Abstract
This study aimed to investigate the influence of hormonal treatment on the vaginal microbiome during fertility treatments. Bacterial vaginosis (BV) could affect fecundity, particularly in the in vitro fertilization (IVF) population, where negative effects on pregnancy outcomes have been reported. It is hypothesized that the hormone treatment during fertility treatments could influence the abundance of Lactobacilli, with negative effects on the pregnancy results. A total of 53 couples attending a fertility clinic in the Netherlands between July 2019 and August 2022 were included in this prospective cohort study. Vaginal samples were collected at start of treatment, oocyte retrieval or insemination from subjects undergoing intra uterine insemination (IUI) with mild ovarian stimulation, and IVF or intra cytoplasmatic sperm injection (ICSI) with controlled ovarian hyperstimulation. AmpliSens® Florocenosis/Bacterial vaginosis-FRT qPCR and 16S rRNA gene-based amplicon sequencing were performed on all samples. In total, 140 swabs were analyzed, with a median of two swabs per person. 33 (24%) tested qPCR BV positive. Lactobacilli percentage decreased during fertility treatments, leading to changes in the vaginal microbiome. Shannon diversity index was not significantly different. Of the total of 53 persons, nine switched from qPCR BV negative to positive during treatment. The persons switching to qPCR BV positive had already a (not significant) higher Shannon diversity index at start of treatment. If the vaginal microbiome of persons deteriorates during fertility treatments, timing of following treatments, lifestyle modifications, or a freeze all strategy could be of possible benefit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The vaginal microbiome is known to change over time, depending on menstrual cycle, suggesting that the vaginal microbiome is influenced by hormones. Lactobacilli are bacteria known to be dependent on hormonal status, especially levels of estrogen, in women. Lack of Lactobacilli is associated with an abnormal microbiome, also called bacterial vaginosis. Incidence of bacterial vaginosis (BV) can be as high as 29%, with a significant portion of persons being asymptomatic [1, 2].
It is hypothesized that the hormones used during assisted reproduction could influence the vaginal microbiome through glycogen accumulation, which is utilized by Lactobacillus [2, 3]. Recent studies in persons undergoing in vitro fertilization (IVF) have shown a negative effect of an abnormal microbiome on pregnancy outcomes [4, 5]. Over the past decade, more research has been published on the endometrial and vaginal microbiome, with a growing understanding of a continuum between the vaginal and endometrial microbiome [6].
Only a few studies have investigated the influence of external hormones on the microbiome over time. One study found no effect of vaginal progesterone treatment during pregnancy on vaginal microbiome [2] and another study reported no microbiome changes during IVF treatment [7]. Other studies reported alterations in the vaginal microbiome during IVF; however, these studies were small [3, 8]. Carosso reported a small decrease in Lactobacilli and higher bacterial diversity during the first IVF treatment session compared to pretreatment. Hyman found changes in microbiome status during IVF; however, all subjects were treated with antibiotics, which could also influence their microbiome. There is currently no literature on the impact of lower dose hormonal stimulation during intra uterine insemination (IUI) cycles on the vaginal microbiome.
This is the first study monitoring the vaginal microbiome using qPCR and sequencing during multiple consecutive fertility treatments (pretreatment and around ovulation) in a subfertile population undergoing IUI, IVF, and intracytoplasmic sperm injection (ICSI). It is hypothesized that Lactobacilli will decrease over time, potentially leading to a higher incidence of bacterial vaginosis.
Materials and Methods
This study was performed on a prospective single center cohort in the Haaglanden Medical Center (HMC) in the Hague, the Netherlands. The fertility department of the HMC collaborates with the IVF laboratory of the Leiden University Medical Center. Persons (18 years or older) undergoing fertility treatments directly after fertility assessment were included between July 2019 and June 2022. Exclusion criteria were inability to understand Dutch or English language, three or more miscarriages, and prophylactic antibiotic treatment. No couples were treated with donor sperm or donor oocytes. Microbiological assessment of vaginal swabs was done by external laboratory of Eurofins NMDL and DDL Diagnostic Laboratory in Rijswijk, The Netherlands.
Sample and Data Collection
When persons signed informed consent at their initial fertility assessment (intake), the first vaginal swab (e-swab, Copan Italia SpA, Breschia, Italy) was taken from the posterior fornix after inserting a speculum. No measurements were performed if the participants were menstruating, on hormonal therapy or post-coital. At consecutive IUI or IVF/ICSI procedures the sampling was performed prior to the ovum pick up or insemination, up to a maximum of four procedures. The vaginal sampling was performed at moment of the last ultrasound before transfer in case of a frozen embryo cycle. No new swabs were collected after an ongoing pregnancy or when switched from IUI to IVF/ICSI. The swabs were used for BV qPCR and microbiota analysis. Fertility doctors and study participants were blinded for the results of the swab. Participants with symptoms of BV were tested separately and treated according to the standard protocol when tested positive.
Follow-up ended at cessation of treatment, ongoing pregnancy or at end of study (August 2022). Data about patient characteristics and fertility treatments were extracted from the electronic patient files. A cloud-based clinical data management service, Castor EDC, was used to collect all data.
Fertility Treatments
All couples starting with IUI or IVF/ICSI at the HMC hospital need to stop smoking before starting treatment and need to have a body mass index (BMI) below 35. Fertility treatment is initiated if indicated based on the results of the fertility assessment. Couples with unexplained infertility started with IUI with mild ovarian stimulation or IVF based on female age. For IVF/ICSI protocols, both long and short agonist and antagonist protocols were applied. Estrogen (E2) levels are measured in blood in most cases before ovum pick up is scheduled. During ovum pick up, antibiotics are not routinely administered, but only given for certain indications (for example with endometriomas).
Nucleic Acid Extraction
In the HMC laboratory, the vaginal samples were frozen within 24 h and afterwards transported to the external laboratory for molecular analysis. DNA was extracted from 200 μl samples and eluted in a final volume of 100 μl with the MagNA Pure 96 instrument using the MagNA Pure 96 DNA and Pathogen Universal small Volume Kit and the Pathogen Universal protocol (Roche Diagnostics, Basel, Switzerland). Appropriate positive and negative control samples were included in testing, which were evaluated in the BV qPCR.
BV qPCR
The extracted DNA was tested according to the manufacturer’s instructions with a CE-IVD marked multiplex quantitative PCR assay (the AmpliSens® Florocenosis/Bacterial vaginosis-FRT PCR kit InterLabService, Moscow, Russia). Based on the presence of Lactobacillus species, Gardnerella vaginalis, Atopobium vaginae (recently reclassified as Fannyhessea vaginae) [9], and total amount of bacteria, swabs were labeled as BV positive (amount of G. vaginalis and/or A. vaginae is almost equal or exceeds the amount of Lactobacillus), BV negative (G. vaginalis and/or A. vaginae are absent or its amount is considerable less than the Lactobacillus amount), unspecified dysbiosis (amount of Lactobacillus is reduced relative to the total amount of bacteria, whereas G. vaginalis and/or A. vaginae are absent or its amount is considerable less than total amount of bacteria), or suspected dysbiosis (amount of G. vaginalis and/or A. vaginae is similar to the amount of Lactobacillus but does not exceed the limit value) using the software tool provided by the kit manufacturer.
Microbiota Analysis
Microbiota analysis was performed on the extracted DNA of swabs of persons with repeated measurements of vaginal microbiome. A fragment of ~421bp of the V1–V2 region of the 16S rRNA gene was amplified using the primers described by Ravel et al. and Walker et al. with Illumina overhang adaptor sequences added [10, 11]. Outcomes were categorized in one of five vaginal microbiome community state types (CST), as reported by Ravel et al. CST I is dominated by L. crispatus, and respectively CST II by L. gasseri, CST III by L. iners, CST IV by non-lactobacilli, CST V by L. jensenii [11]. Previously described literature on this subject also used these CSTs. More details on the microbiome analysis are described in the attachment.
Outcomes
Primary outcome were Lactobacilli amount and Shannon diversity index. Secondary outcomes were change of qPCR BV status or microbiome CST. Shannon diversity index (SDI) is a commonly used weighted measure to analyze the various types of bacteria in a particular environment. The SDI is zero when there is only one type of bacteria present and there is no diversity [12]. Sample size was not calculated upfront. For statistical analysis, IBM SPSS statistics for Macintosh, version 27, was used. Main outcome parameters were analyzed using a paired t test.
Results
Population
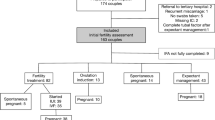
In total, 82 couples were eligible for inclusion; however, of 21 participants, there were no following swabs, and eight participants became pregnant spontaneously before starting fertility treatment. In Fig. 1, the included participants are shown. The remaining couples were multi-ethnic and most had a high socioeconomic status (Table 1). Only two persons had discharge complaints. Twenty-two persons were treated with IVF and 31 persons treated with IUI. In total 53 participants had 87 consecutive treatment cycles (in total 140 samples), with a median of two samples per person.
Microbiota Over Time
Of the 140 samples, 33 (24%) were tested positive for BV qPCR. At intake, 10 of 53 (19%) persons tested BV qPCR positive; and during treatment, 17 of the 53 (32%) tested BV qPCR positive. The 140 samples were also tested with the microbiota analysis (Supplemental figure 1). The details about the microbiome results and the comparison with qPCR are described in another paper [13].
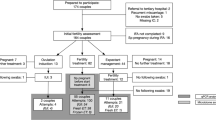
On average, the number of Lactobacilli decreased 4.6% from intake to last treatment, and 8.9% when comparing the intake swab with the lowest following swab during treatment. This decrease in Lactobacilli was significant (p = 0.01, Fig. 2). Shannon diversity index (SDI) was not significantly different from first sample (p = 0.59). During fertility treatment, more than 10% decrease of Lactobacilli can be seen in 17 of the 53 persons (and only a 10% increase in four persons). This appeared in all community state types (CST). E2 levels were measured in most IVF participants but did not show a specific association with changing microbiome (data not shown).
Of the 52 participants, in total, 27 persons had a more than 10% change in Lactobacilli, Gardnerella, or Atopobium/Fannyhessiae over time (Fig. 3). Of these 27 persons, eight, two, seven, and nine persons were classified as CST I, II, III, and IV at intake, respectively. One participant had a microbiome dominated by L. ultenesis/L. johnsonii and could not be classified in the regular CST (in Fig. 3 personID 27).
Deterioration of the Microbiota
In total, 13 persons had a deterioration of qPCR or CST result (including switching to less favorable L. iners) as shown in Table 2 (extended descriptions of which bacteria leaded to a change is shown in Supplement Table 1). Nine of these persons had IVF/ICSI treatment and four had IUI treatment. The decrease of lactobacilli resulted in a switch from BV negative to BV positive in nine persons (personID 10 at second treatment). There were no differences seen compared to the whole group based on ethnicity, reason of subfertility, gonadotropin dose, or IVF protocol (not all data shown). However, five of 14 negatively changed persons had human papillomavirus (HPV) infection last year. Also, two persons were (former) smokers. In swabs that switched from qPCR BV negative to positive, there were already a small number of non-lactobacilli bacteria present in the vaginal microbiome at baseline. The mean Shannon diversity index was 1.02 in the qPCR BV negative baseline samples who switched and 0.87 in the baseline samples that remained qPCR negative (not significant p = 0.46). The main non-lactobacilli bacteria found in these swabs were Gardnerella or Atopobium/Fannyhessiae.
In one person (personID 12), Staphylococcus and Ureaplasma were found and Ureaplasma and Gardnerella increased during treatment. In another person (personID 19), mainly Bifidobacterium, Streptococcus, and Prevotella were found, and Gardnerella was increased during treatment. One subject changed to qPCR BV positive during one of the IUI cycles (personID 5) just after an antibiotic treatment for a urinary tract infection. One person (personID 17) had a dramatic decrease of Lactobacilli during IVF treatment and switched to qPCR BV positive. This person turned back to qPCR BV negative during her frozen embryo transfer in her natural cycle. In total, 11 persons switched from microbiome CST. Two persons (personID 3 and 7) who changed from CST I to CST III had already a low amount of L. iners in the first swab, one of them stopped smoking (personID 7).
Improvement of the Microbiota
Three persons had an improvement of their microbiome. Two persons switched from qPCR BV positive to negative during IUI treatments. One (personID 18) switched to qPCR BV negative because of treatment with antibiotics for discharge complaints. The other person (personID 25) stopped smoking before starting treatment. One person switched (personID 24) from qPCR BV positive to negative during first IVF treatment. The person lost some weight prior to start with IVF (BMI below 35 at start IVF). With the second IVF stimulation, this person switched back to qPCR BV positive again. A different stimulation protocol with urinary FSH was used this second time, compared to recombinant FSH the first stimulation.
Discussion
This is the first study investigating the impact of hormones during multiple fertility treatments on the vaginal microbiome. This study showed that fertility treatment resulted in a decrease in the rate of Lactobacilli, ranging from 4.6 to 8.9% in the worst case. Shannon diversity index did not show a significant difference, which suggests that the decrease in Lactobacilli is most prominent in samples that were already more diverse at intake. At intake, 10 of 53 (19%) persons tested BV qPCR positive; and during treatment, 17 of the 53 (32%) tested BV qPCR positive. In total, 13 persons showed a deterioration in their BV PCR and/or microbiome result (including changing to less favorable L. iners). In contrast, only three persons showed an improvement of their microbiome status. This could be probably intentional because these persons were trying to improve their lifestyle.
A strength of this study are the multiple observations during consecutive treatment cycles. Earlier studies only examined the microbiome prior to IVF treatment and during the first treatment, with less than 30 participants [3, 7, 8]. Carosso et al. did find a decrease of Lactobacilli; however, this was not statistically significant. Possibly, Lactobacilli decrease more pronounced after undergoing multiple treatments. Another strength is the heterogenous population studied, which includes non-Caucasian persons and a mix of IUI and IVF/ICSI treatment protocols. This enhances the applicability of the findings and allows for a broader understanding of the potential impact of fertility treatments on the vaginal microbiome.
This decrease in Lactobacilli and negative change of microbiome status could have a negative effect on ongoing pregnancy rates. Another paper by this research group showed that samples with a consecutive miscarriage had 15% less Lactobacilli compared to samples with a consecutive ongoing pregnancy (however not significant) [13]. The numbers in this study were too low to calculate specific pregnancy outcomes for changing qPCR or microbiome status compared to persons with a stable microbiome. It should be further investigated which amount of decrease of Lactobacilli is clinically relevant. Understanding the fluctuations of the vaginal microbiome and knowing which persons are at risk could improve fertility treatments in the future.
This study is the largest study conducted on this subject to date; however, the numbers of subjects is still limited because this study is a sub-analysis of a larger prospective study. This study did not consider sexual intercourse and the seminal microbiome around time of sampling, which could influence the change of vaginal microbiome as well. Another limitation could be the variability in hormonal treatment protocols utilized. The most common stimulation was with recombinant gonadotropins, and an agonist protocol in IVF. However, the decrease of Lactobacilli was observed in both IUI and IVF, suggesting that it is not solely depending on specific protocols (recombinant or urinary FSH, antagonist or agonist protocol) or on E2 levels, as previously suggested by Hyman et al.
The change of vaginal microbiome could be related to overall health status or the immune system. The persons with a deteriorated microbiome during treatment were found to be more frequently smokers and/or tested positive for HPV (human papillomavirus). The correlation between HPV infections and abnormal microbiota has already been reported in literature before [14]. Healthier lifestyle might have a positive impact on the microbiome. Three cases presented here show this positive effect; however, it is important to note that these examples are anecdotal, and this is insufficient evidence for a broader conclusion. If lifestyle changes could reduce recurrence of BV after treatment or prevent the worsening of the microbiome during fertility treatment, this could serve as additional motivation for persons to adopt healthier lifestyle before conception.
Vaginal microbiome testing to monitor changes in Lactobacilli levels during IUI or IVF treatment could provide valuable insights. Future research should focus on the microbiome and pregnancy results in the context of multiple sequential fertility treatments. This study showed one case in which the vaginal microbiome returned to normal during frozen embryo transfer (personID 17). If future research can show a clinically relevant association between the vaginal microbiome and pregnancy outcomes, it may lead to different treatment strategies. Treatment options such as freeze all strategy, introducing pauses between treatments, vaginal probiotics, and lifestyle interventions could be investigated further to improve pregnancy outcomes.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Ravel J, Brotman RM, Gajer P, Ma B, Nandy M, Fadrosh DW, et al. Daily temporal dynamics of vaginal microbiota before, during and after episodes of bacterial vaginosis. Microbiome. 2013; Available from: http://www.ncbi.nlm.nih.gov/. Accessed 23 Feb 2023.
Kindinger LM, Bennett PR, Lee YS, Marchesi JR, Smith A, Cacciatore S, et al. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome. 2017;5:6. http://microbiomejournal.biomedcentral.com/articles/10.1186/s40168-016-0223-9..
Hyman RW, Herndon CN, Jiang H, Palm C, Fukushima M, Bernstein D, et al. The dynamics of the vaginal microbiome during infertility therapy with in vitro fertilization-embryo transfer. J Assist Reprod Genet. 2012;29:105–15. https://doi.org/10.1007/s10815-011-9694-6.
Koedooder R, Singer M, Schoenmakers S, Savelkoul PHM, Morré SA, de Jonge JD, et al. The vaginal microbiome as a predictor for outcome of in vitro fertilization with or without intracytoplasmic sperm injection: a prospective study. Hum Reprod. 2019;34:1042–54. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=31119299&retmode=ref&cmd=prlinks. Accessed 28 Jul 2022
Moreno I, Garcia-Grau I, Perez-Villaroya D, Gonzalez-Monfort M, Bahceci M, Barrionuevo MJ, et al. Endometrial microbiota composition is associated with reproductive outcome in infertile patients. Microbiome. 2022;10:1–17. https://doi.org/10.1186/s40168-021-01184-w.
Chen C, Song X, Wei W, Zhong H, Dai J, Lan Z, et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat Commun. 2017;8:811–75. Available from: https://www.nature.com/articles/s41467-017-00901-0.
Zhao C, Wei Z, Yang J, Zhang J, Yu C, Yang A, et al. Characterization of the vaginal microbiome in women with infertility and its potential correlation with hormone stimulation during in vitro fertilization surgery. mSystems. 2020;5. https://doi.org/10.1128/mSystems.00450-20.
Carosso A, Revelli A, Gennarelli G, Canosa S, Cosma S, Borella F, et al. Controlled ovarian stimulation and progesterone supplementation affect vaginal and endometrial microbiota in IVF cycles: a pilot study. J Assist Reprod Genet. 2020;37:2315–26. https://doi.org/10.1007/s10815-020-01878-4.
Nouioui I, Carro L, García-López M, Meier-Kolthoff JP, Woyke T, Kyrpides NC, et al. Genome-based taxonomic classification of the phylum Actinobacteria. Front Microbiol. 2018;9:2007. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30186281. Accessed 14 Oct 2022.
Walker AW, Martin JC, Scott P, Parkhill J, Flint HJ, Scott KP. 16S rRNA gene-based profiling of the human infant gut microbiota is strongly influenced by sample processing and PCR primer choice. Microbiome. 2015;3. Available from: https://pubmed.ncbi.nlm.nih.gov/26120470/. Accessed 14 Oct 2022.
Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SSK, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011;108:4680–7. Available from: http://www.pmc/articles/PMC3063603/. Accessed 14 Oct 2022.
Hyman RW, Fukushima M, Jiang H, Fung E, Rand L, Johnson B, et al. Diversity of the vaginal microbiome correlates with preterm birth. Reprod Sci. 2014;21:32–40.
van den Tweel MM, van den Munckhof EHA, van der Zanden M, Molijn AC, van Lith JMM, Le Cessie S, et al. Bacterial vaginosis in a subfertile population undergoing fertility treatments: a prospective cohort study. J Assist Reprod Genet. 2023. https://doi.org/10.1007/s10815-023-03000-w
Moreno E, Ron R, Serrano-Villar S. The microbiota as a modulator of mucosal inflammation and HIV/HPV pathogenesis: from association to causation. Front Immunol. 2023;14:103.
Acknowledgements
A special thanks to the HMC fertility doctors and their team for including participants and retrieving samples.
Funding
This study was funded by a grant from the Research Fund of the Haaglanden Medical Center (2021) and Stichting Researchfonds Bronovo. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
MMvdT: protocol/project development, data collection and management, data analysis, manuscript writing. EHAvdM: protocol/ project development, microbiome data analysis, manuscript editing. MvdZ: including participants and sample collection, data analysis, manuscript editing. AM: protocol/project development, microbiome data analysis, manuscript editing. JMMvL: manuscript editing, critical discussion KEB: protocol/project development, data analysis, manuscript editing.
Corresponding author
Ethics declarations
Ethics Approval
This study was approved by the Medical Ethics Committee of Leiden Den Haag Delft, reference Z21.031.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
Informed consent was obtained from all individual participants included in the study.
Competing Interests
The authors have stated explicitly that they have no conflict of interest in connection with this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary file 1
Supplemental Fig. 1: all microbiome samples arranged per person, categorized on community state type microbiome on basis of their first sample at intake. Persons used in Figure 3 are marked with their personID in this figure as well. (TIFF 62458 kb)
Supplementary file 2
Supplemental table 1: detailed description of persons with changed microbiome (XLSX 10 kb)
Supplementary file 3
(DOCX 27 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van den Tweel, M., van den Munckhof, E., van der Zanden, M. et al. The Vaginal Microbiome Changes During Various Fertility Treatments. Reprod. Sci. 31, 1593–1600 (2024). https://doi.org/10.1007/s43032-024-01484-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-024-01484-0