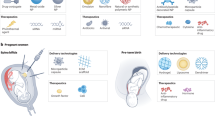
Abstract
More than 20% of pregnant women experience serious complications during pregnancy, that gravely affect the safety of both the mother and the child. Due to the unique state of pregnancy, medication during pregnancy is subject to various restrictions. Nanotechnology is an emerging technology that has been the focus of extensive medical research, and great progress has recently been made in developing sensitive diagnostic modalities and efficient medical treatment. Accumulating evidence has shown that nanodrug delivery systems can significantly improve the targeting, reduce the toxicity and improve the bioavailability of drugs. Recently, some actively targeted nanomedicines have been explored in the treatment of pregnancy-related diseases. This article reviews common types of nanocarriers and active targeting ligands in common pregnancy-related diseases and complications such as preeclampsia, preterm birth, fetal growth restriction, and choriocarcinoma. Finally, the challenges and future prospects in the development of these nanomaterials are discussed, with the aim of providing guidance for future research directions.


Similar content being viewed by others
Data Availability
No data associated in this paper.
References
Usta IM, Nassar AH. Advanced maternal age. Part I: obstetric complications. Am J Perinatol. 2008;25(8):521–34. https://doi.org/10.1055/s-0028-1085620.
Wennberg AL, Opdahl S, Bergh C, Aaris Henningsen AK, Gissler M, Romundstad LB, Pinborg A, Tiitinen A, Skjaerven R, Wennerholm UB. Effect of maternal age on maternal and neonatal outcomes after assisted reproductive technology. Fertil Steril. 2016;106(5):1142–9. https://doi.org/10.1016/j.fertnstert.2016.06.021.
Tang M, Zhang X, Fei W, Xin Y, Zhang M, Yao Y, Zhao Y, Zheng C, Sun D. Advance in placenta drug delivery: concern for placenta-originated disease therapy. Drug Deliv. 2023;30(1):2184315. https://doi.org/10.1080/10717544.2023.2184315.
Centers for Disease Control and Prevention. Preterm birth. 2023. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth.htm
Hawkes N. Trial of Viagra for fetal growth restriction is halted after baby deaths. BMJ. 2018;362:k3247. https://doi.org/10.1136/bmj.k3247.
Mantle A, Yang MJ, Judkins A, Chanthavong I, Yoder BA, Chan B. Association of Intrapartum Drugs with spontaneous intestinal perforation: a single-Center Retrospective Review. Am J Perinatol. 2021. https://doi.org/10.1055/a-1673-0183.
Fisk NM, Atun R. Market failure and the poverty of new drugs in maternal health. PLoS Med. 2008;5(1):e22. https://doi.org/10.1371/journal.pmed.0050022.
Fathi-Karkan S, Arshad R, Rahdar A, Ramezani A, Behzadmehr R, Ghotekar S, Pandey S. Recent advancements in the targeted delivery of etoposide nanomedicine for cancer therapy: a comprehensive review. Eur J Med Chem. 2023;259:115676. https://doi.org/10.1016/j.ejmech.2023.115676.
De Jong WH, Borm PJ. Drug delivery and nanoparticles:applications and hazards. Int J Nanomed. 2008;3(2):133–49. https://doi.org/10.2147/ijn.s596.
Yusuf A, Almotairy ARZ, Henidi H, Alshehri OY, Aldughaim MS. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles’ Physicochemical Properties on Responses in Biological Systems. Polym (Basel). 2023;15(7). https://doi.org/10.3390/polym15071596.
van der Meel R, Vehmeijer LJ, Kok RJ, Storm G, van Gaal EV. Ligand-targeted particulate nanomedicines undergoing clinical evaluation: current status. Adv Drug Deliv Rev. 2013;65(10):1284–98. https://doi.org/10.1016/j.addr.2013.08.012.
Friedman AD, Claypool SE, Liu R. The smart targeting of nanoparticles. Curr Pharm Des. 2013;19(35):6315–29. https://doi.org/10.2174/13816128113199990375.
Barenholz Y. Doxil(R)--the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160(2):117–34. https://doi.org/10.1016/j.jconrel.2012.03.020.
Tian B, Hua S, Liu J. Multi-functional chitosan-based nanoparticles for drug delivery: recent advanced insight into cancer therapy. Carbohydr Polym. 2023;315:120972. https://doi.org/10.1016/j.carbpol.2023.120972.
Zong TX, Silveira AP, Morais JAV, Sampaio MC, Muehlmann LA, Zhang J, Jiang CS, Liu SK. Recent advances in Antimicrobial Nano-Drug Delivery systems. Nanomaterials (Basel). 2022;12(11). https://doi.org/10.3390/nano12111855.
Yang B, Song BP, Shankar S, Guller A, Deng W. Recent advances in liposome formulations for breast cancer therapeutics. Cell Mol Life Sci. 2021;78(13):5225–43. https://doi.org/10.1007/s00018-021-03850-6.
Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M, Nejati-Koshki K. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8(1):102. https://doi.org/10.1186/1556-276X-8-102.
Bulbake U, Doppalapudi S, Kommineni N, Khan W. Liposomal formulations in clinical use. Updated Rev Pharm. 2017;9(2). https://doi.org/10.3390/pharmaceutics9020012.
Zhang J, Leifer F, Rose S, Chun DY, Thaisz J, Herr T, Nashed M, Joseph J, Perkins WR, DiPetrillo K. Amikacin Liposome Inhalation suspension (ALIS) penetrates non-tuberculous mycobacterial biofilms and enhances Amikacin Uptake into macrophages. Front Microbiol. 2018;9:915. https://doi.org/10.3389/fmicb.2018.00915.
Herzog C, Hartmann K, Kunzi V, Kursteiner O, Mischler R, Lazar H, Gluck R. Eleven years of Inflexal V-a virosomal adjuvanted influenza vaccine. Vaccine. 2009;27(33):4381–7. https://doi.org/10.1016/j.vaccine.2009.05.029.
Guimaraes D, Cavaco-Paulo A, Nogueira E. Design of liposomes as drug delivery system for therapeutic applications. Int J Pharm. 2021;601:120571. https://doi.org/10.1016/j.ijpharm.2021.120571.
Bamrungsap S, Zhao Z, Chen T, Wang L, Li C, Fu T, Tan W. Nanotechnology in therapeutics: a focus on nanoparticles as a drug delivery system. Nanomed (Lond). 2012;7(8):1253–71. https://doi.org/10.2217/nnm.12.87.
El-Hammadi MM, Arias JL. Recent advances in the Surface functionalization of PLGA-Based nanomedicines. Nanomaterials (Basel). 2022;12(3). https://doi.org/10.3390/nano12030354.
Florez L, Herrmann C, Cramer JM, Hauser CP, Koynov K, Landfester K, Crespy D, Mailander V. How shape influences uptake: interactions of anisotropic polymer nanoparticles and human mesenchymal stem cells. Small. 2012;8(14):2222–30. https://doi.org/10.1002/smll.201102002.
Wang L, Hao Y, Li H, Zhao Y, Meng D, Li D, Shi J, Zhang H, Zhang Z, Zhang Y. Co-delivery of doxorubicin and siRNA for glioma therapy by a brain targeting system: angiopep-2-modified poly(lactic-co-glycolic acid) nanoparticles. J Drug Target. 2015;23(9):832–46. https://doi.org/10.3109/1061186X.2015.1025077.
Zhang Z, Cheng W, Pan Y, Jia L. An anticancer agent-loaded PLGA nanomedicine with glutathione-response and targeted delivery for the treatment of lung cancer. J Mater Chem B. 2020;8(4):655–65. https://doi.org/10.1039/c9tb02284h.
Kim Y, Park EJ, Na DH. Recent progress in dendrimer-based nanomedicine development. Arch Pharm Res. 2018;41(6):571–82. https://doi.org/10.1007/s12272-018-1008-4.
Kodama Y, Nakamura T, Kurosaki T, Egashira K, Mine T, Nakagawa H, Muro T, Kitahara T, Higuchi N, Sasaki H. Biodegradable nanoparticles composed of dendrigraft poly-L-lysine for gene delivery. Eur J Pharm Biopharm. 2014;87(3):472–9. https://doi.org/10.1016/j.ejpb.2014.04.013.
Zhao K, Rong G, Teng Q, Li X, Lan H, Yu L, Yu S, Jin Z, Chen G, Li Z. Dendrigraft poly-L-lysines delivery of DNA vaccine effectively enhances the immunogenic responses against H9N2 avian influenza virus infection in chickens. Nanomedicine. 2020;27:102209. https://doi.org/10.1016/j.nano.2020.102209.
MacDiarmid JA, Mugridge NB, Weiss JC, Phillips L, Burn AL, Paulin RP, Haasdyk JE, Dickson KA, Brahmbhatt VN, Pattison ST, et al. Bacterially derived 400 nm particles for encapsulation and cancer cell targeting of chemotherapeutics. Cancer Cell. 2007;11(5):431–45. https://doi.org/10.1016/j.ccr.2007.03.012.
MacDiarmid JA, Brahmbhatt H. Minicells: versatile vectors for targeted drug or si/shRNA cancer therapy. Curr Opin Biotechnol. 2011;22(6):909–16. https://doi.org/10.1016/j.copbio.2011.04.008.
Kaitu’u-Lino TJ, Pattison S, Ye L, Tuohey L, Sluka P, MacDiarmid J, Brahmbhatt H, Johns T, Horne AW, Brown J, et al. Targeted nanoparticle delivery of doxorubicin into placental tissues to treat ectopic pregnancies. Endocrinology. 2013;154(2):911–9. https://doi.org/10.1210/en.2012-1832.
Hari SK, Gauba A, Shrivastava N, Tripathi RM, Jain SK, Pandey AK. Polymeric micelles and cancer therapy: an ingenious multimodal tumor-targeted drug delivery system. Drug Deliv Transl Res. 2023;13(1):135–63. https://doi.org/10.1007/s13346-022-01197-4.
Hwang D, Ramsey JD, Kabanov AV. Polymeric micelles for the delivery of poorly soluble drugs: from nanoformulation to clinical approval. Adv Drug Deliv Rev. 2020;156:80–118. https://doi.org/10.1016/j.addr.2020.09.009.
Cabral H, Miyata K, Osada K, Kataoka K. Block Copolymer Micelles in Nanomedicine Applications. Chem Rev. 2018;118(14):6844–92. https://doi.org/10.1021/acs.chemrev.8b00199.
Bae YH. Drug targeting and tumor heterogeneity. J Control Release. 2009;133(1):2–3. https://doi.org/10.1016/j.jconrel.2008.09.074.
Yetisgin AA, Cetinel S, Zuvin M, Kosar A, Kutlu O. Therapeutic nanoparticles and their targeted delivery applications. Molecules. 2020;25(9). https://doi.org/10.3390/molecules25092193.
Cheng Z, Li M, Dey R, Chen Y. Nanomaterials for cancer therapy: current progress and perspectives. J Hematol Oncol. 2021;14(1):85. https://doi.org/10.1186/s13045-021-01096-0.
Pereira KV, Giacomeli R, Gomes de Gomes M, Haas SE. The challenge of using nanotherapy during pregnancy: Technological aspects and biomedical implications. Placenta. 2020;100:75–80. https://doi.org/10.1016/j.placenta.2020.08.005.
Huppertz B. The anatomy of the normal placenta. J Clin Pathol. 2008;61(12):1296–302. https://doi.org/10.1136/jcp.2008.055277.
Kojima J, Ono M, Kuji N, Nishi H. Human chorionic villous differentiation and placental development. Int J Mol Sci. 2022;23(14). https://doi.org/10.3390/ijms23148003.
James JL, Stone PR, Chamley LW. The regulation of trophoblast differentiation by oxygen in the first trimester of pregnancy. Hum Reprod Update. 2006;12(2):137–44. https://doi.org/10.1093/humupd/dmi043.
de Araujo TE, Milian ICB, de Souza G, da Silva RJ, Rosini AM, Guirelli PM, Franco PS, Barbosa BF, Ferro EAV, da Costa IN. Experimental models of maternal-fetal interface and their potential use for nanotechnology applications. Cell Biol Int. 2020;44(1):36–50. https://doi.org/10.1002/cbin.11222.
Pemathilaka RL, Reynolds DE, Hashemi NN. Drug transport across the human placenta: review of placenta-on-a-chip and previous approaches. Interface Focus. 2019;9(5):20190031. https://doi.org/10.1098/rsfs.2019.0031.
Rattanapinyopituk K, Shimada A, Morita T, Sakurai M, Asano A, Hasegawa T, Inoue K, Takano H. Demonstration of the clathrin- and caveolin-mediated endocytosis at the maternal-fetal barrier in mouse placenta after intravenous administration of gold nanoparticles. J Vet Med Sci. 2014;76(3):377–87. https://doi.org/10.1292/jvms.13-0512.
Walker N, Filis P, Soffientini U, Bellingham M, O’Shaughnessy PJ, Fowler PA. Placental transporter localization and expression in the human: the importance of species, sex, and gestational age differencesdagger. Biol Reprod. 2017;96(4):733–42. https://doi.org/10.1093/biolre/iox012.
Mao Q, Chen X. An update on placental drug transport and its relevance to fetal drug exposure. Med Rev (Berl). 2022;2(5):501–11. https://doi.org/10.1515/mr-2022-0025.
Figueroa-Espada CG, Hofbauer S, Mitchell MJ, Riley RS. Exploiting the placenta for nanoparticle-mediated drug delivery during pregnancy. Adv Drug Deliv Rev. 2020;160:244–61. https://doi.org/10.1016/j.addr.2020.09.006.
Whigham CA, MacDonald TM, Walker SP, Hannan NJ, Tong S, Kaitu’u-Lino TJ. The untapped potential of placenta-enriched molecules for diagnostic and therapeutic development. Placenta. 2019;84:28–31. https://doi.org/10.1016/j.placenta.2019.02.002.
Ferretti C, Bruni L, Dangles-Marie V, Pecking AP, Bellet D. Molecular circuits shared by placental and cancer cells, and their implications in the proliferative, invasive and migratory capacities of trophoblasts. Hum Reprod Update. 2007;13(2):121–41. https://doi.org/10.1093/humupd/dml048.
King A, Ndifon C, Lui S, Widdows K, Kotamraju VR, Agemy L, Teesalu T, Glazier JD, Cellesi F, Tirelli N, et al. Tumor-homing peptides as tools for targeted delivery of payloads to the placenta. Sci Adv. 2016;2(5):e1600349. https://doi.org/10.1126/sciadv.1600349.
Cureton N, Korotkova I, Baker B, Greenwood S, Wareing M, Kotamraju VR, Teesalu T, Cellesi F, Tirelli N, Ruoslahti E, et al. Selective targeting of a Novel Vasodilator to the Uterine vasculature to treat impaired Uteroplacental perfusion in pregnancy. Theranostics. 2017;7(15):3715–31. https://doi.org/10.7150/thno.19678.
Zhang B, Tan L, Yu Y, Wang B, Chen Z, Han J, Li M, Chen J, Xiao T, Ambati BK, et al. Placenta-specific drug delivery by trophoblast-targeted nanoparticles in mice. Theranostics. 2018;8(10):2765–81. https://doi.org/10.7150/thno.22904.
Hussain A, Kumar A, Uttam V, Sharma U, Sak K, Saini RV, Saini AK, Haque S, Tuli HS, Jain A, et al. Application of curcumin nanoformulations to target folic acid receptor in cancer: recent trends and advances. Environ Res. 2023;233:116476. https://doi.org/10.1016/j.envres.2023.116476.
Boss SD, Ametamey SM. Development of Folate receptor-targeted PET radiopharmaceuticals for Tumor Imaging-A Bench-to-Bedside Journey. Cancers (Basel). 2020;12(6). https://doi.org/10.3390/cancers12061508.
Liu Y, Zhang Q, Gao X, Wang T. Study on lipid nanomicelles targeting placenta for the treatment of preeclampsia. J Drug Target. 2022;30(8):894–909. https://doi.org/10.1080/1061186X.2022.2068558.
Fuchs AR, Fuchs F, Husslein P, Soloff MS. Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol. 1984;150(6):734–41. https://doi.org/10.1016/0002-9378(84)90677-x.
Refuerzo JS, Leonard F, Bulayeva N, Gorenstein D, Chiossi G, Ontiveros A, Longo M, Godin B. Uterus-targeted liposomes for preterm labor management: studies in pregnant mice. Sci Rep. 2016;6:34710. https://doi.org/10.1038/srep34710.
Holmes VA, Wallace JM. Haemostasis in normal pregnancy: a balancing act? Biochem Soc Trans. 2005;33(Pt 2):428–32. https://doi.org/10.1042/BST0330428.
Zhang N, Ru B, Hu J, Xu L, Wan Q, Liu W, Cai W, Zhu T, Ji Z, Guo R, et al. Recent advances of CREKA peptide-based nanoplatforms in biomedical applications. J Nanobiotechnol. 2023;21(1):77. https://doi.org/10.1186/s12951-023-01827-0.
Agemy L, Sugahara KN, Kotamraju VR, Gujraty K, Girard OM, Kono Y, Mattrey RF, Park JH, Sailor MJ, Jimenez AI, et al. Nanoparticle-induced vascular blockade in human prostate cancer. Blood. 2010;116(15):2847–56. https://doi.org/10.1182/blood-2010-03-274258.
Soper JT. Gestational trophoblastic disease. Obstet Gynecol. 2006;108(1):176–87. https://doi.org/10.1097/01.AOG.0000224697.31138.a1.
Wang Y, Wang Z, Zhu X, Wan Q, Han P, Ying J, Qian J. Intestinal metastasis from choriocarcinoma: a case series and literature review. World J Surg Oncol. 2022;20(1):173. https://doi.org/10.1186/s12957-022-02623-0.
Seckl MJ, Sebire NJ, Berkowitz RS. Gestational trophoblastic disease. Lancet. 2010;376(9742):717–29. https://doi.org/10.1016/S0140-6736(10)60280-2.
Deleuze A, Massard C, Le Du F, You B, Lefeuvre-Plesse C, Bolze PA, de la Motte Rouge T. Management of trophoblastic tumors: review of evidence, current practice, and future directions. Expert Rev Anticancer Ther. 2023;23(7):699–708. https://doi.org/10.1080/14737140.2023.2215438.
Tu W, Luo M, Wang Z, Yan W, Xia Y, Deng H, He J, Han P, Tian D. Upregulation of SATB1 promotes tumor growth and metastasis in liver cancer. Liver Int. 2012;32(7):1064–78. https://doi.org/10.1111/j.1478-3231.2012.02815.x.
Hu B, Zhong L, Weng Y, Peng L, Huang Y, Zhao Y, Liang XJ. Therapeutic siRNA: state of the art. Signal Transduct Target Ther. 2020;5(1):101. https://doi.org/10.1038/s41392-020-0207-x.
Dong J, Cao Y, Shen H, Ma Q, Mao S, Li S, Sun J. EGFR aptamer-conjugated liposome-polycation-DNA complex for targeted delivery of SATB1 small interfering RNA to choriocarcinoma cells. Biomed Pharmacother. 2018;107:849–59. https://doi.org/10.1016/j.biopha.2018.08.042.
Zhou D, Ye C, Pan Z, Deng Y. SATB1 Knockdown inhibits Proliferation and Invasion and decreases Chemoradiation Resistance in Nasopharyngeal Carcinoma cells by reversing EMT and suppressing MMP-9. Int J Med Sci. 2021;18(1):42–52. https://doi.org/10.7150/ijms.49792.
Soto-Cerrato V, Vinals F, Lambert JR, Kelly JA, Perez-Tomas R. Prodigiosin induces the proapoptotic gene NAG-1 via glycogen synthase kinase-3beta activity in human breast cancer cells. Mol Cancer Ther. 2007;6(1):362–9. https://doi.org/10.1158/1535-7163.MCT-06-0266.
Zhao W, Gao D, Ning L, Jiang Y, Li Z, Huang B, Chen A, Wang C, Liu Y. Prodigiosin inhibits the proliferation of glioblastoma by regulating the KIAA1524/PP2A signaling pathway. Sci Rep. 2022;12(1):18527. https://doi.org/10.1038/s41598-022-23186-w.
Zhao K, Li D, Cheng G, Zhang B, Han J, Chen J, Wang B, Li M, Xiao T, Zhang J, et al. Targeted delivery prodigiosin to Choriocarcinoma by peptide-guided dendrigraft Poly-l-lysines nanoparticles. Int J Mol Sci. 2019;20(21). https://doi.org/10.3390/ijms20215458.
Zhang X, Chen Y, Sun D, Zhu X, Ying X, Yao Y, Fei W, Zheng C. Emerging pharmacologic interventions for pre-eclampsia treatment. Expert Opin Ther Targets. 2022;26(8):739–59. https://doi.org/10.1080/14728222.2022.2134779.
Poon LC, Shennan A, Hyett JA, Kapur A, Hadar E, Divakar H, McAuliffe F, da Silva Costa F, von Dadelszen P, McIntyre HD, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: a pragmatic guide for first-trimester screening and prevention. Int J Gynaecol Obstet. 2019;145(Suppl 1):1–33. https://doi.org/10.1002/ijgo.12802.
Ives CW, Sinkey R, Rajapreyar I, Tita ATN, Oparil S. Preeclampsia-Pathophysiology and Clinical presentations: JACC State-of-the-art review. J Am Coll Cardiol. 2020;76(14):1690–702. https://doi.org/10.1016/j.jacc.2020.08.014.
von Dadelszen P, Magee LA. Pre-eclampsia: an update. Curr Hypertens Rep. 2014;16(8). https://doi.org/10.1007/s11906-014-0454-8.
Turanov AA, Lo A, Hassler MR, Makris A, Ashar-Patel A, Alterman JF, Coles AH, Haraszti RA, Roux L, Godinho B, et al. RNAi modulation of placental sFLT1 for the treatment of preeclampsia. Nat Biotechnol. 2018. https://doi.org/10.1038/nbt.4297.
Yu J, Jia J, Guo X, Chen R, Feng L. Modulating circulating sFlt1 in an animal model of preeclampsia using PAMAM nanoparticles for siRNA delivery. Placenta. 2017;58:1–8. https://doi.org/10.1016/j.placenta.2017.07.360.
Li L, Yang H, Chen P, Xin T, Zhou Q, Wei D, Zhang Y, Wang S. Trophoblast-targeted Nanomedicine modulates placental sFLT1 for Preeclampsia Treatment. Front Bioeng Biotechnol. 2020;8:64. https://doi.org/10.3389/fbioe.2020.00064.
Li L, Li H, Xue J, Chen P, Zhou Q, Zhang C. Nanoparticle-mediated simultaneous downregulation of placental Nrf2 and sFlt1 improves maternal and fetal outcomes in a Preeclampsia Mouse Model. ACS Biomater Sci Eng. 2020;6(10):5866–73. https://doi.org/10.1021/acsbiomaterials.0c00826.
Burton GJ, Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. Am J Obstet Gynecol. 2018;218(2S):S745–61. https://doi.org/10.1016/j.ajog.2017.11.577.
Sharma D, Shastri S, Sharma P. Intrauterine growth restriction: antenatal and postnatal aspects. Clin Med Insights Pediatr. 2016;10:67–83. https://doi.org/10.4137/CMPed.S40070.
Fung C, Zinkhan E. Short- and long-term implications of small for gestational age. Obstet Gynecol Clin North Am. 2021;48(2):311–23. https://doi.org/10.1016/j.ogc.2021.02.004.
Vollmer B, Edmonds CJ. School Age neurological and cognitive outcomes of fetal growth retardation or small for gestational age Birth Weight. Front Endocrinol (Lausanne). 2019;10:186. https://doi.org/10.3389/fendo.2019.00186.
Alberry M, Soothill P. Management of fetal growth restriction. Arch Dis Child Fetal Neonatal Ed. 2007;92(1):F62–67. https://doi.org/10.1136/adc.2005.082297.
Constancia M, Hemberger M, Hughes J, Dean W, Ferguson-Smith A, Fundele R, Stewart F, Kelsey G, Fowden A, Sibley C, et al. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature. 2002;417(6892):945–8. https://doi.org/10.1038/nature00819.
Forbes K, Westwood M, Baker PN, Aplin JD. Insulin-like growth factor I and II regulate the life cycle of trophoblast in the developing human placenta. Am J Physiol Cell Physiol. 2008;294(6):C1313–1322. https://doi.org/10.1152/ajpcell.00035.2008.
Li Q, Liu X, Liu W, Zhang Y, Liu W, Wu M, Chen Z, Zhao Y, Zou L. Placenta-targeted nanoparticles loaded with PFKFB3 overexpression plasmids enhance angiogenesis and placental function. Bioeng (Basel). 2022;9(11). https://doi.org/10.3390/bioengineering9110652.
Williams DJ, Vallance PJ, Neild GH, Spencer JA, Imms FJ. Nitric oxide-mediated vasodilation in human pregnancy. Am J Physiol. 1997;272(2 Pt 2):H748–752. https://doi.org/10.1152/ajpheart.1997.272.2.H748.
Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7(2):156–67. https://doi.org/10.1038/nrd2466.
Duffett L, Rodger M. LMWH to prevent placenta-mediated pregnancy complications: an update. Br J Haematol. 2015;168(5):619–38. https://doi.org/10.1111/bjh.13209.
Cheng J, Zhang S, Li C, Li K, Jia X, Wei Q, Qi H, Zhang J. Functionally integrating nanoparticles alleviate deep vein thrombosis in pregnancy and rescue intrauterine growth restriction. Nat Commun. 2022;13(1):7166. https://doi.org/10.1038/s41467-022-34878-2.
WHO. Recommended definitions, terminology and format for statistical tables related to the perinatal period and use of a new certificate for cause of perinatal deaths. Modifications recommended by FIGO as amended October 14, 1976. Acta Obstet Gynecol Scand. 1977;56(3):247–53.
Coler BS, Shynlova O, Boros-Rausch A, Lye S, McCartney S, Leimert KB, Xu W, Chemtob S, Olson D, Li M, et al. Landscape of Preterm Birth Therapeutics and a path Forward. J Clin Med. 2021;10(13). https://doi.org/10.3390/jcm10132912.
Abramovici A, Cantu J, Jenkins SM. Tocolytic therapy for acute preterm labor. Obstet Gynecol Clin North Am. 2012;39(1):77–87. https://doi.org/10.1016/j.ogc.2011.12.003.
Vermillion ST, Landen CN. Prostaglandin inhibitors as tocolytic agents. Semin Perinatol. 2001;25(4):256–62. https://doi.org/10.1053/sper.2001.27549.
Moise KJ Jr., Ou CN, Kirshon B, Cano LE, Rognerud C, Carpenter RJ Jr. Placental transfer of indomethacin in the human pregnancy. Am J Obstet Gynecol. 1990;162(2):549–54. https://doi.org/10.1016/0002-9378(90)90427-9.
Paul JW, Hua S, Ilicic M, Tolosa JM, Butler T, Robertson S, Smith R. (2017) Drug delivery to the human and mouse uterus using immunoliposomes targeted to the oxytocin receptor. Am J Obstet Gynecol, 216(3):283 ehttps://doi.org/10.1016/j.ajog.2016.08.027.
da Fonseca EB, Damiao R, Moreira DA. Preterm birth prevention. Best Pract Res Clin Obstet Gynaecol. 2020;69:40–9. https://doi.org/10.1016/j.bpobgyn.2020.09.003.
Zierden HC, Shapiro RL, DeLong K, Carter DM, Ensign LM. Next generation strategies for preventing preterm birth. Adv Drug Deliv Rev. 2021;174:190–209. https://doi.org/10.1016/j.addr.2021.04.021.
Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2–25. https://doi.org/10.1016/j.addr.2013.11.009.
Joshi MD. Drug delivery during pregnancy: how can nanomedicine be used? Ther Deliv. 2017;8(12):1023–5. https://doi.org/10.4155/tde-2017-0084.
Fliedel L, Alhareth K, Mignet N, Fournier T, Andrieux K. Placental models for evaluation of Nanocarriers as Drug Delivery systems for pregnancy Associated disorders. Biomedicines. 2022;10(5). https://doi.org/10.3390/biomedicines10050936.
Aengenheister L, Favaro RR, Morales-Prieto DM, Furer LA, Gruber M, Wadsack C, Markert UR, Buerki-Thurnherr T. Research on nanoparticles in human perfused placenta: state of the art and perspectives. Placenta. 2021;104:199–207. https://doi.org/10.1016/j.placenta.2020.12.014.
Zhou Y, Ji J, Hong F, Zhuang J, Wang L. Maternal exposure to Nanoparticulate Titanium Dioxide causes inhibition of hippocampal development involving dysfunction of the Rho/NMDAR signaling pathway in offspring. J Biomed Nanotechnol. 2019;15(4):839–47. https://doi.org/10.1166/jbn.2019.2723.
Ataei ML, Ebrahimzadeh-Bideskan AR. The effects of nano-silver and garlic administration during pregnancy on neuron apoptosis in rat offspring hippocampus. Iran J Basic Med Sci. 2014;17(6):411–8.
Hong F, Zhou Y, Ji J, Zhuang J, Sheng L, Wang L. Nano-TiO(2) inhibits development of the Central Nervous System and its mechanism in offspring mice. J Agric Food Chem. 2018;66(44):11767–74. https://doi.org/10.1021/acs.jafc.8b02952.
Su J, Duan X, Qiu Y, Zhou L, Zhang H, Gao M, Liu Y, Zou Z, Qiu J, Chen C. Pregnancy exposure of titanium dioxide nanoparticles causes intestinal dysbiosis and neurobehavioral impairments that are not significant postnatally but emerge in adulthood of offspring. J Nanobiotechnol. 2021;19(1):234. https://doi.org/10.1186/s12951-021-00967-5.
Roset Bahmanyar E, Out HJ, van Duin M. Women and babies are dying from inertia: a collaborative framework for obstetrical drug development is urgently needed. Am J Obstet Gynecol. 2021;225(1):43–50. https://doi.org/10.1016/j.ajog.2021.03.024.
Moses AS, Kadam L, St Lorenz A, Baldwin MK, Morgan T, Hebert J, Park Y, Lee H, Demessie AA, Korzun T, et al. Nano-Theranostic modality for visualization of the Placenta and photo-hyperthermia for potential management of ectopic pregnancy. Small. 2023;19(2):e2202343. https://doi.org/10.1002/smll.202202343.
Acknowledgements
We would like to thank Figdraw (www.figdraw.com) for help in creating the schematic figure.
Funding
This work was funded by National Key Research and Development Program of China (2022YFC2704600), Central Government Guiding Local Science and Technology Development Foundation of Shandong Province (Grant No. YDZX2022096), National Natural Science Foundation of China (Grant No.81741038) and Jinan Science and Technology Program(Grant No.202134014).
Author information
Authors and Affiliations
Contributions
HY reviewed the literature and drafted the manuscript. SW made critical revisions. All authors read and approved the manuscript for publication.
Corresponding author
Ethics declarations
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, H., Wang, S. Actively Targeted Nanomedicines: A New Perspective for the Treatment of Pregnancy-Related Diseases. Reprod. Sci. (2024). https://doi.org/10.1007/s43032-024-01520-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43032-024-01520-z