Abstract
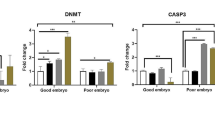
Phthalates are a class of environmental endocrine disrupting chemicals which can cause reproductive system damages. However, data about reproductive toxicity spectrum of phthalate metabolites among Chinese women undergoing in vitro fertilization (IVF) treatments are scarce yet. Previous studies regarding underlying embryo toxicities focused on oxidative stress and apoptosis, while energy metabolism abnormality might be another key cause for embryo developmental disruptions. Here, we found that among the measured eight phthalate metabolites, monomethyl phthalate (MMP) had the second highest urinary concentration in women receiving IVF. Compare to the lowest exposure level group, MMP in tertile 3 was associated with fewer counts of oocyte retrieved and good-quality embryos, and MMP in tertile 2 was correlated with reduced good-quality embryo rate. The direct embryo toxicities of MMP were studied using mouse 2-cell embryos. Consistent to results found in human populations, exposure to MMP induced mouse early embryo developmental delay. Furthermore, MMP exposure led to excessive reactive oxygen species production in early embryos, and antioxidant can partially rescue the early embryo development slow down. Embryo apoptosis could also be caused by oxidative stress. To be noted, elevated apoptosis level was not found in live “slow” embryos but dead embryos, which suggested that apoptosis was not related to early embryo developmental delay. Additionally, MMP exposure depleted adenosine triphosphate (ATP) synthesis of early embryos, which could be reversed by antioxidant. In conclusion, MMP, as the newly found embryonic toxicant in Chinese women, resulted in early embryo development delay, apoptosis, and energy metabolism disruptions via inducing redox imbalance.




Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this article.
Code Availability
Not applicable.
Abbreviations
- IVF:
-
In vitro fertilization
- MMP:
-
Monomethyl phthalate
- MBP:
-
Mono-n-butyl phthalate
- MEP:
-
Monoethyl phthalate
- MEHP:
-
Mono (2-ethylhexyl) phthalate
- MEOHP:
-
Mono (2-ethyl-5-oxohexyl) phthalate
- MEHHP:
-
Mono (2-ethyl-5-hydroxyhexyl) phthalate
- MOP:
-
Mono-n-octyl phthalate
- MBzP:
-
Monobenzyl phthalate
- ROS:
-
Reactive oxygen species
- ATP:
-
Adenosine triphosphate
- NAC:
-
N-acetyl-L-cysteine
References
Qureshi MS, Yusoff ARBM, Wirzal MDH, SirajuddinBarek J, Afridi HI, Üstündag Z. Methods for the determination of endocrine-disrupting phthalate esters. Crit Rev Anal Chem. 2016;46(2):146–59.
Haverinen E, Fernandez MF, Mustieles V, Tolonen H. Metabolic syndrome and endocrine disrupting chemicals: an overview of exposure and health effects. Int J Environ Res Public Health. 2021. https://doi.org/10.3390/ijerph182413047.
Zhang YJ, Guo JL, Xue JC, Bai CL, Guo Y. Phthalate metabolites: characterization, toxicities, global distribution, and exposure assessment. Environ Pollut. 2021. https://doi.org/10.1016/j.envpol.2021.118106.
Calafat AM, Slakman AR, Silva MJ, Herbert AR, Needham LL. Automated solid phase extraction and quantitative analysis of human milk for 13 phthalate metabolites. J Chromatogr B. 2004. https://doi.org/10.1016/j.jchromb.2004.02.006.
Du YY, Fang YL, Wang YX, Zeng Q, Guo N, Zhao H, Li YF. Follicular fluid and urinary concentrations of phthalate metabolites among infertile women and associations with in vitro fertilization parameters. Reprod Toxicol. 2016. https://doi.org/10.1016/j.reprotox.2016.04.005.
Hauser R, Gaskins AJ, Souter I, Smith KW, Dodge LE, Ehrlich S, Meeker JD, Calafat AM, Williams PL, E.S. Team. Urinary phthalate metabolite concentrations and reproductive outcomes among women undergoing in vitro fertilization: results from the EARTH study. Environ Health Perspect. 2016. https://doi.org/10.1289/ehp.1509760.
Machtinger R, Gaskins AJ, Racowsky C, Mansur A, Adir M, Baccarelli AA, Calafat AM, Hauser R. Urinary concentrations of biomarkers of phthalates and phthalate alternatives and IVF outcomes. Environ Int. 2018. https://doi.org/10.1016/j.envint.2017.11.011.
Wu H, Ashcraft L, Whitcomb BW, Rahil T, Tougias E, Sites CK, Pilsner JR. Parental contributions to early embryo development: influences of urinary phthalate and phthalate alternatives among couples undergoing IVF treatment. Hum Reprod. 2017. https://doi.org/10.1093/humrep/dew301.
Wu H, Estill MS, Shershebnev A, Suvorov A, Krawetz SA, Whitcomb BW, Dinnie H, Rahil T, Sites CK, Pilsner JR. Preconception urinary phthalate concentrations and sperm DNA methylation profiles among men undergoing IVF treatment: a cross-sectional study. Hum Reprod. 2017. https://doi.org/10.1093/humrep/dex283.
Spinder N, Bergman JEH, van Tongeren M, Boezen HM, Kromhout H, de Walle HEK. Maternal occupational exposure to endocrine-disrupting chemicals and urogenital anomalies in the offspring. Hum Reprod. 2021. https://doi.org/10.1093/humrep/deab205.
Tang ZR, Xu XL, Deng SL, Lian ZX, Yu K. Oestrogenic endocrine disruptors in the placenta and the fetus. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21041519.
Mose T, Knudsen LE, Hedegaard M, Mortensen GK. Transplacental transfer of monomethyl phthalate and mono(2-ethylhexyl) phthalate in a human placenta perfusion system. Int J Toxicol. 2007. https://doi.org/10.1080/10915810701352721.
Zhang H, Gao F, Ben Y, Su Y. Association between phthalate exposure and risk of spontaneous pregnancy loss: a systematic review and meta-analysis. Environ Pollut. 2020. https://doi.org/10.1016/j.envpol.2020.115446.
Guo J, Wu M, Gao X, Chen J, Li S, Chen B, Dong R. Meconium exposure to phthalates, sex and thyroid hormones, birth size and pregnancy outcomes in 251 mother-infant pairs from Shanghai. Int J Environ Res Public Health. 2020. https://doi.org/10.3390/ijerph17217711.
Deng T, Du Y, Wang Y, Teng X, Hua X, Yuan X, Yao Y, Guo N, Li Y. The associations of urinary phthalate metabolites with the intermediate and pregnancy outcomes of women receiving IVF/ICSI treatments: a prospective single-center study. Ecotoxicol Environ Saf. 2020. https://doi.org/10.1016/j.ecoenv.2019.109884.
Reinsberg J, Wegener-Toper P, van der Ven K, van der Ven H, Klingmueller D. Effect of mono-(2-ethylhexyl) phthalate on steroid production of human granulosa cells. Toxicol Appl Pharm. 2009. https://doi.org/10.1016/j.taap.2009.05.022.
Kar D, Bandyopadhyay A. Targeting peroxisome proliferator activated receptor alpha (PPAR alpha) for the prevention of mitochondrial impairment and hypertrophy in cardiomyocytes. Cell Physiol Biochem. 2018. https://doi.org/10.1159/000492875.
Cajas YN, Canon-Beltran K, Ladron de Guevara M, Millan de la Blanca MG, Ramos-Ibeas P, Gutierrez-Adan A, Rizos D, Gonzalez EM. Antioxidant nobiletin enhances oocyte maturation and subsequent embryo development and quality. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21155340.
Zhang G, Yang W, Jiang F, Zou P, Zeng Y, Ling X, Zhou Z, Cao J, Ao L. PERK regulates Nrf2/ARE antioxidant pathway against dibutyl phthalate-induced mitochondrial damage and apoptosis dependent of reactive oxygen species in mouse spermatocyte-derived cells. Toxicol Lett. 2019. https://doi.org/10.1016/j.toxlet.2019.03.007.
Yuan X-Q, Du Y-Y, Liu C, Guo N, Teng X-M, Hua X, Yao Y-C, Deng Y-L, Zeng Q, Deng T-R, Li Y-F. Phthalate metabolites and biomarkers of oxidative stress in the follicular fluid of women undergoing in vitro fertilization. Sci Total Environ. 2020. https://doi.org/10.1016/j.scitotenv.2020.139834.
Chu DP, Tian S, Qi L, Hao CJ, Xia HF, Ma X. Abnormality of maternal-to-embryonic transition contributes to MEHP-induced mouse 2-cell block. J Cell Physiol. 2013. https://doi.org/10.1002/jcp.24222.
Chu DP, Tian S, Sun DG, Hao CJ, Xia HF, Ma X. Exposure to mono-n-butyl phthalate disrupts the development of preimplantation embryos. Reprod Fertil Dev. 2013. https://doi.org/10.1071/RD12178.
Zhu L, Xi Q, Zhang H, Li Y, Ai J, Jin L. Blastocyst culture and cryopreservation to optimize clinical outcomes of warming cycles. Reprod Biomed Online. 2013. https://doi.org/10.1016/j.rbmo.2013.04.006.
Bonilla E, del Mazo J. Deregulation of the Sod1 and Nd1 genes in mouse fetal oocytes exposed to mono-(2-ethylhexyl) phthalate (MEHP). Reprod Toxicol. 2010. https://doi.org/10.1016/j.reprotox.2010.04.008.
Wang W, Craig ZR, Basavarajappa MS, Gupta RK, Flaws JA. Di (2-ethylhexyl) phthalate inhibits growth of mouse ovarian antral follicles through an oxidative stress pathway. Toxicol Appl Pharmacol. 2012. https://doi.org/10.1016/j.taap.2011.11.008.
Wang W, Craig ZR, Basavarajappa MS, Hafner KS, Flaws JA. Mono-(2-ethylhexyl) phthalate induces oxidative stress and inhibits growth of mouse ovarian antral follicles. Biol Reprod. 2012. https://doi.org/10.1095/biolreprod.112.102467.
Zhou C, Flaws JA. Effects of an environmentally relevant phthalate mixture on cultured mouse antral follicles. Toxicol Sci. 2017. https://doi.org/10.1093/toxsci/kfw245.
Stojkovic M, Machado SA, Stojkovic P, Zakhartchenko V, Hutzler P, Goncalves PB, Wolf E. Mitochondrial distribution and adenosine triphosphate content of bovine oocytes before and after in vitro maturation: correlation with morphological criteria and developmental capacity after in vitro fertilization and culture. Biol Reprod. 2001. https://doi.org/10.1095/biolreprod64.3.904.
Piras AR, Menendez-Blanco I, Soto-Heras S, Catala MG, Izquierdo D, Bogliolo L, Paramio MT. Resveratrol supplementation during in vitro maturation improves embryo development of prepubertal goat oocytes selected by brilliant cresyl blue staining. J Reprod Dev. 2019. https://doi.org/10.1262/jrd.2018-077.
Zhao MH, Liang S, Kim SH, Cui XS, Kim NH. Fe(III) Is essential for porcine embryonic development via mitochondrial function maintenance. PLoS ONE. 2015. https://doi.org/10.1371/journal.pone.0130791.
Acknowledgements
The authors thank Dr. Deng and Dr. Li for their guidance on experiment design and paper writing; Dr. Yuan and Dr. Teng for clinical data collection; Dr. Du for valuable guidance throughout animal experiments; Ms. Liao and Mr. Li for suggestions on data statistical analysis; Ms. Yao and Ms. Li for suggestions on manuscript writing; Ms. Guo for helping establishing the IVF and in vitro culture systems of mouse embryos; all the doctors, embryologists, and nurses for following up the participants and collecting clinical data. This work was supported by the National Natural Science Foundation of China [No. 81571508; No. 81771654].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics Approval
All included patients gave their oral and written informed consent. The study project was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China (No. TJ-IRB20180607; [2019] S934).
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
ESM1
(DOCX 15.2 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tian, W., Liao, H., Li, N. et al. Monomethyl Phthalate Causes Early Embryo Development Delay, Apoptosis, and Energy Metabolism Disruptions Through Inducing Redox Imbalance. Reprod. Sci. 31, 139–149 (2024). https://doi.org/10.1007/s43032-023-01309-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-023-01309-6