Abstract
Purpose of Review
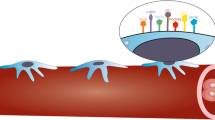
Capillaries are composed of endothelial cells that are partially wrapped by a mural cell known as a pericyte. There is growing interest in the functional roles of pericytes within the skeletal muscle microcirculation and how they might ultimately influence skeletal muscle functions.
Recent Findings
Generally, pericytes are thought to stabilize the capillary structure, modify endothelial cell functions, and participate in the process of new capillary formation, angiogenesis. Based on their structural organization and phenotypic behaviors, pericytes are postulated to perform a broad set of roles within the skeletal muscle that may include coordinating tissue homeostasis in healthy muscle and during the regeneration processes in damaged muscle. These functions indicate potential therapeutic opportunities targeting or utilizing pericytes to improve muscle health under pathophysiological conditions such as diabetes or muscle ischemia.
Summary
This review will discuss the current knowledge of skeletal muscle pericyte phenotype and function and the evidence supporting the influence of pericytes in skeletal muscle health under physiological and pathological states.




Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
DeFronzo RA, Gunnarsson R, Björkman O, Olsson M, Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest. 1985;76:149–55.
Krogh A. The number and distribution of capillaries in muscles with calculations of the oxygen pressure head necessary for supplying the tissue. J Physiol. 1919;52:409–15.
Romanul FC. Capillary supply and metabolism of muscle fibers. Arch Neurol. 1965;12:497–509.
Gray SD, Renkin EM. Microvascular supply in relation to fiber metabolic type in mixed skeletal muscles on rabbits. Microvasc Res. 1978;16:406–25.
Bruns RR, Palade GE. Studies on blood capillaries. I. General organization of blood capillaries in muscle. J Cell Biol. 1968;37:244–76.
Poole DC, Copp SW, Ferguson SK, Musch TI. Skeletal muscle capillary function: contemporary observations and novel hypotheses. Exp Physiol. 2013;98:1645–58.
Haas TL, Nwadozi E. Regulation of skeletal muscle capillary growth in exercise and disease. Appl Physiol Nutr Metab. 2015;40:1221–32.
Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–23.
Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215.
Díaz-Flores L, Gutiérrez R, Madrid JF, Varela H, Valladares F, Acosta E, et al. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24:909–69.
Lindahl P, Johansson BR, Levéen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–5.
Courtoy PJ, Boyles J. Fibronectin in the microvasculature: localization in the pericyte-endothelial interstitium. J Ultrastruct Res. 1983;83:258–73.
Iendaltseva O, Orlova VV, Mummery CL, Danen EHJ, Schmidt T. Fibronectin patches as anchoring points for force sensing and transmission in human induced pluripotent stem cell-derived pericytes. Stem Cell Reports. 2020;14:1107–22.
Tilton RG, Kilo C, Williamson JR. Pericyte-endothelial relationships in cardiac and skeletal muscle capillaries. Microvascular Research. Elsevier Science. 1979;18:325–35.
Papapetropoulos A, Fulton D, Mahboubi K, Kalb RG, O’Connor DS, Li F, et al. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem. 2000;275:9102–5.
Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6:460–3.
Wakui S, Yokoo K, Muto T, Suzuki Y, Takahashi H, Furusato M, et al. Localization of Ang-1, -2, Tie-2, and VEGF expression at endothelial-pericyte interdigitation in rat angiogenesis. Lab Invest. 2006;86:1172–84.
Eilken HM, Diéguez-Hurtado R, Schmidt I, Nakayama M, Jeong H-W, Arf H, et al. Pericytes regulate VEGF-induced endothelial sprouting through VEGFR1. Nat Commun. 2017;8:1574.
Gerhardt H, Wolburg H, Redies C. N-cadherin mediates pericytic-endothelial interaction during brain angiogenesis in the chicken. Dev Dyn. 2000;218:472–9.
Tillet E, Vittet D, Féraud O, Moore R, Kemler R, Huber P. N-cadherin deficiency impairs pericyte recruitment, and not endothelial differentiation or sprouting, in embryonic stem cell-derived angiogenesis. Exp Cell Res. 2005;310:392–400.
Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, et al. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell. 2006;124:161–73.
Liu H, Zhang W, Kennard S, Caldwell RB, Lilly B. Notch3 is critical for proper angiogenesis and mural cell investment. Circ Res. 2010;107:860–70.
Goumans M-J, Lebrin F, Valdimarsdottir G. Controlling the angiogenic switch: a balance between two distinct TGF-b receptor signaling pathways. Trends Cardiovasc Med. 2003;13:301–7.
Papetti M, Shujath J, Riley KN, Herman IM. FGF-2 antagonizes the TGF-beta1-mediated induction of pericyte alpha-smooth muscle actin expression: a role for myf-5 and Smad-mediated signaling pathways. Invest Ophthalmol Vis Sci. 2003;44:4994–5005.
Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro-oncology. 2005;7:452–64.
Tilton RG, Hoffmann PL, Kilo C, Williamson JR. Pericyte degeneration and basement membrane thickening in skeletal muscle capillaries of human diabetics. Diabetes. 1981;30:326–34.
Pollack AA, Wood EH. Venous pressure in the saphenous vein at the ankle in man during exercise and changes in posture. J Appl Physiol. 1949;1:649–62.
Birbrair A, Zhang T, Wang Z-M, Messi ML, Enikolopov GN, Mintz A, et al. Skeletal muscle pericyte subtypes differ in their differentiation potential. Stem Cell Res. 2013;10:67–84.
Egginton S, Zhou AL, Brown MD, Hudlická O. Unorthodox angiogenesis in skeletal muscle. Cardiovasc Res. 2001;49:634–46.
Haas TL, Milkiewicz M, Davis SJ, Zhou AL, Egginton S, Brown MD, et al. Matrix metalloproteinase activity is required for activity-induced angiogenesis in rat skeletal muscle. Am J Physiol Heart Circ Physiol. 2000;279:H1540–7.
Zhou A, Egginton S, Hudlická O, Brown MD. Internal division of capillaries in rat skeletal muscle in response to chronic vasodilator treatment with alpha1-antagonist prazosin. Cell Tissue Res. 1998;293:293–303.
Djonov VG, Galli AB, Burri PH. Intussusceptive arborization contributes to vascular tree formation in the chick chorio-allantoic membrane. Anat Embryol (Berl). 2000;202:347–57.
Styp-Rekowska B, Hlushchuk R, Pries AR, Djonov V. Intussusceptive angiogenesis: pillars against the blood flow. Acta Physiol (Oxf). 2011;202:213–23.
Milkiewicz M, Brown MD, Egginton S, Hudlicka O. Association between shear stress, angiogenesis, and VEGF in skeletal muscles in vivo. Microcirculation. 2001;8:229–41.
Rivilis I, Milkiewicz M, Boyd P, Goldstein J, Brown MD, Egginton S, et al. Differential involvement of MMP-2 and VEGF during muscle stretch- versus shear stress-induced angiogenesis. Am J Physiol Heart Circ Physiol. 2002;283:H1430–8.
Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307.
Egginton S, Hudlicka O, Brown MD, Graciotti L, Granata AL. In vivo pericyte-endothelial cell interaction during angiogenesis in adult cardiac and skeletal muscle. Microvasc Res. 1996;51:213–28.
Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60.
Teichert M, Milde L, Holm A, Stanicek L, Gengenbacher N, Savant S, et al. Pericyte-expressed Tie2 controls angiogenesis and vessel maturation. Nat Commun. 2017;8:16106.
Lloyd PG, Prior BM, Yang HT, Terjung RL. Angiogenic growth factor expression in rat skeletal muscle in response to exercise training. American Journal of Physiology-Heart and Circulatory Physiology. American Physiological Society. 2003;284:H1668–78.
Gustafsson T, Rundqvist H, Norrbom J, Rullman E, Jansson E, Sundberg CJ. The influence of physical training on the angiopoietin and VEGF-A systems in human skeletal muscle. J Appl Physiol. 2007;103:1012–20.
Hoier B, Nordsborg N, Andersen S, Jensen L, Nybo L, Bangsbo J, et al. Pro- and anti-angiogenic factors in human skeletal muscle in response to acute exercise and training. J Physiol. 2012;590:595–606.
Pichiule P, Chavez JC, LaManna JC. Hypoxic regulation of angiopoietin-2 expression in endothelial cells. J Biol Chem. 2004;279:12171–80.
Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, et al. The Tie-2 ligand Angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 2004;103:4150–6.
Scholz A, Plate KH, Reiss Y. Angiopoietin-2: a multifaceted cytokine that functions in both angiogenesis and inflammation. Ann N Y Acad Sci. 2015;1347:45–51.
Mofarrahi M, Hussain SNA. Expression and functional roles of angiopoietin-2 in skeletal muscles. PLOS ONE. 2011;6:e22882.
Koller A, Sun D, Kaley G. Role of shear stress and endothelial prostaglandins in flow- and viscosity-induced dilation of arterioles in vitro. Circ Res. 1993;72:1276–84.
Perrot CY, Herrera JL, Fournier-Goss AE, Komatsu M. Prostaglandin E2 breaks down pericyte–endothelial cell interaction via EP1 and EP4-dependent downregulation of pericyte N-cadherin, connexin-43, and R-Ras. Scientific Reports. 2020;10:11186.
Cantelmo AR, Conradi L-C, Brajic A, Goveia J, Kalucka J, Pircher A, et al. Inhibition of the glycolytic activator PFKFB3 in endothelium induces tumor vessel normalization, impairs metastasis, and improves chemotherapy. Cancer Cell. 2016;30:968–85.
Nwadozi E, Rudnicki M, Haas TL. Metabolic coordination of pericyte phenotypes: therapeutic implications. Front Cell Dev Biol. 2020;8:77.
Onogi Y, Wada T, Okekawa A, Matsuzawa T, Watanabe E, Ikeda K, et al. Pro-inflammatory macrophages coupled with glycolysis remodel adipose vasculature by producing platelet-derived growth factor-B in obesity. Scientific Reports. 2020;10:670.
Nehls V, Denzer K, Drenckhahn D. Pericyte involvement in capillary sprouting during angiogenesis in situ. Cell Tissue Res. 1992;270:469–74.
Ribatti D, Nico B, Crivellato E. The role of pericytes in angiogenesis. Int J Dev Biol. 2011;55:261–8.
Stapor PC, Murfee WL. Identification of class III β-tubulin as a marker of angiogenic perivascular cells. Microvasc Res. 2012;83:257–62.
Hoier B, Prats C, Qvortrup K, Pilegaard H, Bangsbo J, Hellsten Y. Subcellular localization and mechanism of secretion of vascular endothelial growth factor in human skeletal muscle. FASEB J. 2013;27:3496–504.
Reinmuth N, Liu W, Jung YD, Ahmad SA, Shaheen RM, Fan F, et al. Induction of VEGF in perivascular cells defines a potential paracrine mechanism for endothelial cell survival. FASEB J. 2001;15:1239–41.
Darland DC, Massingham LJ, Smith SR, Piek E, Saint-Geniez M, D’Amore PA. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev Biol. 2003;264:275–88.
Timmons JA, Jansson E, Fischer H, Gustafsson T, Greenhaff PL, Ridden J, et al. Modulation of extracellular matrix genes reflects the magnitude of physiological adaptation to aerobic exercise training in humans. BMC Biol. 2005;3:19.
Burri PH, Tarek MR. A novel mechanism of capillary growth in the rat pulmonary microcirculation. Anat Rec. 1990;228:35–45.
Djonov V, Baum O, Burri PH. Vascular remodeling by intussusceptive angiogenesis. Cell Tissue Res. 2003;314:107–17.
Kurz H, Burri PH, Djonov VG. Angiogenesis and vascular remodeling by intussusception: from form to function. News Physiol Sci. 2003;18:65–70.
Kurz H, Fehr J, Nitschke R, Burkhardt H. Pericytes in the mature chorioallantoic membrane capillary plexus contain desmin and alpha-smooth muscle actin: relevance for non-sprouting angiogenesis. Histochem Cell Biol. 2008;130:1027–40.
Zhan K, Bai L, Wang G, Zuo B, Xie L, Wang X. Different angiogenesis modes and endothelial responses in implanted porous biomaterials. Integr Biol. 2018;10:406–18.
Egginton S, Zhou AL, Brown MD, Hudlická O. The role of pericytes in controlling angiogenesis in vivo. Adv Exp Med Biol. 2000;476:81–99.
• Gianni-Barrera R, Butschkau A, Uccelli A, Certelli A, Valente P, Bartolomeo M, et al. PDGF-BB regulates splitting angiogenesis in skeletal muscle by limiting VEGF-induced endothelial proliferation. Angiogenesis. 2018;21:883–900 This study provides evidence that PDGF-BB retains pericyte coverage of capillaries during VEGF-A stimulation in skeletal muscle, which correlates with effective expansion of capillary networks via splitting angiogenesis.
Yao Y, Chen Z-L, Norris EH, Strickland S. Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat Commun. 2014;5:3413.
Yao Y, Norris EH, Mason CE, Strickland S. Laminin regulates PDGFRβ(+) cell stemness and muscle development. Nat Commun. 2016;7:11415.
Gautam J, Nirwane A, Yao Y. Laminin differentially regulates the stemness of type I and type II pericytes. Stem Cell Res Ther. 2017;8:28.
Reynolds LE, D’Amico G, Lechertier T, Papachristodoulou A, Muñoz-Félix JM, De Arcangelis A, et al. Dual role of pericyte α6β1-integrin in tumour blood vessels. J Cell Sci. 2017;130:1583–95.
Skalak TC, Price RJ, Zeller PJ. Where do new arterioles come from? Mechanical forces and microvessel adaptation. Microcirculation. 1998;5:91–4.
Peirce SM, Skalak TC. Microvascular remodeling: a complex continuum spanning angiogenesis to arteriogenesis. Microcirculation. 2003;10:99–111.
Volz KS, Jacobs AH, Chen HI, Poduri A, McKay AS, Riordan DP, et al. Pericytes are progenitors for coronary artery smooth muscle. Elife. 2015;4:e10036. https://doi.org/10.7554/eLife.10036.
Hansen-Smith F, Egginton S, Zhou AL, Hudlicka O. Growth of arterioles precedes that of capillaries in stretch-induced angiogenesis in skeletal muscle. Microvasc Res. 2001;62:1–14.
Birbrair A, Zhang T, Wang Z-M, Messi ML, Enikolopov GN, Mintz A, et al. Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells Dev. 2013;22:2298–314.
Birbrair A, Zhang T, Wang Z-M, Messi ML, Mintz A, Delbono O. Type-1 pericytes participate in fibrous tissue deposition in aged skeletal muscle. Am J Physiol Cell Physiol. 2013;305:C1098–113.
Birbrair A, Zhang T, Files DC, Mannava S, Smith T, Wang Z-M, et al. Type-1 pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner. Stem Cell Res Ther. 2014;5:122.
Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–67.
Dellavalle A, Maroli G, Covarello D, Azzoni E, Innocenzi A, Perani L, et al. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun. 2011;2:499.
Madonna R, Balistreri CR, Geng Y-J, De Caterina R. Diabetic microangiopathy: pathogenetic insights and novel therapeutic approaches. Vascul Pharmacol. 2017;90:1–7.
Hayden MR, Yang Y, Habibi J, Bagree SV, Sowers JR. Pericytopathy: oxidative stress and impaired cellular longevity in the pancreas and skeletal muscle in metabolic syndrome and type 2 diabetes. Oxid Med Cell Longev. 2010;3:290–303.
Beltramo E, Porta M. Pericyte loss in diabetic retinopathy: mechanisms and consequences. Curr Med Chem. 2013;20:3218–25.
Hammes H-P, Lin J, Wagner P, Feng Y, Vom Hagen F, Krzizok T, et al. Angiopoietin-2 causes pericyte dropout in the normal retina: evidence for involvement in diabetic retinopathy. Diabetes. 2004;53:1104–10.
Pfister F, Feng Y, vom Hagen F, Hoffmann S, Molema G, Hillebrands J-L, et al. Pericyte migration: a novel mechanism of pericyte loss in experimental diabetic retinopathy. Diabetes. 2008;57:2495–502.
Solomon TPJ, Haus JM, Li Y, Kirwan JP. Progressive hyperglycemia across the glucose tolerance continuum in older obese adults is related to skeletal muscle capillarization and nitric oxide bioavailability. J Clin Endocrinol Metab. 2011;96:1377–84.
Groen BBL, Hamer HM, Snijders T, van Kranenburg J, Frijns D, Vink H, et al. Skeletal muscle capillary density and microvascular function are compromised with aging and type 2 diabetes. J Appl Physiol (1985). 2014;116:998–1005.
Tilton RG, Faller AM, Burkhardt JK, Hoffmann PL, Kilo C, Williamson JR. Pericyte degeneration and acellular capillaries are increased in the feet of human diabetic patients. Diabetologia. 1985;28:895–900.
• Baum O, Bernd J, Becker S, Odriozola A, Zuber B, Tschanz SA, et al. Structural microangiopathies in skeletal muscle related to systemic vascular pathologies in humans. Front Physiol. 2020;11:28 This study demonstrates ultrastructural abnormalities in the endothelial-pericyte contacts in capillaries from individuals with diabetes and with peripheral artery disease and postulates that pericyte processes can penetrate through the endothelial cell to interact directly with plasma.
Faulkner A, Tamiato A, Cathery W, Rampin A, Caravaggi CM, Jover E, et al. Dimethyl-2-oxoglutarate improves redox balance and mitochondrial function in muscle pericytes of individuals with diabetes mellitus. Diabetologia. 2020;63:2205–17.
Lillioja S, Bogardus C. Insulin resistance in Pima Indians. A combined effect of genetic predisposition and obesity-related skeletal muscle cell hypertrophy. Acta Med Scand Suppl. 1988;723:103–19.
Pipinos II, Judge AR, Selsby JT, Zhu Z, Swanson SA, Nella AA, et al. The myopathy of peripheral arterial occlusive disease: part 1. Functional and histomorphological changes and evidence for mitochondrial dysfunction. Vasc Endovascular Surg. 2007;41:481–9.
Pipinos II, Judge AR, Selsby JT, Zhu Z, Swanson SA, Nella AA, et al. The myopathy of peripheral arterial occlusive disease: Part 2. Oxidative stress, neuropathy, and shift in muscle fiber type. Vasc Endovascular Surg. 2008;42:101–12.
Dumont NA, Bentzinger CF, Sincennes MC, Rudnicki MA. Satellite cells and skeletal muscle regeneration. Compr Physiol. 2015;5:1027–59.
Weiss DJ, Casale GP, Koutakis P, Nella AA, Swanson SA, Zhu Z, et al. Oxidative damage and myofiber degeneration in the gastrocnemius of patients with peripheral arterial disease. J Transl Med. 2013;11:230.
Brandão D, Costa C, Canedo A, Vaz G, Pignatelli D. Endogenous vascular endothelial growth factor and angiopoietin-2 expression in critical limb ischemia. Int Angiol. 2011;30:25–34.
Arpino J-M, Nong Z, Li F, Yin H, Ghonaim N, Milkovich S, et al. Four-dimensional microvascular analysis reveals that regenerative angiogenesis in ischemic muscle produces a flawed microcirculation. Circ Res. 2017;120:1453–65.
Nwadozi E, Rudnicki M, De Ciantis M, Milkovich S, Pulbere A, Roudier E, et al. High-fat diet pre-conditioning improves microvascular remodelling during regeneration of ischaemic mouse skeletal muscle. Acta Physiol (Oxf). 2020;229:e13449.
Ylä-Herttuala S, Bridges C, Katz MG, Korpisalo P. Angiogenic gene therapy in cardiovascular diseases: dream or vision? European Heart Journal. 2017;38:1365–71.
Lee RJ, Springer ML, Blanco-Bose WE. Shaw Robin, Ursell Philip C., Blau Helen M. VEGF gene delivery to myocardium. Circulation. 2000;102:898–901.
Gianni-Barrera R, Trani M, Fontanellaz C, Heberer M, Djonov V, Hlushchuk R, et al. VEGF over-expression in skeletal muscle induces angiogenesis by intussusception rather than sprouting. Angiogenesis. 2013;16:123–36.
Baum O, Torchetti E, Malik C, Hoier B, Walker M, Walker PJ, et al. Capillary ultrastructure and mitochondrial volume density in skeletal muscle in relation to reduced exercise capacity of patients with intermittent claudication. Am J Physiol Regul Integr Comp Physiol. 2016;310:R943–51.
• Mietus CJ, Lackner TJ, Karvelis PS, Willcockson GT, Shields CM, Lambert NG, et al. Abnormal microvascular architecture, fibrosis, and pericyte characteristics in the calf muscle of peripheral artery disease patients with claudication and critical limb ischemia. J Clin Med. 2020;9:2575. https://doi.org/10.3390/jcm9082575This study demonstrates increased collagen deposition and basement membrane thickening in microvessels of ischemic muscle. Pericyte coverage of microvessels was increased and postulated to contribute to this pathological fibrosis.
Dar A, Domev H, Ben-Yosef O, Tzukerman M, Zeevi-Levin N, Novak A, et al. Multipotent vasculogenic pericytes from human pluripotent stem cells promote recovery of murine ischemic limb. Circulation. 2012;125:87–99.
Cathery W, Faulkner A, Maselli D, Madeddu P. Concise review: the regenerative journey of pericytes toward clinical translation. Stem Cells. 2018;36:1295–310.
Hayes KL, Messina LM, Schwartz LM, Yan J, Burnside AS, Witkowski S. Type 2 diabetes impairs the ability of skeletal muscle pericytes to augment postischemic neovascularization in db/db mice. Am J Physiol, Cell Physiol. 2018;314:C534–44.
Munroe M, Dvoretskiy S, Lopez A, Leong J, Dyle MC, Kong H, et al. Pericyte transplantation improves skeletal muscle recovery following hindlimb immobilization. FASEB J. 2019;33:7694–706.
Vono R, Fuoco C, Testa S, Pirrò S, Maselli D, Ferland McCollough D, et al. Activation of the Pro-Oxidant PKCβII-p66Shc signaling pathway contributes to pericyte dysfunction in skeletal muscles of patients with diabetes with critical limb ischemia. Diabetes. 2016;65:3691–704.
Birbrair A, Zhang T, Wang Z-M, Messi ML, Olson JD, Mintz A, et al. Type-2 pericytes participate in normal and tumoral angiogenesis. Am J Physiol Cell Physiol. 2014;307:C25–38.
• Kovacs-Oller T, Ivanova E, Bianchimano P, Sagdullaev BT. The pericyte connectome: spatial precision of neurovascular coupling is driven by selective connectivity maps of pericytes and endothelial cells and is disrupted in diabetes. Cell Discov. 2020;6:1–18 This study demonstrates directional signaling between pericytes and endothelial cells in the retina that participates in precise blood flow control. This functional coupling is significantly disrupted by chronic severe hyperglycemia.
Williamson JR, Tilton RG, Kilo C, Yu S. Immunofluorescent imaging of capillaries and pericytes in human skeletal muscle and retina. Microvascular Research. 1980;20:233–41.
•• Corliss BA, Ray HC, Doty R, Mathews C, Sheybani N, Fitzgerald K, et al. Pericyte Bridges in Homeostasis and hyperglycemia: reconsidering pericyte dropout and microvascular structures. Diabetes. 2020;10:15808. https://doi.org/10.1038/s41598-020-72875-xThis study documents the presence of dynamic pericyte processes connect multiple capillaries in the retinal and skeletal muscle microvasculature and provides evidence that decreased pericyte coverage in response to hyperglycemia can be explained by pericyte movement away from the capillary.
Alarcon-Martinez L, Villafranca-Baughman D, Quintero H, Kacerovsky JB, Dotigny F, Murai KK, et al. Interpericyte tunnelling nanotubes regulate neurovascular coupling. Nature. 2020;585:91–5.
Tilton RG, Kilo C, Williamson JR, Murch DW. Differences in pericyte contractile function in rat cardiac and skeletal muscle microvasculatures. Microvasc Res. 1979;18:336–52.
Sims DE, Westfall JA. Analysis of relationships between pericytes and gas exchange capillaries in neonatal and mature bovine lungs. Microvasc Res. 1983;25:333–42.
• Nwadozi E, Ng A, Strömberg A, Liu H-Y, Olsson K, Gustafsson T, et al. Leptin is a physiological regulator of skeletal muscle angiogenesis and is locally produced by PDGFRα and PDGFRβ expressing perivascular cells. Angiogenesis. 2019;22:103–15 This study provides evidence that leptin contributes to physiological skeletal muscle angiogenesis via regulation of skeletal muscle VEGF-A production. Further, pericytes produce leptin in response to nutrient excess, suggesting a novel nutrient sensing role of pericytes.
Muoio DM, Dohm GL, Fiedorek FT, Tapscott EB, Coleman RA, Dohn GL. Leptin directly alters lipid partitioning in skeletal muscle. Diabetes. 1997;46:1360–3.
Acknowledgements
Some elements within the schematic figures were generated using Biorender.com. Funding to TLH from Natural Science and Research Council of Canada.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All reported experiments with animal subjects were performed in accordance with all applicable ethical standards including the Helsinki declaration and its amendments, institutional research committee standards, and international/national/institutional guidelines.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pericytes
Rights and permissions
About this article
Cite this article
Nwadozi, E., Haas, T.L. Emerging Roles of Pericytes in Coordinating Skeletal Muscle Functions: Implications and Therapeutic Potential. Curr. Tissue Microenviron. Rep. 2, 29–39 (2021). https://doi.org/10.1007/s43152-021-00029-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43152-021-00029-w