10.1016/j.arr.2020.101235.
Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten C, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–68. https://doi.org/10.1183/09031936.05.00035205.
Miller MR. Structural and physiological age-associated changes in aging lungs. Semin Respir Crit Care Med. 2010;31(5):521–7. https://doi.org/10.1055/s-0030-1265893.
Ho JC, Chan KN, Hu WH, Lam WK, Zheng L, Tipoe GL, et al. The effect of aging on nasal mucociliary clearance, beat frequency, and ultrastructure of respiratory cilia. Am J Respir Crit Care Med. 2001;163(4):983–8. https://doi.org/10.1164/ajrccm.163.4.9909121.
Grootaert MOJ, Moulis M, Roth L, Martinet W, Vindis C, Bennett MR, de Meyer GRY. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc Res. 2018;114(4):622–34. https://doi.org/10.1093/cvr/cvy007.
Engbers MJ, van Hylckama VA, Rosendaal FR. Venous thrombosis in the elderly: incidence, risk factors and risk groups. J Thromb Haemost. 2010;8(10):2105–12. https://doi.org/10.1111/j.1538-7836.2010.03986.x.
Yayan J. Relative risk of pulmonary embolism in the very elderly compared with the elderly. Clin Interv Aging. 2013;8:861–70. https://doi.org/10.2147/CIA.S46572.
Turetz M, Sideris AT, Friedman OA, Triphathi N, Horowitz JM. Epidemiology, pathophysiology, and natural history of pulmonary embolism. Semin Interv Radiol. 2018;35(2):92–8. https://doi.org/10.1055/s-0038-1642036.
Yamada M, Fujino N, Ichinose M. Inflammatory responses in the initiation of lung repair and regeneration: their role in stimulating lung resident stem cells. Inflamm Regen. 2016;36(1):15. https://doi.org/10.1186/s41232-016-0020-7.
Schulte H, Mühlfeld C, Brandenberger C. Age-related structural and functional changes in the mouse lung. Front Physiol. 2019;10(1466). https://doi.org/10.3389/fphys.2019.01466.
Chow RD, Majety M, Chen S. The aging transcriptome and cellular landscape of the human lung in relation to SARS-CoV-2. Nat Commun. 2021;12(1):4. https://doi.org/10.1038/s41467-020-20323-9.
Korfei M, MacKenzie B, Meiners S. The ageing lung under stress. Eur Respir Rev. 2020;29(156):200126. https://doi.org/10.1183/16000617.0126-2020.
Cardús J, Burgos F, Diaz O, Roca J, Barberà JA, Marrades RM, et al. Increase in pulmonary ventilation-perfusion inequality with age in healthy individuals. Am J Respir Crit Care Med. 1997;156(2 Pt 1):648–53. https://doi.org/10.1164/ajrccm.156.2.9606016.
Basisty N, Kale A, Jeon OH, Kuehnemann C, Payne T, Rao C, Holtz A, Shah S, Sharma V, Ferrucci L, Campisi J, Schilling B. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020;18(1):e3000599. https://doi.org/10.1371/journal.pbio.3000599.
Coppé J-P, Desprez P-Y, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. https://doi.org/10.1146/annurev-pathol-121808-102144.
Davalos AR, Coppe J-P, Campisi J, Desprez P-Y. Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev. 2010;29(2):273–83. https://doi.org/10.1007/s10555-010-9220-9.
Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, van Steeg H, Dollé MET, Hoeijmakers JHJ, de Bruin A, Hara E, Campisi J. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31(6):722–33. https://doi.org/10.1016/j.devcel.2014.11.012.
Omori S, Wang TW, Johmura Y, Kanai T, Nakano Y, Kido T, et al. Generation of a p16 reporter mouse and its use to characterize and target p16(high) cells in vivo. Cell Metab. 2020;32(5):814–28.e6. https://doi.org/10.1016/j.cmet.2020.09.006.
Reyes de Mochel N, Cheong KN, Cassandras M, Wang C, Krasilnikov M, Matatia P, et al. Sentinel p16INK4a+ cells in the basement membrane form a reparative niche in the lung. bioRxiv. 2020:2020.06.10.142893. https://doi.org/10.1101/2020.06.10.142893.
Kohli J, Wang B, Brandenburg SM, Basisty N, Evangelou K, Varela-Eirin M, Campisi J, Schilling B, Gorgoulis V, Demaria M. Algorithmic assessment of cellular senescence in experimental and clinical specimens. Nat Protoc. 2021;16(5):2471–98. https://doi.org/10.1038/s41596-021-00505-5.
Hansel C, Jendrossek V, Klein D. Cellular senescence in the lung: the central role of senescent epithelial cells. Int J Mol Sci. 2020;21(9):3279. https://doi.org/10.3390/ijms21093279.
Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530(7589):184–9. https://doi.org/10.1038/nature16932. The role of senescent cells in aging was first introduced with this study.
Harkema L, Youssef SA, de Bruin A. Pathology of mouse models of accelerated aging. Vet Pathol. 2016;53(2):366–89. https://doi.org/10.1177/0300985815625169.
Parimon T, Hohmann MS, Yao C. Cellular senescence: pathogenic mechanisms in lung fibrosis. Int J Mol Sci. 2021;22(12):6214. https://doi.org/10.3390/ijms22126214.
Naikawadi RP, Disayabutr S, Mallavia B, Donne ML, Green G, La JL, et al. Telomere dysfunction in alveolar epithelial cells causes lung remodeling and fibrosis. JCI Insight. 2016;1(14). https://doi.org/10.1172/jci.insight.86704.
Venosa A. Senescence in pulmonary fibrosis: between aging and exposure. Front Med (Lausanne). 2020;7:606462. https://doi.org/10.3389/fmed.2020.606462.
Meyer KC, Ershler W, Rosenthal NS, Lu XG, Peterson K. Immune dysregulation in the aging human lung. Am J Respir Crit Care Med. 1996;153(3):1072–9. https://doi.org/10.1164/ajrccm.153.3.8630547.
Ball M, Hossain M, Padalia D. Anatomy, airway. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021.
Campisi J. Cellular Senescence and lung function during aging. Yin and Yang. Ann Am Thorac Soc. 2016;13 Suppl 5(Suppl 5):S402–S6. https://doi.org/10.1513/AnnalsATS.201609-703AW.
Melo-Narváez MC, Stegmayr J, Wagner DE, Lehmann M. Lung regeneration: implications of the diseased niche and ageing. Eur Respir Rev. 2020;29(157):200222. https://doi.org/10.1183/16000617.0222-2020.
Kathiriya JJ, Wang C, Zhou M, Brumwell A, Cassandras M, Le Saux CJ, et al. Human alveolar type 2 epithelium transdifferentiates into metaplastic KRT5(+) basal cells. Nat Cell Biol. 2022;24(1):10–23. https://doi.org/10.1038/s41556-021-00809-4.
Faget DV, Ren Q, Stewart SA. Unmasking senescence: context-dependent effects of SASP in cancer. Nat Rev Cancer. 2019;19(8):439–53. https://doi.org/10.1038/s41568-019-0156-2.
Waters DW, Blokland KEC, Pathinayake PS, Burgess JK, Mutsaers SE, Prele CM, Schuliga M, Grainge CL, Knight DA. Fibroblast senescence in the pathology of idiopathic pulmonary fibrosis. Am J Physiol-Lung Cell Mol Physiol. 2018;315(2):L162–L72. https://doi.org/10.1152/ajplung.00037.2018.
Wilkinson HN, Hardman MJ. Senescence in wound repair: emerging strategies to target chronic healing wounds. Front Cell Dev Biol. 2020;8(773). https://doi.org/10.3389/fcell.2020.00773.
van der Feen DE, Berger RMF, Bartelds B. Converging paths of pulmonary arterial hypertension and cellular senescence. Am J Respir Cell Mol Biol. 2019;61(1):11–20. https://doi.org/10.1165/rcmb.2018-0329TR.
Ramadhiani R, Ikeda K, Miyagawa K, Ryanto GRT, Tamada N, Suzuki Y, et al. Endothelial cell senescence exacerbates pulmonary hypertension through Notch-mediated juxtacrine signaling. bioRxiv. 2021:2021.02.02.429321. https://doi.org/10.1101/2021.02.02.429321.
McQuattie-Pimentel AC, Ren Z, Joshi N, Watanabe S, Stoeger T, Chi M, et al. The lung microenvironment shapes a dysfunctional response of alveolar macrophages in aging. J Clin Investig. 2021;131(4). https://doi.org/10.1172/JCI140299.
Rana T, Jiang C, Liu G, Miyata T, Antony V, Thannickal VJ, Liu RM. PAI-1 regulation of TGF-β1–induced alveolar type II cell senescence, SASP secretion, and SASP-mediated activation of alveolar macrophages. Am J Respir Cell Mol Biol. 2019;62(3):319–30. https://doi.org/10.1165/rcmb.2019-0071OC.
Neri F, Basisty N, Desprez P-Y, Campisi J, Schilling B. Quantitative proteomic analysis of the senescence-associated secretory phenotype by data-independent acquisition. Curr Protoc. 2021;1(2):e32. https://doi.org/10.1002/cpz1.32.
Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15(12):786–801. https://doi.org/10.1038/nrm3904.
Rosmark O, Åhrman E, Müller C, Elowsson Rendin L, Eriksson L, Malmström A, Hallgren O, Larsson-Callerfelt AK, Westergren-Thorsson G, Malmström J. Quantifying extracellular matrix turnover in human lung scaffold cultures. Sci Rep. 2018;8(1):5409. https://doi.org/10.1038/s41598-018-23702-x.
Blokland KEC, Pouwels SD, Schuliga M, Knight DA, Burgess JK. Regulation of cellular senescence by extracellular matrix during chronic fibrotic diseases. Clin Sci (Lond). 2020;134(20):2681–706. https://doi.org/10.1042/cs20190893.
Turner NA. Inflammatory and fibrotic responses of cardiac fibroblasts to myocardial damage associated molecular patterns (DAMPs). J Mol Cell Cardiol. 2016;94:189–200. https://doi.org/10.1016/j.yjmcc.2015.11.002.
Shukla SD, Budden KF, Neal R, Hansbro PM. Microbiome effects on immunity, health and disease in the lung. Clin Transl Immunol. 2017;6(3):e133. https://doi.org/10.1038/cti.2017.6.
Lloyd CM, Marsland BJ. Lung homeostasis: influence of age, microbes, and the immune system. Immunity. 2017;46(4):549–61. https://doi.org/10.1016/j.immuni.2017.04.005.
Berg G, Rybakova D, Fischer D, Cernava T, Vergès M-CC, Charles T, et al. Microbiome definition re-visited: old concepts and new challenges. Microbiome. 2020;8(1):103. https://doi.org/10.1186/s40168-020-00875-0. This study describes a paradigm shift in the understanding of the microbiome and provides important insight into potential participation of the lung microbiome in the pathogenesis of pulmonary fibrosis.
Beck JM, Young VB, Huffnagle GB. The microbiome of the lung. Transl Res. 2012;160(4):258–66. https://doi.org/10.1016/j.trsl.2012.02.005.
Pettigrew MM, Tanner W, Harris AD. The lung microbiome and pneumonia. J Infect Dis. 2021;223(Supplement_3):S241–S5. https://doi.org/10.1093/infdis/jiaa702. The recognition that microbial interactions influence lung infections highlights the importance of the microbiome in lung homeostasis.
Nagpal R, Mainali R, Ahmadi S, Wang S, Singh R, Kavanagh K, Kitzman DW, Kushugulova A, Marotta F, Yadav H. Gut microbiome and aging: physiological and mechanistic insights. Nutr Healthy Aging. 2018;4(4):267–85. https://doi.org/10.3233/NHA-170030.
Morris A, Gibson K, Collman RG. The lung microbiome in idiopathic pulmonary fibrosis. What does it mean and what should we do about it? Am J Respir Crit Care Med. 2014;190(8):850–2. https://doi.org/10.1164/rccm.201409-1626ED.
Molyneaux PL, Cox MJ, Willis-Owen SA, Mallia P, Russell KE, Russell AM, et al. The role of bacteria in the pathogenesis and progression of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014;190(8):906–13. https://doi.org/10.1164/rccm.201403-0541OC.
Spagnolo P, Molyneaux PL, Bernardinello N, Cocconcelli E, Biondini D, Fracasso F, et al. The role of the lung’s microbiome in the pathogenesis and progression of idiopathic pulmonary fibrosis. Int J Mol Sci. 2019;20(22). https://doi.org/10.3390/ijms20225618.
Zhang Y-G, Zhu X, Lu R, Messer JS, Xia Y, Chang EB, Sun J. Intestinal epithelial HMGB1 inhibits bacterial infection via STAT3 regulation of autophagy. Autophagy. 2019;15(11):1935–53. https://doi.org/10.1080/15548627.2019.1596485.
Brevi A, Cogrossi LL, Grazia G, Masciovecchio D, Impellizzieri D, Lacanfora L, et al. Much more than IL-17A: cytokines of the IL-17 family between microbiota and cancer. Front Immunol. 2020;11(2914). https://doi.org/10.3389/fimmu.2020.565470.
Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30(6):492–506. https://doi.org/10.1038/s41422-020-0332-7.
Schmitt V, Rink L, Uciechowski P. The Th17/Treg balance is disturbed during aging. Exp Gerontol. 2013;48(12):1379–86. https://doi.org/10.1016/j.exger.2013.09.003.
Rodrigues DM, Sousa AJ, Hawley SP, Vong L, Gareau MG, Kumar SA, Johnson-Henry KC, Sherman PM. Matrix metalloproteinase 9 contributes to gut microbe homeostasis in a model of infectious colitis. BMC Microbiol. 2012;12:105. https://doi.org/10.1186/1471-2180-12-105.
Hewitt RJ, Molyneaux PL. The respiratory microbiome in idiopathic pulmonary fibrosis. Ann Transl Med. 2017;5(12):250. https://doi.org/10.21037/atm.2017.01.56.
Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med. 2018;379(8):797–8. https://doi.org/10.1056/NEJMc1807508.
Chilosi M, Carloni A, Rossi A, Poletti V. Premature lung aging and cellular senescence in the pathogenesis of idiopathic pulmonary fibrosis and COPD/emphysema. Transl Res. 2013;162(3):156–73. https://doi.org/10.1016/j.trsl.2013.06.004.
Minagawa S, Araya J, Numata T, Nojiri S, Hara H, Yumino Y, Kawaishi M, Odaka M, Morikawa T, Nishimura SL, Nakayama K, Kuwano K. Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-beta-induced senescence of human bronchial epithelial cells. Am J Phys Lung Cell Mol Phys. 2011;300(3):L391–401. https://doi.org/10.1152/ajplung.00097.2010.
Kim SK, Jung SM, Park K-S, Kim K-J. Integrative analysis of lung molecular signatures reveals key drivers of idiopathic pulmonary fibrosis. BMC Pulm Med. 2021;21(1):404. https://doi.org/10.1186/s12890-021-01749-3.
DJ DP, JAV H, Morshead KB, Sun K-H, Modrusan Z, Teng G, et al. Molecular mapping of interstitial lung disease reveals a phenotypically distinct senescent basal epithelial cell population. JCI Insight. 2021;6(8). https://doi.org/10.1172/jci.insight.143626.
Habermann AC, Gutierrez AJ, Bui LT, Yahn SL, Winters NI, Calvi CL, et al. Single-cell RNA sequencing reveals profibrotic roles of distinct epithelial and mesenchymal lineages in pulmonary fibrosis. Sci Adv. 2020;6(28):eaba1972. https://doi.org/10.1126/sciadv.aba1972.
Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, Oberg AL, Birch J, Salmonowicz H, Zhu Y, Mazula DL, Brooks RW, Fuhrmann-Stroissnigg H, Pirtskhalava T, Prakash YS, Tchkonia T, Robbins PD, Aubry MC, Passos JF, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017;8:14532. https://doi.org/10.1038/ncomms14532.
Lehmann M, Korfei M, Mutze K, Klee S, Skronska-Wasek W, Alsafadi HN, et al. Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur Respir J. 2017;50(2). https://doi.org/10.1183/13993003.02367-2016.
Pan J, Li D, Xu Y, Zhang J, Wang Y, Chen M, Lin S, Huang L, Chung EJ, Citrin DE, Wang Y, Hauer-Jensen M, Zhou D, Meng A. Inhibition of Bcl-2/xl with ABT-263 selectively kills senescent type II pneumocytes and reverses persistent pulmonary fibrosis induced by ionizing radiation in mice. Int J Radiat Oncol Biol Phys. 2017;99(2):353–61. https://doi.org/10.1016/j.ijrobp.2017.02.216.
Hohmann MS, Habiel DM, Coelho AL, Verri WA Jr, Hogaboam CM. Quercetin enhances ligand-induced apoptosis in senescent idiopathic pulmonary fibrosis fibroblasts and reduces lung fibrosis in vivo. Am J Respir Cell Mol Biol. 2019;60(1):28–40. https://doi.org/10.1165/rcmb.2017-0289OC.
Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK, et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioedicine. 2019;40:554–63. https://doi.org/10.1016/j.ebiom.2018.12.052.
Funding
CJLS is supported by NIH U01 – U01HL134766 and NIH R35 – R35HL150767. Additionally, this work is funded by the Advanced Clinician Scientist Program (IZKF, ACP02 for SDE).
Author information
Authors and Affiliations
Contributions
Both authors have contributed to the writing of this review.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Lung Injury & Fibrosis
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Deinhardt-Emmer, S., Le Saux, C.J. The Aging Microenvironment in Lung Fibrosis. Curr. Tissue Microenviron. Rep. 3, 67–76 (2022). https://doi.org/10.1007/s43152-022-00038-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43152-022-00038-3