Abstract
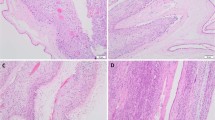
Histological chorioamnionitis (HC) is a common placental finding that represents acute/chronic inflammation and can affect neonatal outcomes. We aimed to examine the effect of HC on neonatal outcomes in pregnancies complicated by preeclampsia. All pregnancies with the diagnosis of preeclampsia at 24–42 weeks between 2008 and 2019 were reviewed. Placental lesions were classified according to the “Amsterdam” criteria. Composite adverse neonatal outcome included ≥1 early complication. Maternal and neonatal outcomes were compared between cases with and without HC. Multivariable regression analysis was performed to identify independent associations with adverse neonatal outcome. Compared to preeclampsia without HC (n=517), preeclampsia with HC (n=55) was characterized by a more advanced gestational age (p<0.001) and a higher rate of nulliparity (p=0.02). Diabetes was more prevalent in preeclampsia without HC (p=0.039) as was a history of previous preeclampsia (p=0.048). Neonates in the preeclampsia with HC group had higher rates of adverse neonatal outcome (p<0.001) and Apgar scores <7 at 5 min (p=0.034) despite a higher birthweight (p=0.005). Preeclampsia without HC was associated with smaller placentas and a higher rate of placental maternal vascular malperfusion lesions (p=0.014 and p<0.001 respectively). By multivariate analysis, gestational age and HC were independently associated with adverse neonatal outcome (aOR 0.88 95% CI 0.76–0.96, and aOR 1.33, 95% CI 1.11–3.09, respectively). In preeclampsia, concomitant HC was associated with adverse neonatal outcome despite a more advanced gestational age and higher neonatal birthweight. This inflammatory mechanism probably plays a role in a more severe subgroup of preeclampsia cases, causing an additional risk for the developing fetus.
Similar content being viewed by others
Abbreviations
- PE:
-
Preeclampsia
- HC:
-
Histological chorioamnionitis
- MVM:
-
maternal vascular malperfusion
References
Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013;170:1–7. https://doi.org/10.1016/j.ejogrb.2013.05.005.
Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36:56–9. https://doi.org/10.1053/j.semperi.2011.09.011.
Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33:130–7. https://doi.org/10.1053/j.semperi.2009.02.010.
Amaral LM, Wallace K, Owens M, Lamarca B. Pathophysiology and current clinical management of preeclampsia. Curr Hypertens Rep. 2017;19. https://doi.org/10.1007/s11906-017-0757-7.Pathophysiology.
Burton GJ, Woods AW, Jauniaux E, Kingdom JCP. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30:473–82. https://doi.org/10.1016/j.placenta.2009.02.009.
Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:1–15. https://doi.org/10.1136/bmj.l2381.
Falco ML, Sivanathan J, Laoreti A, Thilaganathan B, Khalil A. Placental histopathology associated with pre-eclampsia: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2017;50:295–301. https://doi.org/10.1002/uog.17494.
Redline RW. The clinical implications of placental diagnoses. Semin Perinatol. 2015;39:2–8. https://doi.org/10.1053/j.semperi.2014.10.002.
Kalagiri RR, Carder T, Choudhury MSS, Vora BSN, Ballard AR, Govande V, et al. Inflammation in Complicated Pregnancy and Its Outcome. Am J Perinatol. 2016;33:1337–56.
Rana S, Lemoine E, Granger J, Karumanchi SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. 2019;124:1094–112. https://doi.org/10.1161/CIRCRESAHA.118.313276.
Rambaldi MP, Weiner E, Mecacci F, Bar J, Petraglia F. Immunomodulation and preeclampsia. Best Pract Res Clin Obstet Gynaecol. 2019. https://doi.org/10.1016/j.bpobgyn.2019.06.005.
Khong TY, Mooney EE, Ariel I, Balmus NCM, Boyd TK, Brundler MA, et al. Sampling and definitions of placental lesions Amsterdam placental workshop group consensus statement. Arch Pathol Lab Med 2016;140:698–713. https://doi.org/10.5858/arpa.2015-0225-CC.
Jacques SM, Qureshi F. Chronic chorioamnionitis: a clinicopathologic and immunohistochemical study. Hum Pathol. 1998;29:1457–61. https://doi.org/10.1016/S0046-8177(98)90016-8.
Chong Jai Kim, Roberto Romero, Piya Chaemsaithong and J-SK. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Physiol Behav 2017;176:139–48. https://doi.org/10.1016/j.physbeh.2017.03.040.
Cataño Sabogal CP, Fonseca J, García-Perdomo HA. Validation of diagnostic tests for histologic chorioamnionitis: systematic review and meta-analysis. vol. 228. Elsevier Ireland Ltd.; 2018. https://doi.org/10.1016/j.ejogrb.2018.05.043.
Menon R, Taylor RN, Fortunato SJ. Chorioamnionitis – a complex pathophysiologic syndrome. Placenta. 2010;31:113–20. https://doi.org/10.1016/j.placenta.2009.11.012.
Conti N, Torricelli M, Voltolini C, Vannuccini S, Clifton VL, Bloise E, et al. Term histologic chorioamnionitis: a heterogeneous condition. Eur J Obstet Gynecol Reprod Biol 2015;188:34–8. https://doi.org/10.1016/j.ejogrb.2015.02.034.
James M Roberts, Phyllis A August, George Bakris, John R Barton, Ira M Bernstein, Maurice Druzin, Robert R Gaiser, Joey P Granger, Arun Jeyabalan, Donna D Johnson, S Ananth Karumanchi, Marshall Lindheimer, Michelle Y Owens, George R Saade, Baha M Sibai, C KN. Hypertension in pregnancy. Obstet Gynecol 2013;122:1122–31. https://doi.org/10.1016/j.ccl.2012.04.005.
Dollberg S, Haklai Z, Mimouni FB, Gorfein I, Gordon ES. Birthweight standards in the live-born population in Israel. Isr Med Assoc J. 2005;7:311–4.
Pinar H, Sung CJ, Oyer CE, Singer DB. Reference values for singleton and twin placental weights. Pediatr Pathol Lab Med. 1996;16:901–7. https://doi.org/10.1080/15513819609168713.
Strong THJ, Jarles DL, Vega JS, Feldman DB. The umbilical coiling index. Am J Obstet Gynecol. 1994;170:29–32.
Garrido-gomez T, Dominguez F, Quiñonero A, Diaz-gimeno P, Kapidzic M. Defective decidualization during and after severe preeclampsia reveals a possible maternal contribution to the etiology. Proc Natl Acad Sci U S A. 2017;114:E8468–77. https://doi.org/10.1073/pnas.1706546114.
Kadyrov M, Kingdom JCP, Huppertz B. Divergent trophoblast invasion and apoptosis in placental bed spiral arteries from pregnancies complicated by maternal anemia and early-onset preeclampsia/intrauterine growth restriction. Am J Obstet Gynecol. 2006;194:557–63. https://doi.org/10.1016/j.ajog.2005.07.035.
Gleicher N. Why much of the pathophysiology of preeclampsia-eclampsia must be of an autoimmune nature. Am J Obstet Gynecol 2007;196:5.e1–7. https://doi.org/10.1016/j.ajog.2006.09.016.
Hung T-H, Charnock-Jones DS, Skepper JN, Burton GJ. Secretion of tumor necrosis factor-alpha from human placental tissues induced by hypoxia-reoxygenation causes endothelial cell activation in vitro. a potential mediator of the inflammatory response in preeclampsia. Am J Pathol. 2004;164:1049–61. https://doi.org/10.1016/s0002-9440(10)63192-6.
Toldi G, Svec P, Vásárhelyi B, Mészáros G, Rigó J, Tulassay T, et al. Decreased number of FoxP3+ regulatory T cells in preeclampsia. Acta Obstet Gynecol Scand 2008;87:1229–33. https://doi.org/10.1080/00016340802389470.
Prins JR, Boelens HM, Heimweg J, Van der Heide S, Dubois AE, Van Oosterhout AJ, et al. Preeclampsia is associated with lower percentages of regulatory T cells in maternal blood. Hypertens Pregnancy 2009;28:300–11. https://doi.org/10.1080/10641950802601237.
Lau J, Magee F, Fcrcp C, Qiu Z, Dadelszen P Von, Lee SK. Chorioamnionitis with a fetal inflammatory response is associated with higher neonatal mortality, morbidity, and resource use than chorioamnionitis displaying a maternal inflammatory response only. Am J Obs Gynecol 2005;193:708–713. https://doi.org/10.1016/j.ajog.2005.01.017.
Bierstone D, Wagenaar N, Gano DL, Guo T, Georgio G, Groenendaal F, et al. Association of histologic chorioamnionitis with perinatal brain injury and early childhood neurodevelopmental outcomes among preterm neonates. JAMA Pediatr. 2018;172:534–41. https://doi.org/10.1001/jamapediatrics.2018.0102.
Kovo M, Gonen N, Schreiber L, Hochman R, Noy LK, Levy M, et al. Histologic chorioamnionitis concomitant placental abruption and its effects on pregnancy outcome. Placenta 2020;94:39–43. https://doi.org/10.1016/j.placenta.2020.03.012.
Mittal A, Nanda S, Sen J. Antenatal umbilical coiling index as a predictor of perinatal outcome. Arch Gynecol Obstet. 2015;291:763–8. https://doi.org/10.1007/s00404-014-3456-5.
Levy M, Kovo M, Schreiber L, Kleiner I, Koren L, Barda G, et al. Pregnancy outcomes in correlation with placental histopathology in subsequent pregnancies complicated by preeclampsia. Pregnancy Hypertens 2019;18:163–8. https://doi.org/10.1016/j.preghy.2019.09.021.
Bujold, Emmanuel MD, MSc, Roberge, Stéphanie, MSc, Yves Lacasse, MD, MSc, Marc Bureau, MD, Franc¸oisAudibert, MD, MSc, Sylvie Marcoux, MD, PhD, Jean-Claude Forest, MD, PhD, and Yves Gigue’re, MD P. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy. Obstet Gynecol 2010;116:402–14. https://doi.org/10.1097/AOG.0b013e3181e9322a.
Ye J, Chen Y, Zhu J, Chen C, Zhu X, Feng L, et al. Aspirin use during pregnancy and hypoxia-related placental pathology. Pregnancy Hypertens 2018;14:177–88. https://doi.org/10.1016/j.preghy.2018.07.009.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval and Consent to Participate
Ethical approval was waived by the local Ethics Committee (approval number: 0042-10-WOMC) in view of the retrospective nature of the study and all the procedures being performed were part of the routine care.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Levy, M., Mor, L., Kovo, M. et al. Histologic Chorioamnionitis in Pregnancies Complicated by Preeclampsia and the Effect on Neonatal Outcomes. Reprod. Sci. 28, 2029–2035 (2021). https://doi.org/10.1007/s43032-021-00469-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00469-7