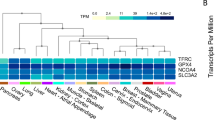
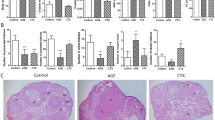
Abstract
Cuproptosis is a recently discovered mode of cell death that has garnered attention due to its association with various diseases. However, the intricate genetic relationship between cuproptosis and ovarian aging has remained largely unexplored. This study aimed to bridge this knowledge gap by leveraging data sets related to ovarian aging and cuproptosis. Through comprehensive bioinformatics analyses, facilitated by R software, we uncovered FDX1 as a potential cuproptosis-related gene with relevance to ovarian aging. To gain insights into FDX1's role, we conducted spatial transcriptome analyses in the ovaries of both young and aged female mice. These experiments revealed a significant reduction in FDX1 expression in the aging group compared to the young group. To substantiate these findings at the genetic level, we turned to clinical infertility biopsies. Impressively, we observed consistent results in biopsies from elderly infertile patients, reinforcing the link between FDX1 and ovarian aging. Moreover, we delved into the pharmacogenomics of ovarian cell lines and discovered that FDX1 expression levels were intricately associated with heightened sensitivity to specific small molecule drugs. This observation suggests that modulating FDX1 could potentially be a strategy to influence drug responses in ovarian-related therapies. In sum, this study marks a pioneering effort in identifying FDX1 as a cuproptosis-related gene implicated in ovarian aging. These findings hold substantial promise, not only in shedding light on the underlying mechanisms of ovarian aging but also in positioning FDX1 as a potential diagnostic biomarker and therapeutic target. With further research, FDX1 could play a pivotal role in advancing precision medicine and therapies for ovarian-related conditions.





Similar content being viewed by others
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Li CJ, Lin LT, Tsai HW, Chern CU, Wen ZH, Wang PH, et al. The Molecular Regulation in the Pathophysiology in Ovarian Aging. Aging Dis. 2021;12:934–49.
Hansen KR, Craig LB, Zavy MT, Klein NA, Soules MR. Ovarian primordial and nongrowing follicle counts according to the Stages of Reproductive Aging Workshop (STRAW) staging system. Menopause. 2012;19:164–71.
Nelson SM, Telfer EE, Anderson RA. The ageing ovary and uterus: new biological insights. Hum Reprod Update. 2013;19:67–83.
Gleicher N, Weghofer A, Barad DH. Defining ovarian reserve to better understand ovarian aging. Reprod Biol Endocrinol. 2011;9:23.
Chen X, Cai Q, Liang R, Zhang D, Liu X, Zhang M, et al. Copper homeostasis and copper-induced cell death in the pathogenesis of cardiovascular disease and therapeutic strategies. Cell Death Dis. 2023;14:105.
Chen L, Min J, Wang F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct Target Ther. 2022;7:378.
Linder MC, Hazegh-Azam M. Copper biochemistry and molecular biology. Am J Clin Nutr. 1996;63:797S-811S.
Cox DW, Moore SD. Copper transporting P-type ATPases and human disease. J Bioenerg Biomembr. 2002;34:333–8.
Arredondo M, Nunez MT. Iron and copper metabolism. Mol Aspects Med. 2005;26:313–27.
Stern BR. Essentiality and toxicity in copper health risk assessment: overview, update and regulatory considerations. J Toxicol Environ Health A. 2010;73:114–27.
Li F, Wang Y, Xu M, Hu N, Miao J, Zhao Y, et al. Single-nucleus RNA Sequencing reveals the mechanism of cigarette smoke exposure on diminished ovarian reserve in mice. Ecotoxicol Environ Saf. 2022;245:114093.
Zhou J, Lin L, Cai H, Liu L, Wang H, Zhang J, et al. SP1 impacts the primordial to primary follicle transition by regulating cholesterol metabolism in granulosa cells. FASEB J. 2023;37:e22767.
Li L, Pei Z, Wu R, Zhang Y, Pang Y, Hu H, et al. FDX1 regulates leydig cell ferroptosis mediates PM(2.5)-induced testicular dysfunction of mice. Ecotoxicol Environ Saf. 2023;263:115309.
Lu J, Ling X, Sun Y, Liu L, Liu L, Wang X, et al. FDX1 enhances endometriosis cell cuproptosis via G6PD-mediated redox homeostasis. Apoptosis. 2023;28:1128–40.
Xing J, Qiao G, Luo X, Liu S, Chen S, Ye G, et al. Ferredoxin 1 regulates granulosa cell apoptosis and autophagy in polycystic ovary syndrome. Clin Sci (Lond). 2023;137:453–68.
Li CJ, Chang CH, Tsang YL, Fang SH, Chen SN, Chiang AJ. Prognostic significance of ferroptosis pathway gene signature and correlation with macrophage infiltration in cervical squamous cell carcinoma. Int Immunopharmacol. 2022;112:109273.
Li CJ, Lin LT, Tsai HW, Wen ZH, Tsui KH. Phosphoglycerate mutase family member 5 maintains oocyte quality via mitochondrial dynamic rearrangement during aging. Aging Cell. 2022;21:e13546.
Finch CE, Holmes DJ. Ovarian aging in developmental and evolutionary contexts. Ann N Y Acad Sci. 2010;1204:82–94.
Hansen KR, Knowlton NS, Thyer AC, Charleston JS, Soules MR, Klein NA. A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause. Hum Reprod. 2008;23:699–708.
Mazhar M, Din AU, Ali H, Yang G, Ren W, Wang L, et al. Implication of ferroptosis in aging. Cell Death Discov. 2021;7:149.
Wang Z, Dong H, Yang L, Yi P, Wang Q, Huang D. The role of FDX1 in granulosa cell of Polycystic ovary syndrome (PCOS). BMC Endocr Disord. 2021;21:119.
Imamichi Y, Mizutani T, Ju Y, Matsumura T, Kawabe S, Kanno M, et al. Transcriptional regulation of human ferredoxin 1 in ovarian granulosa cells. Mol Cell Endocrinol. 2013;370:1–10.
Lin J, Zheng J, Zhang H, Chen J, Yu Z, Chen C, et al. Cytochrome P450 family proteins as potential biomarkers for ovarian granulosa cell damage in mice with premature ovarian failure. Int J Clin Exp Pathol. 2018;11:4236–46.
SreerangarajaUrs DB, Wu WH, Komrskova K, Postlerova P, Lin YF, Tzeng CR, et al. Mitochondrial Function in Modulating Human Granulosa Cell Steroidogenesis and Female Fertility. Int J Mol Sci. 2020;21(10):3592.
Tsui KH, Li CJ. Mitoquinone shifts energy metabolism to reduce ROS-induced oxeiptosis in female granulosa cells and mouse oocytes. Aging (Albany NY). 2023;15:246–60.
Su WP, Li CJ, Lin LT, Lin PH, Wen ZH, Sheu JJ, et al. Boosting mitochondrial function and metabolism in aging female germ cells with dual ROCK/ROS inhibition. Biomed Pharmacother. 2023;163:114888.
Lin PH, Su WP, Li CJ, Lin LT, Sheu JJ, Wen ZH, et al. Investigating the Role of Ferroptosis-Related Genes in Ovarian Aging and the Potential for Nutritional Intervention. Nutrients. 2023;15(11):2461.
Lin PH, Li CJ, Lin LT, Su WP, Sheu JJ, Wen ZH, et al. Unraveling the Clinical Relevance of Ferroptosis-Related Genes in Human Ovarian Aging. Reprod Sci. 2023;30(12):3529–36.
Lin PH, Lin LT, Li CJ, Kao PG, Tsai HW, Chen SN, et al. Combining Bioinformatics and Experiments to Identify CREB1 as a Key Regulator in Senescent Granulosa Cells. Diagnostics (Basel). 2020;10(5):295.
Tsui KH, Wang PH, Lin LT, Li CJ. DHEA protects mitochondria against dual modes of apoptosis and necroptosis in human granulosa HO23 cells. Reproduction. 2017;154:101–10.
Goldman KN. The quest for biomarkers linking ovarian aging and longevity. Fertil Steril. 2022;118:134–5.
Kelsey T. Models and Biomarkers for Ovarian Ageing. Subcell Biochem. 2023;103:185–99.
Acknowledgements
We thank Ching-Yu Chu for supporting data collection and statistical analysis.
Author information
Authors and Affiliations
Contributions
Conceptualization, C.-J.L., Z.-H.W. and L.-T.L.; Methodology, C.-J.L.; Formal Analysis, C.-C.W. and L.-T.L.; Writing-Original Draft Preparation, C.-C.W., J.-T.C.; Writing-Review & Editing, J.-T.C. and K.-H.T.; Supervision, K.-H.T.
Corresponding authors
Ethics declarations
Formatting of funding sources
This research was funded by the Ministry of Science Technology (MOST 111–2314-B-075B-014-MY3) and Kaohsiung Veterans General Hospital (VGHKS111-144, 112–089 and 112-D07).
Conflicts of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Identification of Cuproptosis-Related Genes as Diagnostic Markers for Ovarian Aging
• Predictive Value of Cuproptosis-Related Genes in Infertility Patients
• Clinical Biopsy Validates the Link Between Cuproptosis-Related Genes and Ovarian Aging
• Cuproptosis-Related Genes as Potential Biomarkers for Ovarian Aging
• The Role of Cuproptosis-Related Genes in Assessing Ovarian Aging through Clinical Biopsy
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, CC., Li, CJ., Lin, LT. et al. Cuproptosis-Related Gene FDX1 Identified as a Potential Target for Human Ovarian Aging. Reprod. Sci. (2024). https://doi.org/10.1007/s43032-024-01573-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43032-024-01573-0