Abstract
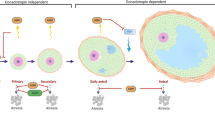
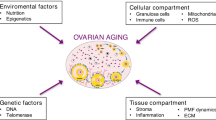
Female fertility decreases during aging. The development of effective therapeutic strategies to address the age-related decline in oocyte quality and quantity and its accurate diagnosis remain major challenges. In this review, we summarize our current understanding of the study of aging and infertility, focusing primarily on the molecular basis of energy metabolism, mitochondrial function, and redox homeostasis in granulosa cells and oocytes, and discuss perspectives on future research directions. Mitochondria serve as a central hub sensing a multitude of physiological processes, including energy production, cellular redox homeostasis, aging, and senescence. Young granulosa cells favor glycolysis and actively produce pyruvate, NADPH, and other metabolites. Oocytes rely on oxidative phosphorylation fueled by nutrients, metabolites, and antioxidants provided by the adjacent granulosa cells. A reduced cellular energy metabolism phenotype, including both aerobic glycolysis and mitochondrial respiration, is characteristic of older female granulosa cells compared with younger female granulosa cells. Aged oocytes become more susceptible to oxidative damage to cells and mitochondria because of further depletion of antioxidant-dependent ROS scavenging systems. Molecular perturbations of gene expression caused by a subtle change in the follicular fluid microenvironment adversely affect energy metabolism and mitochondrial dynamics in granulosa cells and oocytes, further causing redox imbalance and accelerating aging and senescence. Furthermore, recent advances in technology are beginning to identify biofluid molecular markers that may influence follicular development and oocyte quality. Accumulating evidence suggests that redox imbalance caused by abnormal energy metabolism and/or mitochondrial dysfunction is closely linked to the pathophysiology of age-related subfertility.


Similar content being viewed by others
Data Availability
No new data were created.
References
Secomandi L, Borghesan M, Velarde M, Demaria M. The role of cellular senescence in female reproductive aging and the potential for senotherapeutic interventions. Hum Reprod Update. 2022;28(2):172–89.
Cecchino GN, García-Velasco JA, Rial E. Reproductive senescence impairs the energy metabolism of human luteinized granulosa cells. Reprod Biomed Online. 2021;43(5):779–87.
Kansaku K, Takeo S, Itami N, Kin A, Shirasuna K, Kuwayama T, et al. Maternal aging affects oocyte resilience to carbonyl cyanide-m-chlorophenylhydrazone-induced mitochondrial dysfunction in cows. PLoS ONE. 2017;12(11):e0188099.
Ferraretti AP, Goossens V, de Mouzon J, Bhattacharya S, Castilla JA, Korsak V, et al. European IVF-monitoring (EIM); Consortium for European Society of Human Reproduction and Embryology (ESHRE). Assisted reproductive technology in Europe, 2008: results generated from European registers by ESHRE. Hum Reprod. 2012;27(9):2571–84.
Ahelik A, Mändar R, Korrovits P, Karits P, Talving E, Rosenstein K, et al. Systemic oxidative stress could predict assisted reproductive technique outcome. J Assist Reprod Genet. 2015;32(5):699–704.
Kil IS, Huh TL, Lee YS, Lee YM, Park JW. Regulation of replicative senescence by NADP+ -dependent isocitrate dehydrogenase. Free Radic Biol Med. 2006;40(1):110–9.
Dumollard R, Carroll J, Duchen MR, Campbell K, Swann K. Mitochondrial function and redox state in mammalian embryos. Semin Cell Dev Biol. 2009;20(3):346–53.
Cetica PD, Pintos LN, Dalvit GC, Beconi MT. Antioxidant enzyme activity and oxidative stress in bovine oocyte in vitro maturation. IUBMB Life. 2001;51(1):57–64.
Nishihara T, Matsumoto K, Hosoi Y, Morimoto Y. Evaluation of antioxidant status and oxidative stress markers in follicular fluid for human in vitro fertilization outcome. Reprod Med Biol. 2018;17(4):481–6.
Gong Y, Zhang K, Xiong D, Wei J, Tan H, Qin S. Growth hormone alleviates oxidative stress and improves the IVF outcomes of poor ovarian responders: a randomized controlled trial. Reprod Biol Endocrinol. 2020;18(1):91.
Wang L, Tang J, Wang L, Tan F, Song H, Zhou J, et al. Oxidative stress in oocyte aging and female reproduction. J Cell Physiol. 2021;236(12):7966–83.
Liu J, Liu M, Ye X, Liu K, Huang J, Wang L, et al. Delay in oocyte aging in mice by the antioxidant N-acetyl-L-cysteine (NAC). Hum Reprod. 2012;27(5):1411–20.
Shi L, Zhang J, Lai Z, Tian Y, Fang L, Wu M, et al. Long-term moderate oxidative stress decreased ovarian reproductive function by reducing follicle quality and rogesterone production. PLoS ONE. 2016;11(9):e0162194.
Meseguer M, Martínez-Conejero JA, O’Connor JE, Pellicer A, Remohí J, Garrido N. The significance of sperm DNA oxidation in embryo development and reproductive outcome in an oocyte donation program: a new model to study a male infertility prognostic factor. Fertil Steril. 2008;89(5):1191–9.
Rizzo A, Roscino MT, Binetti F, Sciorsci RL. Roles of reactive oxygen species in female reproduction. Reprod Domest Anim. 2012;47(2):344–52.
Park SU, Walsh L, Berkowitz KM. Mechanisms of ovarian aging. Reproduction. 2021;162(2):R19–33.
Xie HL, Zhu S, Zhang J, Wen J, Yuan HJ, Pan LZ, et al. Glucose metabolism during in vitro maturation of mouse oocytes: an study using RNA interference. J Cell Physiol. 2018;233(9):6952–64.
Fontana J, Martínková S, Petr J, Žalmanová T, Trnka J. Metabolic cooperation in the ovarian follicle. Physiol Res. 2020;69(1):33–48.
Kirillova A, Smitz JEJ, Sukhikh GT, Mazunin I. the role of mitochondria in oocyte maturation. Cells. 2021;10(9):2484.
Immediata V, Ronchetti C, Spadaro D, Cirillo F, Levi-Setti PE. Oxidative stress and human ovarian response-from somatic ovarian cells to oocytes damage: a clinical comprehensive narrative review. Antioxidants (Basel). 2022;11(7):1335.
Bisht S, Faiq M, Tolahunase M, Dada R. Oxidative stress and male infertility. Nat Rev Urol. 2017;14:470–85.
Lenaz G. Mitochondria and reactive oxygen species. Which role in physiology and pathology? Adv Exp Med Biol. 2012;942:93–136.
Wiener-Megnazi Z, Vardi L, Lissak A, Shnizer S, Reznick AZ, Ishai D, et al. Oxidative stress indices in follicular fluid as measured by the thermochemiluminescence assay correlate with outcome parameters in in vitro fertilization. Fertil Steril. 2004;82(Suppl 3):1171–6.
Hiona A, Leeuwenburgh C. The role of mitochondrial DNA mutations in aging and sarcopenia: implications for the mitochondrial vicious cycle theory of aging. Exp Gerontol. 2008;43(1):24–33.
Guérin P, El Mouatassim S, Ménézo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update Mar-Apr. 2001;7(2):175–89.
Talalay P. Chemoprotection against cancer by induction of Phase 2 enzymes. BioFactors. 2000;12:5–11.
Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J. 2016;15(1):71.
Nagata S, Tatematsu K, Kansaku K, Inoue Y, Kobayashi M, Shirasuna K, et al. Effect of aging on mitochondria and metabolism of bovine granulosa cells. J Reprod Dev. 2020;66(6):547–54.
Koh JH, Kim YW, Seo DY, Sohn TS. Mitochondrial TFAM as a signaling regulator between cellular organelles: a perspective on metabolic diseases. Diabetes Metab J. 2021;45(6):853–65.
Wang S, Zheng Y, Li J, Yu Y, Zhang W, Song M, et al. Single-cell transcriptomic atlas of primate ovarian aging. Cell. 2020;180(3):585-600.e19.
Qian Y, Shao L, Yuan C, Jiang CY, Liu J, Gao C, et al. Implication of differential peroxiredoxin 4 expression with age in ovaries of mouse and human for ovarian aging. Curr Mol Med. 2016;16(3):243–51.
Perheentupa A, Huhtaniemi I. Aging of the human ovary and testis. Mol Cell Endocrinol. 2009;299:2–13.
Debbarh H, Louanjli N, Aboulmaouahib S, Jamil M, Ahbbas L, Kaarouch I, et al. Antioxidant activities and lipid peroxidation status in human follicular fluid: age-dependent change. Zygote. 2021;29(6):490–4.
Zorn B, Vidmar G, Meden-Vrtovec H. Seminal reactive oxygen species as predictors of fertilization, embryo quality and pregnancy rates after conventional in vitro fertilization and intracytoplasmic sperm injection. Int J Androl. 2003;26:279–85.
Fan L, Guan F, Ma Y, Zhang Y, Li L, Sun Y, et al. N-Acetylcysteine improves oocyte quality through modulating the Nrf2 signaling pathway to ameliorate oxidative stress caused by repeated controlled ovarian hyperstimulation. Reprod Fertil Dev. 2022;34(10):736–50.
Lin X, Dai Y, Tong X, Xu W, Huang Q, Jin X, et al. Excessive oxidative stress in cumulus granulosa cells induced cell senescence contributes to endometriosis-associated infertility. Redox Biol. 2020;30:101431.
Budani MC, Carletti E, Tiboni GM. Cigarette smoke is associated with altered expression of antioxidant enzymes in granulosa cells from women undergoing in vitro fertilization. Zygote. 2017;25(3):296–303.
Costantino S, Paneni F, Cosentino F. Ageing, metabolism and cardiovascular disease. J Physiol. 2016;594(8):2061–73.
Dumollard R, Campbell K, Halet G, Carroll J, Swann K. Regulation of cytosolic and mitochondrial ATP levels in mouse eggs and zygotes. Dev Biol. 2008;316:431–40.
Li R, Albertini DF. The road to maturation: somatic cell interaction and self-organization of the mammalian oocyte. Nat Rev Mol Cell Biol. 2013;14:141–52.
Rimon-Dahari N, Yerushalmi-Heinemann L, Alyagor L, Dekel N. Ovarian folliculogenesis. Results Probl Cell Differ. 2016;58:167–90.
Orisaka M, Tajima K, Tsang BK, Kotsuji F. Oocyte-granulosa-theca cell interactions during preantral follicular development. J Ovarian Res. 2009;2(1):9.
Wang Q, Chi MM, Schedl T, Moley KH. An intercellular pathway for glucose transport into mouse oocytes. Am J Physiol Endocrinol Metab. 2012;302(12):E1511–8.
Simerman AA, Hill DL, Grogan TR, Elashoff D, Clarke NJ, Goldstein EH, et al. Intrafollicular cortisol levels inversely correlate with cumulus cell lipid content as a possible energy source during oocyte meiotic resumption in women undergoing ovarian stimulation for in vitro fertilization. Fertil Steril. 2015;103(1):249–57.
Johnson MT, Freeman EA, Gardner DK, Hunt PA. Oxidative metabolism of pyruvate is required for meiotic maturation of murine oocytes in vivo. Biol Reprod. 2007;77(1):2–8.
Appeltant R, Somfai T, Nakai M, Bodó S, Maes D, Kikuchi K, et al. Interactions between oocytes and cumulus cells during in vitro maturation of porcine cumulus-oocyte complexes in a chemically defined medium: effect of denuded oocytes on cumulus expansion and oocyte maturation. Theriogenology. 2015;83(4):567–76.
Redding GP, Bronlund JE, Hart AL. Theoretical investigation into the dissolved oxygen levels in follicular fluid of the developing human follicle using mathematical modelling. Reprod Fertil Dev. 2008;20(3):408–17.
Shiratsuki S, Hara T, Munakata Y, Shirasuna K, Kuwayama T, Iwata H. Low oxygen level increases proliferation and metabolic changes in bovine granulosa cells. Mol Cell Endocrinol. 2016;5(437):75–85.
Kind KL, Tam KK, Banwell KM, Gauld AD, Russell DL, Macpherson AM, et al. Oxygen-regulated gene expression in murine cumulus cells. Reprod Fertil Dev. 2015;27(2):407–18.
Baptista I, Karakitsou E, Cazier JB, Günther UL, Marin S, Cascante M. TKTL1 Knockdown impairs hypoxia-induced glucose-6-phosphate dehydrogenase and glyceraldehyde-3-phosphate dehydrogenase overexpression. Int J Mol Sci. 2022;23(7):3574.
Guo J, Min CG, Zhang KY, Zhan CL, Wang YC, Hou SK, et al. Tetrabromobisphenol exposure impairs bovine oocyte maturation by inducing mitochondrial dysfunction. Molecules. 2022;27(22):8111.
Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3(3):177–85.
Martin E, Rosenthal RE, Fiskum G. Pyruvate dehydrogenase complex: metabolic link to ischemic brain injury and target of oxidative stress. J Neurosci Res. 2005;79(1–2):240–7.
Imanaka S, Shigetomi H, Kobayashi H. Reprogramming of glucose metabolism of cumulus cells and oocytes and its therapeutic significance. Reprod Sci. 2022;29(3):653–67.
Kansaku K, Itami N, Kawahara-Miki R, Shirasuna K, Kuwayama T, Iwata H. Differential effects of mitochondrial inhibitors on porcine granulosa cells and oocytes. Theriogenology. 2017;103:98–103.
Xie HL, Wang YB, Jiao GZ, Kong DL, Li Q, Li H, et al. Effects of glucose metabolism during in vitro maturation on cytoplasmic maturation of mouse oocytes. Sci Rep. 2016;9(6):20764.
Park SH, Lee AR, Choi K, Joung S, Yoon JB, Kim S. TOMM20 as a potential therapeutic target of colorectal cancer. BMB Rep. 2019;52(12):712–7.
Yu Y, Dumollard R, Rossbach A, Lai FA, Swann K. Redistribution of mitochondria leads to bursts of ATP production during spontaneous mouse oocyte maturation. J Cell Physiol. 2010;224:672–80.
Dalton CM, Szabadkai G, Carroll J. Measurement of ATP in single oocytes: impact of maturation and cumulus cells on levels and consumption. J Cell Physiol. 2014;229(3):353–61.
Wang T, Zhang M, Jiang Z. Seli. Mitochondrial dysfunction and ovarian aging. Am J Reprod Immunol. 2017;77:5.
Uhde K, van Tol HTA, Stout TAE, Roelen BAJ. Metabolomic profiles of bovine cumulus cells and cumulus-oocyte-complex-conditioned medium during maturation in vitro. Sci Rep. 2018;8(1):9477.
Pizarro BM, Cordeiro A, Reginatto MW, Campos SPC, Mancebo ACA, Areas PCF, et al. Estradiol and progesterone levels are related to redox status in the follicular fluid during in vitro fertilization. J Endocr Soc. 2020;4(7):bvaa064.
Luti S, Fiaschi T, Magherini F, Modesti PA, Piomboni P, Governini L, et al. Relationship between the metabolic and lipid profile in follicular fluid of women undergoing in vitro fertilization. Mol Reprod Dev. 2020;87(9):986–97.
Cambi M, Tamburrino L, Marchiani S, Olivito B, Azzari C, Forti G, et al. Development of a specific method to evaluate 8-hydroxy, 2-deoxyguanosine in sperm nuclei: relationship with semen quality in a cohort of 94 subjects. Reproduction. 2013;145(3):227–35.
Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. 2008;44(3):280–7.
Mukheef MA, Ali RA, Alheidery HHA. Follicular fluid 8-Hydroxy-2-Deoxyguanosine (8-OHdG) as biomarker for oxidative stress in intracytoplasmic sperm injection. J Med Invest. 2022;69(1.2):112–6.
Oral O, Kutlu T, Aksoy E, Fiçicioğlu C, Uslu H, Tuğrul S. The effects of oxidative stress on outcomes of assisted reproductive techniques. J Assist Reprod Genet. 2006;23(2):81–5.
Borowiecka M, Wojsiat J, Polac I, Radwan M, Radwan P, Zbikowska HM. Oxidative stress markers in follicular fluid of women undergoing in vitro fertilization and embryo transfer. Syst Biol Reprod Med. 2012;58(6):301–5.
Jozwik M, Wolczynski S, Jozwik M, Szamatowicz ML. Oxidative stress markers in preovulatory follicular fluid in humans. Mol Hum Reprod. 1999;5:409–13.
Rubio CP, Hernández-Ruiz J, Martinez-Subiela S, Tvarijonaviciute A, Ceron JJ. Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: an update. BMC Vet Res. 2016;12(1):166.
Appasamy M, Jauniaux E, Serhal P, Al-Qahtani A, Groome NP, Muttukrishna S. Evaluation of the relationship between follicular fluid oxidative stress, ovarian hormones, and response to gonadotropin stimulation. Fertil Steril. 2008;89(4):912–21.
Jana SK, NB K, Chattopadhyay R, Chakravarty B, Chaudhury K. Upper control limit of reactive oxygen species in follicular fluid beyond which viable embryo formation is not favorable. Reprod Toxicol. 2010;29:447–51.
Pasqualotto EB, Agarwal A, Sharma RK, Izzo VM, Pinotti JA, Joshi NJ, Rose BI. Effect of oxidative stress in follicular fluid on the outcome of assisted reproductive procedures. Fertil Steril. 2004;81(4):973–6.
Kreheľová A, Kovaříková V, Domoráková I, Solár P, Pastornická A, Pavliuk-Karachevtseva A, et al. Characterization of glutathione peroxidase 4 in rat oocytes, preimplantation embryos, and selected maternal tissues during early development and implantation. Int J Mol Sci. 2021;22(10):5174.
El Mouatassim S, Guerin P, Menezo Y. Expression of genes encoding antioxidant enzymes in human and mouse oocytes during the final stages of maturation. Mol Hum Reprod. 1999;5:720–5.
Koli R, Chowdary H, Gupta S, Williams J, Agarwal A, Combelles C. Correlation between the dynamics of total antioxidant capacity (TAC) and glutathione peroxidase (GPx) activity and the sizes of bovine antral follicles and follicle dominance. Fertil Steril. 2007;88:S303.
Carbone MC, Tatone C, Delle Monache S, Marci R, Caserta D, Colonna R, et al. Antioxidant enzymatic defences in human follicular fluid: characterization and age-dependent changes. Mol Hum Reprod. 2003;9:639–43.
Jingyun Z, Zhaoyan N, Xianglong K, Liqian, Na Z, Lvcuiting, et al. Study on the relationship between SlRTl and oxidative stress in aged patients undergoing in vitro fertilization and embryo transfer cycles. J Gynecol Obstet Hum Reprod. 2023;52(1):102516.
Paszkowski T, Traub AI, Robinson SY, McMaster D. Selenium dependent glutathione peroxidase activity in human follicular fluid. Clin Chim Acta. 1995;236:173–80.
von Mengden L, De Bastiani MA, Arruda LS, Link CA, Klamt F. Cumulus cell antioxidant system is modulated by patients’ clinical characteristics and correlates with embryo development. J Assist Reprod Genet. 2022;39(6):1277–95.
Soria-Tiedemann M, Michel G, Urban I, Aldrovandi M, O’Donnell VB, Stehling S, et al. Unbalanced expression of glutathione peroxidase 4 and arachidonate 15-lipoxygenase affects acrosome reaction and in vitro fertilization. Int J Mol Sci. 2022;23(17):9907.
Al-Saleh I, Coskun S, Al-Doush I, Al-Rajudi T, Al-Rouqi R, Abduljabbar M, et al. Exposure to phthalates in couples undergoing in vitro fertilization treatment and its association with oxidative stress and DNA damage. Environ Res. 2019;169:396–408.
Younis A, Clower C, Nelsen D, Butler W, Carvalho A, Hok E, et al. The relationship between pregnancy and oxidative stress markers on patients undergoing ovarian stimulations. J Assist Reprod Genet. 2012;29(10):1083–9.
Lazzarino G, Pallisco R, Bilotta G, Listorti I, Mangione R, Saab MW, et al. Altered follicular fluid metabolic pattern correlates with female infertility and outcome measures of in vitro fertilization. Int J Mol Sci. 2021;22(16):8735.
Yuan C, Li Z, Zhao Y, Wang X, Chen L, Zhao Z, et al. Follicular fluid exosomes: important modulator in proliferation and steroid synthesis of porcine granulosa cells. FASEB J. 2021;35(5):e21610.
Acknowledgements
Figures were created by Toyomi Kobayashi (Ms.Clinic MayOne, Nara, Japan; https://www.mscl-mayone.com/; accessed on 9 July 2023).
Author information
Authors and Affiliations
Contributions
Conception and design, H.K. Acquisition of data, S.M., C.Y., and H.S. Analysis and Interpretation of data, S.I. Drafting of the manuscript, H.K. Critical revision of the manuscript for important intellectual content, S.M., C.Y., H.S., and S.I. Statistical analysis, S.I. Administrative technical or material support, H.K. Supervision, S.I. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The submitted paper is a review article and has not been approved by the Institutional Review Board and the Research and Ethical Committee of Nara Medical University Graduate School of Medicine, Kashihara, Japan.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kobayashi, H., Yoshimoto, C., Matsubara, S. et al. Altered Energy Metabolism, Mitochondrial Dysfunction, and Redox Imbalance Influencing Reproductive Performance in Granulosa Cells and Oocyte During Aging. Reprod. Sci. 31, 906–916 (2024). https://doi.org/10.1007/s43032-023-01394-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-023-01394-7