Abstract
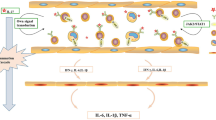
The objective of this study is to investigate the effect of IL-27 on Th1 cells infiltration in human fetal membranes (FMs) in preterm labor (PL). The expression of Th1 cells specific transcription factor (T-bet), Th1 cells infiltration related molecules (CXCL9, CXCL10, CXCL11, and ICAM-1), and IL-27 receptor α subunit (IL-27Rα) was compared in human FMs from pregnant women in PL group and term labor (TL) group. In vitro, rhIL-27 was added to the culture medium of amniotic epithelial cells (WISH cells) to detect the expression of CXCL9, CXCL10, CXCL11, and ICAM-1. Furthermore, the underlying signaling pathway was detected by single-sample gene set enrichment analysis and western blot analysis. The expression of T-bet and CXCL9, CXCL10, CXCL11, and ICAM-1 as well as IL-27Rα was higher in human FMs from PL group than TL group. In vitro, rhIL-27 could upregulate the expression of CXCL9, CXCL10, CXCL11, and ICAM-1 in WISH cells. Using gene-set enrichment analysis of FMs, JAK/STAT signaling pathway was found to be activated by IL-27 signaling in PL. Using western blot analysis, JAK2/STAT1/STAT3 signaling pathway was confirmed to be enhanced in rhIL-27 treated WISH cells. In addition, AG490 (JAK2 inhibitor) could inhibit the secretion of CXCL9, CXCL10, and CXCL11 in WISH cells stimulated by rhIL-27. Our results suggested that IL-27 may promote Th1 cells infiltration in human FMs in PL, by promoting the expression of CXCL9, CXCL10, and CXCL11 at least partly through JAK2/STAT1/STAT3 signaling pathway.





Similar content being viewed by others
Availability of Data and Material
Data and materials would be provided if requested.
Code Availability
GraphPad Prism software version 8.0 (GraphPad Software Inc., La Jolla, CA, USA).
Abbreviations
- FMs:
-
Fetal membranes
- IL-27Rα:
-
IL-27 receptor a subunit
- PL:
-
Preterm labor
- TL:
-
Term labor
- Th1 cells:
-
Type 1 helper cells
- PROM:
-
Premature rupture of membranes
- ssGSEA:
-
Single-sample gene set enrichment analysis
- CCL:
-
C-C motif ligand
- CXCL:
-
C-X-C motif chemokine ligand
- ICAM-1:
-
Intercellular adhesion molecule-1
- JAK:
-
Janus kinase
- STAT:
-
Signal transducer and activator of transcription
References
Romero R, Dey SK. Fisher S J Preterm labor: one syndrome, many causes. Science. 2014;345:760–5. https://doi.org/10.1126/science.1251816.
Blencowe H, Cousens S, Chou D, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10(Suppl 1):S2. https://doi.org/10.1186/1742-4755-10-s1-s2.
Vogel JP, Chawanpaiboon S, Moller AB, et al. The global epidemiology of preterm birth. Best Pract Res Clin Obstet Gynaecol. 2018;52:3–12. https://doi.org/10.1016/j.bpobgyn.2018.04.003.
Green ES. Arck P C Pathogenesis of preterm birth: bidirectional inflammation in mother and fetus. Semin Immunopathol. 2020;42:413–29. https://doi.org/10.1007/s00281-020-00807-y.
Sindram-Trujillo A, Scherjon S, Kanhai H, et al. Increased T-cell activation in decidua parietalis compared to decidua basalis in uncomplicated human term pregnancy. Am J Reprod Immunol. 2003;49:261–8. https://doi.org/10.1034/j.1600-0897.2003.00041.x.
Gomez-Lopez N, Vadillo-Perez L, Hernandez-Carbajal A, et al. Specific inflammatory microenvironments in the zones of the fetal membranes at term delivery. Am J Obstet Gynecol. 2011;205(235):e15-24. https://doi.org/10.1016/j.ajog.2011.04.019.
Gomez-Lopez N, Vega-Sanchez R, Castillo-Castrejon M, et al. Evidence for a role for the adaptive immune response in human term parturition. Am J Reprod Immunol. 2013;69:212–30. https://doi.org/10.1111/aji.12074.
Sindram-Trujillo AP, Scherjon SA, van Hulst-van MPP, et al. Comparison of decidual leukocytes following spontaneous vaginal delivery and elective cesarean section in uncomplicated human term pregnancy. J Reprod Immunol. 2004;62:125–37. https://doi.org/10.1016/j.jri.2003.11.007.
Tilburgs T,Roelen D L,van der Mast B J, et al. Differential distribution of CD4(+)CD25(bright) and CD8(+)CD28(-) T-cells in decidua and maternal blood during human pregnancy. Placenta 2006; 27 Suppl A: S47–53. https://doi.org/10.1016/j.placenta.2005.11.008.
Tilburgs T, Scherjon SA, Roelen DL, et al. Decidual CD8+CD28- T cells express CD103 but not perforin. Human immunology. 2009;70:96–100. https://doi.org/10.1016/j.humimm.2008.12.006.
Tilburgs T,Schonkeren D,Eikmans M, et al. Human decidual tissue contains differentiated CD8+ effector-memory T cells with unique properties. Journal of immunology (Baltimore, Md. : 1950)2010; 185: 4470–7. https://doi.org/10.4049/jimmunol.0903597.
Powell R M,Lissauer D,Tamblyn J, et al. Decidual T cells exhibit a highly differentiated phenotype and demonstrate potential fetal specificity and a strong transcriptional response to IFN. Journal of immunology (Baltimore, Md. : 1950)2017; 199: 3406–3417. https://doi.org/10.4049/jimmunol.1700114.
van der Zwan A, Bi K, Norwitz ER, et al. Mixed signature of activation and dysfunction allows human decidual CD8(+) T cells to provide both tolerance and immunity. Proc Natl Acad Sci U S A. 2018;115:385–90. https://doi.org/10.1073/pnas.1713957115.
Gomez-Lopez N, Romero R, Arenas-Hernandez M, et al. In vivo T-cell activation by a monoclonal αCD3ε antibody induces preterm labor and birth. Am J Reprod Immunol. 2016;76:386–90. https://doi.org/10.1111/aji.12562.
Arenas-Hernandez M,Romero R,Xu Y, et al. Effector and activated T cells induce preterm labor and birth that is prevented by treatment with progesterone. Journal of immunology (Baltimore, Md. : 1950)2019; 202: 2585–2608. https://doi.org/10.4049/jimmunol.1801350.
Ferran C, Sheehan K, Dy M, et al. Cytokine-related syndrome following injection of anti-CD3 monoclonal antibody: further evidence for transient in vivo T cell activation. Eur J Immunol. 1990;20:509–15. https://doi.org/10.1002/eji.1830200308.
Wang W, Sung N, Gilman-Sachs A, et al. T helper (Th) cell profiles in pregnancy and recurrent pregnancy losses: Th1/Th2/Th9/Th17/Th22/Tfh cells. Frontiers in immunology. 2020;11:2025. https://doi.org/10.3389/fimmu.2020.02025.
Sykes L, MacIntyre DA, Yap XJ, et al. The Th1:Th2 dichotomy of pregnancy and preterm labour. Mediators of inflammation. 2012;2012:967629. https://doi.org/10.1155/2012/967629.
Saito S, Nakashima A, Shima T, et al. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63:601–10. https://doi.org/10.1111/j.1600-0897.2010.00852.x.
Griffith JW, Sokol CL. Luster A D Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annual review of immunology. 2014;32:659–702. https://doi.org/10.1146/annurev-immunol-032713-120145.
Rahman A. Fazal F Hug tightly and say goodbye: role of endothelial ICAM-1 in leukocyte transmigration. Antioxid Redox Signal. 2009;11:823–39. https://doi.org/10.1089/ars.2008.2204.
Hosokawa Y, Hosokawa I, Ozaki K, et al. Oncostatin M synergistically induces CXCL10 and ICAM-1 expression in IL-1beta-stimulated-human gingival fibroblasts. J Cell Biochem. 2010;111:40–8. https://doi.org/10.1002/jcb.22648.
Pflanz S, Timans JC, Cheung J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–90. https://doi.org/10.1016/s1074-7613(02)00324-2.
Wang Q,Liu J Regulation and immune function of IL-27. Journal of immunology (Baltimore, Md. : 1950)2016; 941: 191–211. https://doi.org/10.4049/jimmunol.1601094.
Yoshida H,Hamano S,Senaldi G, et al. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity 2001; 15: 569–78. https://doi.org/10.1016/s1074-7613(01)00206-0.
Cao Y,Doodes P D,Glant T T, et al. IL-27 induces a Th1 immune response and susceptibility to experimental arthritis. Journal of immunology (Baltimore, Md. : 1950)2008; 180: 922–30. https://doi.org/10.4049/jimmunol.180.2.922.
Hosokawa Y, Hosokawa I, Ozaki K, et al. IL-27 Modulates chemokine production in TNF-alpha-stimulated human oral epithelial cells. Cell Physiol Biochem. 2017;43:1198–206. https://doi.org/10.1159/000481760.
Wong CK, Chen DP, Tam LS, et al. Effects of inflammatory cytokine IL-27 on the activation of fibroblast-like synoviocytes in rheumatoid arthritis. Arthrit Res Ther. 2010;12:R129. https://doi.org/10.1186/ar3067.
Yin N,Wang H,Zhang H, et al. IL-27 induces a pro-inflammatory response in human fetal membranes mediating preterm birth. International immunopharmacology2017; 50: 361–369. https://doi.org/10.1007/s12253-017-0295-2.
Menon R, Lappas M. Zakar T Editorial: the role of the fetal membranes in pregnancy and birth. Front Physiol. 2021;12: 653084. https://doi.org/10.3389/fphys.2021.653084.
Gomez-Lopez N, Estrada-Gutierrez G, Jimenez-Zamudio L, et al. Fetal membranes exhibit selective leukocyte chemotaxic activity during human labor. J Reprod Immunol. 2009;80:122–31. https://doi.org/10.1016/j.jri.2009.01.002.
Gomez-Lopez N, Vadillo-Perez L, Nessim S, et al. Choriodecidua and amnion exhibit selective leukocyte chemotaxis during term human labor. Am J Obstet Gynecol. 2011;204(364):e9-16. https://doi.org/10.1016/j.ajog.2010.11.010.
Kim CJ, Romero R, Chaemsaithong P, et al. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015;213:S29-52. https://doi.org/10.1016/j.ajog.2015.08.040.
Hall AO, Silver JS. Hunter C A The immunobiology of IL-27. Adv Immunol. 2012;115:1–44. https://doi.org/10.1016/b978-0-12-394299-9.00001-1.
Zhang Z, Chen F, Li J, et al. 1,25(OH)(2)D(3) suppresses proinflammatory responses by inhibiting Th1 cell differentiation and cytokine production through the JAK/STAT pathway. Am J Transl Res. 2018;10:2737–46.
Jacques SM. Qureshi F Chronic chorioamnionitis: a clinicopathologic and immunohistochemical study. Hum Pathol. 1998;29:1457–61. https://doi.org/10.1016/s0046-8177(98)90016-8.
Vega-Sanchez R, Gomez-Lopez N, Flores-Pliego A, et al. Placental blood leukocytes are functional and phenotypically different than peripheral leukocytes during human labor. J Reprod Immunol. 2010;84:100–10. https://doi.org/10.1016/j.jri.2009.08.002.
Ito M, Nakashima A, Hidaka T, et al. A role for IL-17 in induction of an inflammation at the fetomaternal interface in preterm labour. J Reprod Immunol. 2010;84:75–85. https://doi.org/10.1016/j.jri.2009.09.005.
Gomez-Lopez N, Arenas-Hernandez M, Romero R, et al. Regulatory T cells play a role in a subset of idiopathic preterm labor/birth and adverse neonatal outcomes. Cell Rep. 2020;32: 107874. https://doi.org/10.1016/j.celrep.2020.107874.
Arefieva A, Nikolaeva M, Stepanova E, et al. Association of CD200 expression in paternal lymphocytes with female Th1/Th2 balance and pregnancy establishment at immunotherapy of recurrent spontaneous abortion. Am J Reprod Immunol. 2021;85: e13355. https://doi.org/10.1111/aji.13355.
Makhseed M, Raghupathy R, Azizieh F, et al. Th1 and Th2 cytokine profiles in recurrent aborters with successful pregnancy and with subsequent abortions. Hum Reprod. 2001;16:2219–26. https://doi.org/10.1093/humrep/16.10.2219.
Saito S. Sakai M Th1/Th2 balance in preeclampsia. J Reprod Immunol. 2003;59:161–73. https://doi.org/10.1016/s0165-0378(03)00045-7.
El-Shazly S, Makhseed M, Azizieh F, et al. Increased expression of pro-inflammatory cytokines in placentas of women undergoing spontaneous preterm delivery or premature rupture of membranes. Am J Reprod Immunol. 2004;52:45–52. https://doi.org/10.1111/j.1600-0897.2004.00181.x.
Makhseed M, Raghupathy R, El-Shazly S, et al. Pro-inflammatory maternal cytokine profile in preterm delivery. Am J Reprod Immunol. 2003;49:308–18. https://doi.org/10.1034/j.1600-0897.2003.00038.x.
Chuileannáin FN. Brennecke S Prediction of preterm labour in multiple pregnancies. Baillieres Clin Obstet Gynaecol. 1998;12:53–66. https://doi.org/10.1016/s0950-3552(98)80039-4.
Kim CJ, Romero R, Kusanovic JP, et al. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol. 2010;23:1000–11. https://doi.org/10.1038/modpathol.2010.73.
Hasheminia S J,Tolouei S,Zarkesh-Esfahani S H, et al. Cytokines gene expression in newly diagnosed multiple sclerosis patients. Iranian journal of allergy, asthma, and immunology2015; 14: 208–16
Frascoli M,Coniglio L,Witt R, et al. Alloreactive fetal T cells promote uterine contractility in preterm labor via IFN-gamma and TNF-alpha. Sci. Transl. Med.2018; 10. https://doi.org/10.1126/scitranslmed.aan2263.
Hantoushzadeh S, Anvari Aliabad R. Norooznezhad A H Antibiotics, inflammation, and preterm labor: a missed conclusion. J Inflamm Res. 2020;13:245–54. https://doi.org/10.2147/jir.S248382.
Hirsch E, Filipovich Y. Mahendroo M Signaling via the type I IL-1 and TNF receptors is necessary for bacterially induced preterm labor in a murine model. Am J Obstet Gynecol. 2006;194:1334–40. https://doi.org/10.1016/j.ajog.2005.11.004.
Romero R,Chaemsaithong P,Chaiyasit N, et al. CXCL10 and IL-6: markers of two different forms of intra-amniotic inflammation in preterm labor. Am. J. Reprod. Immunol.2017; 78. https://doi.org/10.1111/aji.12685.
Pan J, Tian X, Huang H, et al. Proteomic study of fetal membrane: inflammation-triggered proteolysis of extracellular matrix may present a pathogenic pathway for spontaneous preterm birth. Front Physiol. 2020;11:800. https://doi.org/10.3389/fphys.2020.00800.
He J, Zhang Q, Zhang W, et al. The interleukin-27-964A>G polymorphism enhances sepsis-induced inflammatory responses and confers susceptibility to the development of sepsis. Crit Care. 2018;22:248. https://doi.org/10.1186/s13054-018-2180-0.
Xu F,Liu Q,Lin S, et al. IL-27 is elevated in acute lung injury and mediates inflammation. Journal of clinical immunology2013; 33: 1257–68. https://doi.org/10.1007/s10875-013-9923-0.
Fan J, Zhang YC, Zheng DF, et al. IL-27 is elevated in sepsis with acute hepatic injury and promotes hepatic damage and inflammation in the CLP model. Cytokine. 2020;127: 154936. https://doi.org/10.1016/j.cyto.2019.154936.
Yin N, Zhang H, Luo X, et al. IL-27 activates human trophoblasts to express IP-10 and IL-6: implications in the immunopathophysiology of preeclampsia. Mediators of inflammation. 2014;2014:926875. https://doi.org/10.1155/2014/926875.
Shibata S, Tada Y, Asano Y, et al. IL-27 activates Th1-mediated responses in imiquimod-induced psoriasis-like skin lesions. J Invest Dermatol. 2013;133:479–88. https://doi.org/10.1038/jid.2012.313.
Qiu H N,Liu B,Liu W, et al. Interleukin-27 enhances TNF-alpha-mediated activation of human coronary artery endothelial cells. Molecular and cellular biochemistry2016; 411: 1–10. https://doi.org/10.1007/s11010-015-2563-3.
Nakanishi Y, Lu B, Gerard C, et al. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462:510–3. https://doi.org/10.1038/nature08511.
Aguilar HN. Mitchell B F Physiological pathways and molecular mechanisms regulating uterine contractility. Hum Reprod Update. 2010;16:725–44. https://doi.org/10.1093/humupd/dmq016.
Capece A, Vasieva O, Meher S, et al. Pathway analysis of genetic factors associated with spontaneous preterm birth and pre-labor preterm rupture of membranes. PloS one. 2014;9:e108578. https://doi.org/10.1371/journal.pone.0108578.
Jiang K, Chen Y. Jarvis J N Soluble factors from LPS- and PHA-activated PBMC induce MAPK, Stat1 and Stat3 phosphorylation in primary cultures of human term placental trophoblasts: implications for infection and prematurity. Placenta. 2007;28:538–42. https://doi.org/10.1016/j.placenta.2006.06.013.
Zhou X, Jiang Z, Zou Y, et al. Role of SOCS3 in the Jak/stat3 pathway in the human placenta: different mechanisms for preterm and term labor. Acta Obstet Gynecol Scand. 2015;94:1112–7. https://doi.org/10.1111/aogs.12708.
Meka RR, Venkatesha SH, Dudics S, et al. IL-27-induced modulation of autoimmunity and its therapeutic potential. Autoimmun Rev. 2015;14:1131–41. https://doi.org/10.1016/j.autrev.2015.08.001.
Lucas S,Ghilardi N,Li J, et al. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci USA2003; 100: 15047–52. https://doi.org/10.1073/pnas.2536517100.
Basset L, Chevalier S, Danger Y, et al. Interleukin-27 and IFNgamma regulate the expression of CXCL9, CXCL10, and CXCL11 in hepatitis. Journal of molecular medicine (Berlin, Germany). 2015;93:1355–67. https://doi.org/10.1007/s00109-015-1319-6.
Wittmann M, Zeitvogel J, Wang D, et al. IL-27 is expressed in chronic human eczematous skin lesions and stimulates human keratinocytes. J Allergy Clin Immunol. 2009;124:81–9. https://doi.org/10.1016/j.jaci.2009.04.026.
Fasshauer M, Kralisch S, Klier M, et al. Insulin resistance-inducing cytokines differentially regulate SOCS mRNA expression via growth factor- and Jak/Stat-signaling pathways in 3T3-L1 adipocytes. J Endocrinol. 2004;181:129–38. https://doi.org/10.1677/joe.0.1810129.
Khan D, Dai R, Karpuzoglu E, et al. Estrogen increases, whereas IL-27 and IFN-gamma decrease, splenocyte IL-17 production in WT mice. Eur J Immunol. 2010;40:2549–56. https://doi.org/10.1002/eji.201040303.
Wang H, Meng R, Li Z, et al. IL-27 induces the differentiation of Tr1-like cells from human naive CD4+ T cells via the phosphorylation of STAT1 and STAT3. Immunology letters. 2011;136:21–8. https://doi.org/10.1016/j.imlet.2010.11.007.
Wang H, Li Z, Yang B, et al. IL-27 suppresses the production of IL-22 in human CD4(+) T cells by inducing the expression of SOCS1. Immunology letters. 2013;152:96–103. https://doi.org/10.1016/j.imlet.2013.05.001.
Funding
This work was supported by research funding from National Natural Science Foundation of China for Youth (81801483) and the National Key R&D Program of China (No. 2016YFC1000407).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics Approval
Ethics approval was gained from the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (2019–137).
Consent to Participate
Informed consent for participation was obtained from all participants.
Consent for Publication
Written informed consent for participation was obtained from the participants.
Competing Interests
The authors declare no competing interests.
Supplementary Information
Rights and permissions
About this article
Cite this article
Mei, Y., Ran, Y., Liu, Z. et al. IL-27 Mediates Th1 Cells Infiltration in Fetal Membranes in Preterm Labor. Reprod. Sci. 29, 1764–1775 (2022). https://doi.org/10.1007/s43032-021-00803-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00803-z