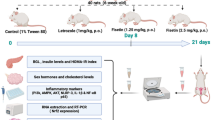
Abstract
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders in women. This study aimed to investigate the therapeutic effects and mechanism of Jujuboside A on PCOS using a dehydroepiandrosterone (DHEA)-induced PCOS mouse model. Estrogen and androgen homeostasis was evaluated in serum from both clinical samples and PCOS mice. The stages of the estrous cycle were determined based on vaginal cytology. The ovarian morphology was observed by stained with hematoxylin and eosin. Moreover, we analyzed protein expression of cytochrome P450 1A1 (CYP1A1), cytochrome P450 1A2 (CYP1A2) and aryl hydrocarbon receptor (AhR) in ovary and KGN cells. Molecular docking, immunofluorescence, and luciferase assay were performed to confirm the activation of AhR by Jujuboside A. Jujuboside A effectively alleviated the disturbance of estrogen homeostasis and restored ovarian function, leading to an improvement in the occurrence and progression of PCOS. Furthermore, the protective effect of JuA against PCOS was dependent on increased CYP1A2 levels regulated by AhR. Our findings suggest that Jujuboside A improves estrogen disorders and may be a potential therapeutic agent for the treatment of PCOS.
Graphical Abstract







Similar content being viewed by others
Data Availability
The data supporting the findings of this study are available on reasonable request from the corresponding authors.
References
Jayasena CN, Franks S. The management of patients with polycystic ovary syndrome. Nat Rev Endocrinol. 2014;10(10):624–36. https://doi.org/10.1038/nrendo.2014.102
Azziz R, Carmina E, Chen ZJ, et al. Polycystic ovary syndrome. Nat Rev Dis Prim. 2016;2:16057. https://doi.org/10.1038/nrdp.2016.57
Kumariya S, Ubba V, Jha RK, Gayen JR. Autophagy in ovary and polycystic ovary syndrome: role, dispute and future perspective. Autophagy. 2021;17(10):2706–32. https://doi.org/10.1080/15548627.2021.1938914
MohanKumar SMJ, Balasubramanian P, Subramanian M, MohanKumar PS. Chronic estradiol exposure - harmful effects on behavior, cardiovascular and reproductive functions. Reproduction. 2018;156(5):R169–86. https://doi.org/10.1530/REP-18-0116
Med ASR. The clinical relevance of luteal phase deficiency: a committee opinion. Fertil Steril. 2012;98(5):1112–7. https://doi.org/10.1016/j.fertnstert.2012.06.050
De Souza MJ, Miller BE, Loucks AB, et al. High frequency of luteal phase deficiency and anovulation in recreational women runners: Blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. J Clin Endocrinol Metab. 1998;83(12):4220–32. https://doi.org/10.1210/jc.83.12.4220
Qi J, Wang Y, Zhu QL, et al. Novel role of CXCL14 in modulating STAR expression in luteinized granulosa cells: implication for progesterone synthesis in PCOS patients. Transl Res. 2021;230:55–67. https://doi.org/10.1016/j.trsl.2020.10.009
Harlow CR, Shaw HJ, Hillier SG, Hodges JK. Factors influencing follicle-stimulating hormone-responsive steroidogenesis in marmoset granulosa cells: effects of androgens and the stage of follicular maturity. Endocrinology. 1988;122(6):2780–7. https://doi.org/10.1210/endo-122-6-2780
Bertoldo MJ, Caldwell ASL, Riepsamen AH, et al. A Hyperandrogenic Environment Causes Intrinsic Defects That Are Detrimental to Follicular Dynamics in a PCOS Mouse Model. Endocrinology. 2019;160(3):699–715. https://doi.org/10.1210/en.2018-00966
Rosenfield RL, Ehrmann DA. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr Rev. 2016;37(5):467–520. https://doi.org/10.1210/er.2015-1104
Wang L, Xiao Y, Tian T, et al. Digenic variants of planar cell polarity genes in human neural tube defect patients (vol 124, pg 94, 2018). Mol Genet Metab. 2021;132(3):211. https://doi.org/10.1016/j.ymgme.2021.01.010
Simpson ER, Clyne C, Rubin G, et al. Aromatase–a brief overview. Annu Rev Physiol. 2002;64:93–127. https://doi.org/10.1146/annurev.physiol.64.081601.142703
Ailawadi RK, Jobanputra S, Kataria M, Gurates B, Bulun SE. Treatment of endometriosis and chronic pelvic pain with letrozole and norethindrone acetate: a pilot study. Fertil Steril. 2004;81(2):290–6. https://doi.org/10.1016/j.fertnstert.2003.09.029
Geffner ME. Aromatase inhibitors to augment height: continued caution and study required. J Clin Res Pediatr Endocrinol. 2009;1(6):256–61. https://doi.org/10.4274/jcrpe.v1i6.256
Arlt W. Dehydroepiandrosterone replacement therapy. Semin Reprod Med. 2004;22(4):379–88. https://doi.org/10.1055/s-2004-861554
Lambard S, Galeraud-Denis I, Bouraima H, et al. Expression of aromatase in human ejaculated spermatozoa: a putative marker of motility. Mol Hum Reprod. 2003;9(3):117–24. https://doi.org/10.1093/molehr/gag020
Tsuchiya Y, Nakajima M, Yokoi T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005;227(2):115–24. https://doi.org/10.1016/j.canlet.2004.10.007
Okino ST, Pookot D, Basak S, Dahiya R. Toxic and chemopreventive ligands preferentially activate distinct aryl hydrocarbon receptor pathways: implications for cancer prevention. Cancer Prev Res (Phila). 2009;2(3):251–6. https://doi.org/10.1158/1940-6207.CAPR-08-0146
Arentz S, Abbott JA, Smith CA, Bensoussan A. Herbal medicine for the management of polycystic ovary syndrome (PCOS) and associated oligo/amenorrhoea and hyperandrogenism; a review of the laboratory evidence for effects with corroborative clinical findings. Bmc Complem Altern M. 2014;14:511. https://doi.org/10.1186/1472-6882-14-511
Raja-Khan N, Stener-Victorin E, Wu X, Legro RS. The physiological basis of complementary and alternative medicines for polycystic ovary syndrome. Am J Physiol Endocrinol Metab. 2011;301(1):E1–10. https://doi.org/10.1152/ajpendo.00667.2010
Kousta E, White DM, Franks S. Modern use of clomiphene citrate in induction of ovulation. Hum Reprod Updat. 1997;3(4):359–65. https://doi.org/10.1093/humupd/3.4.359
Mulligan K, Yang Y, Wininger DA, et al. Effects of metformin and rosiglitazone in HIV-infected patients with hyperinsulinemia and elevated waist/hip ratio. AIDS. 2007;21(1):47–57. https://doi.org/10.1097/QAD.0b013e328011220e
Maged AM, Elsawah H, Abdelhafez A, Bakry A, Al MW. The adjuvant effect of metformin and N-acetylcysteine to clomiphene citrate in induction of ovulation in patients with Polycystic Ovary Syndrome. Gynecol Endocrinol. 2015;31(8):635–8. https://doi.org/10.3109/09513590.2015.1037269
Ding J, Xu Y, Ma XP, et al. Estrogenic effect of the extract of Renshen (Radix Ginseng) on reproductive tissues in immature mice. J Tradit Chin Med. 2015;35(4):460–7.
Cho J, Park W, Lee S, Ahn W, Lee Y. Ginsenoside-Rb1 from Panax ginseng C.A. Meyer activates estrogen receptor-alpha and -beta, independent of ligand binding. J Clin Endocrinol Metab. 2004;89(7):3510–5. https://doi.org/10.1210/jc.2003-031823
Wu JY, Pan ZF, Wang ZQ, et al. Ginsenoside Rg1 protection against beta-amyloid peptide-induced neuronal apoptosis via estrogen receptor alpha and glucocorticoid receptor-dependent anti-protein nitration pathway. Neuropharmacology. 2012;63(3):349–61. https://doi.org/10.1016/j.neuropharm.2012.04.005
Choi JH, Jang M, Kim EJ, et al. Korean Red Ginseng alleviates dehydroepiandrosterone-induced polycystic ovarian syndrome in rats via its antiinflammatory and antioxidant activities. J Ginseng Res. 2020;44(6):790–8. https://doi.org/10.1016/j.jgr.2019.08.007
Yoshikawa M, Murakami T, Ikebata A, et al. Bioactive saponins and glycosides. 10. On the constituents of Zizyphi Spinosi Semen, the seeds of Zizyphus jujuba Mill, var spinosa Hu. 1. Structures and histamine release-inhibitory effects of jujubosides A(1) and C and acetyljujuboside B. Chem Pharm Bull. 1997;45(7):1186–92.
Li HT, Li JN, Zhang T, Xie XY, Gong JY. Antidepressant effect of Jujuboside A on corticosterone-induced depression in mice. Biochem Bioph Res Co. 2022;620:56–62. https://doi.org/10.1016/j.bbrc.2022.06.076.
Di Emidio G, Rea F, Placidi M, et al. Regulatory functions of L-Carnitine, acetyl, and propionyl L-Carnitine in a PCOS mouse model: focus on antioxidant/antiglycative molecular pathways in the ovarian microenvironment. Antioxidants (Basel). 2020;9(9). https://doi.org/10.3390/antiox9090867
Zhou X, Zheng Z, Xu C, et al. Disturbance of Mammary UDP-Glucuronosyltransferase Represses Estrogen Metabolism and Exacerbates Experimental Breast Cancer. J Pharm Sci. 2017;106(8):2152–62. https://doi.org/10.1016/j.xphs.2017.04.073
Hao Z, Xu J, Zhao H, et al. The inhibition of tamoxifen on UGT2B gene expression and enzyme activity in rat liver contribute to the estrogen homeostasis dysregulation. BMC Pharmacol Toxicol. 2022;23(1):33. https://doi.org/10.1186/s40360-022-00574-6
Sakurai S, Shimizu T, Ohto U. The crystal structure of the AhRR-ARNT heterodimer reveals the structural basis of the repression of AhR-mediated transcription. J Biol Chem. 2017;292(43):17609–16. https://doi.org/10.1074/jbc.M117.812974
Rodgers RJ, Suturina L, Lizneva D, et al. Is polycystic ovary syndrome a 20th Century phenomenon? Med Hypotheses. 2019;124:31–4. https://doi.org/10.1016/j.mehy.2019.01.019
Zeng X, Xie YJ, Liu YT, Long SL, Mo ZC. Polycystic ovarian syndrome: Correlation between hyperandrogenism, insulin resistance and obesity. Clin Chim Acta. 2020;502:214–21. https://doi.org/10.1016/j.cca.2019.11.003
Patel S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J Steroid Biochem Mol Biol. 2018;182:27–36. https://doi.org/10.1016/j.jsbmb.2018.04.008
Jazani AM, Azgomi HND, Azgomi AND, Azgomi RND. A comprehensive review of clinical studies with herbal medicine on polycystic ovary syndrome (PCOS). Daru. 2019;27(2):863–77. https://doi.org/10.1007/s40199-019-00312-0
Krishnan A, Muthusami S. Hormonal alterations in PCOS and its influence on bone metabolism. J Endocrinol. 2017;232(2):R99–113. https://doi.org/10.1530/Joe-16-0405
Balthazart J, Cornil CA, Charlier TD, Taziaux M, Ball GF. Estradiol, a Key Endocrine Signal in the Sexual Differentiation and Activation of Reproductive Behavior in Quail. J Exp Zool Part A. 2009;311a(5):323–45. https://doi.org/10.1002/jez.464
Reinen J, Vermeulen NP. Biotransformation of endocrine disrupting compounds by selected phase I and phase II enzymes–formation of estrogenic and chemically reactive metabolites by cytochromes P450 and sulfotransferases. Curr Med Chem. 2015;22(4):500–27. https://doi.org/10.2174/0929867321666140916123022
Kaderbhai MA, Kelly SL, Kaderbhai NN. Towards engineered topogenesis of cytochrome b(5) and P450 for in vivo transformation of xenobiotics. Biochem Soc T. 2006;34:1231–5. https://doi.org/10.1042/Bst0341231
Cribb AE, Knight MJ, Dryer D, et al. Role of polymorphic human cytochrome P450 enzymes in estrone oxidation. Cancer Epidem Biomar. 2006;15(3):551–8. https://doi.org/10.1158/1055-9965.Epi-05-0801
Daujat M, Clair P, Astier C, et al. Induction, regulation and messenger half-life of cytochromes P450 IA1, IA2 and IIIA6 in primary cultures of rabbit hepatocytes. CYP 1A1, 1A2 and 3A6 chromosome location in the rabbit and evidence that post-transcriptional control of gene IA2 does not involve mRNA stabilization. Eur J Biochem. 1991;200(2):501–10. https://doi.org/10.1111/j.1432-1033.1991.tb16211.x
Tompkins LM, Wallace AD. Mechanisms of cytochrome P450 induction. J Biochem Mol Toxic. 2007;21(4):176–81. https://doi.org/10.1002/jbt.20180
Vogel CFA, Van Winkle LS, Esser C, Haarmann-Stemmann T. The aryl hydrocarbon receptor as a target of environmental stressors - Implications for pollution mediated stress and inflammatory responses. Redox Biol. 2020;34:101530. https://doi.org/10.1016/j.redox.2020.101530
Couse JF, Yates MM, Deroo BJ, Korach KS. Estrogen receptor-beta is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology. 2005;146(8):3247–62. https://doi.org/10.1210/en.2005-0213
Horling K, Santos AN, Fischer B. The AhR is constitutively activated and affects granulosa cell features in the human cell line KGN. Mol Hum Reprod. 2011;17(2):104–14. https://doi.org/10.1093/molehr/gaq074
Mottershead DG, Pulkki MM, Muggalla P, et al. Characterization of recombinant human growth differentiation factor-9 signaling in ovarian granulosa cells. Mol Cell Endocrinol. 2008;283(1–2):58–67. https://doi.org/10.1016/j.mce.2007.11.007
Funding
This work was supported by the Natural Science Foundation of China (No. 82173883, China); the Science and Technology Foundation of Xuzhou (No. KC23255, China); the administration of Traditional Chinese Medicine in Jiangsu Province (No. MS2023175, China); the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (No. 18KA350002, China); the Provincial Commission of Health and Family Planning in Jiangsu Province (No. H2017079, China) and the Science and Technology Planning Project of Jiangsu Province (No. BE2019636, China).
Author information
Authors and Affiliations
Contributions
Conceptualization: Xueyan Zhou; Methodology: Nan Zhou, Wenqiang Lv, Qing He, Jiachen Ma; Formal analysis and investigation: Wenqiang Lv, Linnan Chen, Guangyan Xie; Writing—original draft: Nan Zhou, Linnan Chen; Writing—review & editing: Xueyan Zhou, Bei Zhang; Funding Acquisition: Xueyan Zhou, Bei Zhang; Supervision: Yijuan Cao.
Corresponding authors
Ethics declarations
Ethics Approval
The study was approved by the Ethics Committee of Xuzhou Central Hospital, China. (approval number: XZXY-LK-20210901–024).
Competing Interests
The authors report there are no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, N., Lv, W., Chen, L. et al. Jujuboside A Attenuates Polycystic Ovary Syndrome Based on Estrogen Metabolism Through Activating AhR-mediated CYP1A2 Expression. Reprod. Sci. (2024). https://doi.org/10.1007/s43032-024-01511-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43032-024-01511-0