Abstract
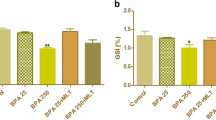
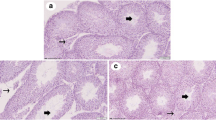
Gestational bisphenol A (BPA) exposure induced multiple programmed diseases in the adult offsprings. Thus, this study targeted exploring the physiological impacts of melatonin (MEL) as a reprogramming strategy against in utero BPA exposure on reproductive capacity of adult F1 female rat offspring. Forty adult pregnant albino female rats were divided equally into 5 groups (n = 8): group I (control), group II (low-dose BPA; 25 μg BPA/kg B.w.t.), group III (low-dose BPA + 10 mg MEL/kg B.w.t.), group IV (high-dose BPA; 250 μg/kg B.w.t.), and group V (high-dose BPA + MEL). Treatments were given daily by subcutaneous (s/c) injection from the fourth day of pregnancy until full term. After delivery, female offspring were selected, and on postnatal day 60, adult offspring were examined for estrus regularity and then were sacrificed at estrus to collect blood and tissue samples. Findings clarified that in utero BPA exposure (both doses) increased significantly (P < 0.05) the ovarian weights and the serum levels of estrogen but decreased that of triiodothyronine (T3) compared to control groups. Significant increasing of serum malondialdehyde (MDA) and decreasing of total antioxidant capacity (TAC) were also detected. Both doses of BPA disturbed remarkably the estrus cycles and caused marked aberrations in ovarian and uterine tissues. Interestingly, prenatal MEL co-treatment with BPA mitigated significantly all of these degenerative changes. Thus, this study first demonstrated that prenatal MEL therapy could be used as a potent reprogramming intervention against BPA-induced reproductive disorders in the adult F1 female rat offspring.




Similar content being viewed by others
References
Fowden AL, Giussani DA, Forhead AJ. Endocrine and metabolic programming during intrauterine development. Early Hum Dev. 2005;81:723–34.
Hill CE, Sapouckey SA, Suvorov A, Vandenberg LN. Developmental exposures to bisphenol S, a BPA replacement, alter estrogen-responsiveness of the female reproductive tract: a pilot study. Cogent Med. 2017;4:1317690.
Huang YQ, Wong CKC, Zheng JS, Bouwman H, Barra R, Wahlström B, et al. Bisphenol A (BPA) in China: a review of sources, environmental levels, and potential human health impacts. Environ Int. 2012;42:91–9.
Vom Saal FS, Nagel SC, Coe BL, Angle BM, Taylor JA. The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Mol Cell Endocrinol. 2012;354:74–84.
Biedermann S, Tschudin P, Grob K. Transfer of bisphenol A from thermal printer paper to the skin. Anal Bioanal Chem. 2010;398:571–6.
Jurek A, Leitner E. Analytical determination of bisphenol A (BPA) and bisphenol analogues in paper products by LC-MS/MS. Food Addit Contam Part A. 2018;35:2256–69.
Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJR, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Cien Saude Colet. 2012;17:407–34.
Miyazaki I, Kikuoka R, Isooka N, Takeshima M, Sonobe K, Arai R, et al. Effects of maternal bisphenol A diglycidyl ether exposure during gestation and lactation on behavior and brain development of the offspring. Food Chem Toxicol. 2020;111235.
Bloom MS, Mok-Lin E, Fujimoto VY. Bisphenol a and ovarian steroidogenesis. Fertil Steril. 2016;106:857–63.
Stoker C, Andreoli MF, Kass L, Bosquiazzo VL, Rossetti MF, Canesini G, et al. Perinatal exposure to bisphenol A (BPA) impairs neuroendocrine mechanisms regulating food intake and kisspeptin system in adult male rats. Evidences of metabolic disruptor hypothesis. Mol Cell Endocrinol. 2020;499:110614.
Wei Y, Han C, Li S, Cui Y, Bao Y, Shi W. Maternal exposure to bisphenol A during pregnancy interferes ovaries development of F1 female mice. Theriogenology. 2020;142:138–48.
Signorile PG, Spugnini EP, Mita L, Mellone P, D’Avino A, Bianco M, et al. Pre-natal exposure of mice to bisphenol A elicits an endometriosis-like phenotype in female offspring. Gen Comp Endocrinol. 2010;168:318–25.
Santamaría C, Durando M, de Toro MM, Luque EH, Rodriguez HA. Ovarian dysfunctions in adult female rat offspring born to mothers perinatally exposed to low doses of bisphenol A. J Steroid Biochem Mol Biol. 2016;158:220–30.
Shi M, Sekulovski N, MacLean JA, Whorton A, Hayashi K. Prenatal exposure to bisphenol A analogues on female reproductive functions in mice. Toxicol Sci. 2019;168:561–71.
Reiter RJ, Tan DX, Korkmaz A, Rosales-Corral SA. Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum Reprod Update. 2014;20:293–307.
Tain Y-L, Huang L-T, Chan JYH. Transcriptional regulation of programmed hypertension by melatonin: an epigenetic perspective. Int J Mol Sci. 2014;15:18484–95.
Chen Y-C, Sheen J-M, Tiao M-M, Tain Y-L, Huang L-T. Roles of melatonin in fetal programming in compromised pregnancies. Int J Mol Sci. 2013;14:5380–401.
Wu T-H, Kuo H-C, Lin I-C, Chien S-J, Huang L-T, Tain Y-L. Melatonin prevents neonatal dexamethasone induced programmed hypertension: histone deacetylase inhibition. J Steroid Biochem Mol Biol. 2014;144:253–9.
Lanoix D, Guérin P, Vaillancourt C. Placental melatonin production and melatonin receptor expression are altered in preeclampsia: new insights into the role of this hormone in pregnancy. J Pineal Res. 2012;53:417–25.
Forrestel AC, Miedlich SU, Yurcheshen M, Wittlin SD, Sellix MT. Chronomedicine and type 2 diabetes: shining some light on melatonin. Diabetologia. 2017;60:808–22.
Reiter RJ, Mayo JC, Tan D, Sainz RM, Alatorre-Jimenez M, Qin L. Melatonin as an antioxidant: under promises but over delivers. J Pineal Res. 2016;61:253–78.
Tain Y-L, Joles JA. Reprogramming: a preventive strategy in hypertension focusing on the kidney. Int J Mol Sci. 2016;17:23.
Uyanıkgil Y. Neuroprotective effects of melatonin upon the offspring cerebellar cortex in the rat model of BCNU-induced cortical dysplasia. J Perinat Med. 2009;37.
Tain Y-L, Lee C-T, Chan JYH, Hsu C-N. Maternal melatonin or N-acetylcysteine therapy regulates hydrogen sulfide-generating pathway and renal transcriptome to prevent prenatal NG-nitro-L-arginine-methyl ester (L-NAME)-induced fetal programming of hypertension in adult male offspring. Am J Obstet Gynecol. 2016;215:636–e1.
Bagheri F, Goudarzi I, Lashkarbolouki T, Salmani ME. Melatonin prevents oxidative damage induced by maternal ethanol administration and reduces homocysteine in the cerebellum of rat pups. Behav Brain Res. 2015;287:215–25.
Acevedo N, Davis B, Schaeberle CM, Sonnenschein C, Soto AM. Perinatally administered bisphenol a as a potential mammary gland carcinogen in rats. Environ Health Perspect. 2013;121:1040–6.
El-Bakry HA, Abd-Elghany MI, Soliman SS. Preventive effect of Commiphora molmol on rat mammary carcinogenesis induced by 7, 12-dimethylbenz (A) anthracene (DMBA) in comparison with melatonin. Glob J Pharmacol. 2013;7:398–411.
Jahnke G, Marr M, Myers C, Wilson R, Travlos G, Price C. Maternal and developmental toxicity evaluation of melatonin administered orally to pregnant Sprague-Dawley rats. Toxicol Sci an Off J Soc Toxicol. 1999;50:271–9.
Rowe SA, Kennaway DJ. Melatonin in rat milk and the likelihood of its role in postnatal maternal entrainment of rhythms. Am J Physiol Integr Comp Physiol. 2002;282:R797–804.
Hardeland R, Reiter RJ, Poeggeler B, Tan D-X. The significance of the metabolism of the neurohormone melatonin: antioxidative protection and formation of bioactive substances. Neurosci Biobehav Rev. 1993;17:347–57.
Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84.
Tokmak M, Şehitoğlu MH, Yüksel Y, Güven M, Akman T, Aras AB, et al. The axon protective effects of syringic acid on ischemia/reperfusion injury in a rat sciatic nerve model 2017.
Bancroft JD, Gamble M. Theory and practice of histological techniques. Elsevier health sciences; 2008.
Armonk NY. IBM Corp. SPSS Statistics for windows, Version 20.0 2011.
Wu L-H, Zhang X-M, Wang F, Gao C-J, Chen D, Palumbo JR, et al. Occurrence of bisphenol S in the environment and implications for human exposure: a short review. Sci Total Environ. 2018;615:87–98.
Pan X, Wang X, Sun Y, Dou Z, Li Z. Inhibitory effects of preimplantation exposure to bisphenol-A on blastocyst development and implantation. Int J Clin Exp Med. 2015;8:8720.
Müller JE, Meyer N, Santamaria CG, Schumacher A, Luque EH, Zenclussen ML, et al. Bisphenol A exposure during early pregnancy impairs uterine spiral artery remodeling and provokes intrauterine growth restriction in mice. Sci Rep. 2018;8:1–10.
Richter HG, Hansell JA, Raut S, Giussani DA. Melatonin improves placental efficiency and birth weight and increases the placental expression of antioxidant enzymes in undernourished pregnancy. J Pineal Res. 2009;46:357–64.
Zhang L, Zhang Z, Wang F, Tian X, Ji P, Liu G. Effects of melatonin administration on embryo implantation and offspring growth in mice under different schedules of photoperiodic exposure. Reprod Biol Endocrinol. 2017;15:78.
Evans TJ. Endocrine disruption. Reprod Dev Toxicol, Elsevier; 2017, p. 1091–110.
Aydin V, Ömeroğlu S, Kartal B, Coşkun-Akçay N, Akarca-Dizakar SÖ, Türkoğlu İ, et al. The effects of antioxidant melatonin on rat ovary neonatal exposure to bisphenol A. Düzce Tıp Fakültesi Derg. 2017;19:33–7.
Ahsan N, Ullah H, Ullah W, Jahan S. Comparative effects of bisphenol S and bisphenol A on the development of female reproductive system in rats; a neonatal exposure study. Chemosphere. 2018;197:336–43.
Mendoza-Rodríguez CA, García-Guzmán M, Baranda-Avila N, Morimoto S, Perrot-Applanat M, Cerbón M. Administration of bisphenol A to dams during perinatal period modifies molecular and morphological reproductive parameters of the offspring. Reprod Toxicol. 2011;31:177–83.
Veiga-Lopez A, Luense LJ, Christenson LK, Padmanabhan V. Developmental programming: gestational bisphenol-A treatment alters trajectory of fetal ovarian gene expression. Endocrinology. 2013;154:1873–84.
Fernández M, Bourguignon N, Lux-Lantos V, Libertun C. Neonatal exposure to bisphenol a and reproductive and endocrine alterations resembling the polycystic ovarian syndrome in adult rats. Environ Health Perspect. 2010;118:1217–22.
Lombardi LA, de Mattos LS, Simões RS, Florencio-Silva R, Sasso GR da S, Carbonel AAF, et al. Melatonin may prevent or reverse polycystic ovary syndrome in rats. Rev Assoc Med Bras. 2019;65:1008–14.
Maganhin CC, Fuchs LFP, Simões RS, Oliveira-Filho RM, de Jesus SM, Baracat EC, et al. Effects of melatonin on ovarian follicles. Eur J Obstet Gynecol Reprod Biol. 2013;166:178–84.
Delclos KB, Camacho L, Lewis SM, Vanlandingham MM, Latendresse JR, Olson GR, et al. Toxicity evaluation of bisphenol A administered by gavage to Sprague Dawley rats from gestation day 6 through postnatal day 90. Toxicol Sci. 2014;139:174–97.
Caserta D, Mantovani A, Marci R, Fazi A, Ciardo F, La Rocca C, et al. Environment and women’s reproductive health. Hum Reprod Update. 2011;17:418–33.
Lite C, Ahmed SSSJ, Santosh W, Seetharaman B. Prenatal exposure to bisphenol-A altered miRNA-224 and protein expression of aromatase in ovarian granulosa cells concomitant with elevated serum estradiol levels in F1 adult offspring. J Biochem Mol Toxicol. 2019;33:e22317.
Wu G, Song D, Wei Q, Xing J, Shi X, Shi F. Melatonin mitigates bisphenol A-induced estradiol production and proliferation by porcine ovarian granulosa cells in vitro. Anim Reprod Sci. 2018;192:91–8. https://doi.org/10.1016/J.ANIREPROSCI.2018.02.018.
de Lemos AJJM, Costa FS, Peixoto CA, Teixeira ÁAC, da Silva SB, Ferreira CGM, et al. Combination of melatonin and metformin hydrochloride for treatment polycystic ovarian in female rats. Acta Sci Vet. 2016;44:1–10.
Moriyama K, Tagami T, Akamizu T, Usui T, Saijo M, Kanamoto N, et al. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J Clin Endocrinol Metab. 2002;87:5185–90.
Chevrier J, Gunier RB, Bradman A, Holland NT, Calafat AM, Eskenazi B, et al. Maternal urinary bisphenol a during pregnancy and maternal and neonatal thyroid function in the CHAMACOS study. Environ Health Perspect. 2013;121:138–44.
Romano ME, Webster GM, Vuong AM, Zoeller RT, Chen A, Hoofnagle AN, et al. Gestational urinary bisphenol A and maternal and newborn thyroid hormone concentrations: the HOME study. Environ Res. 2015;138:453–60.
Ahmed RG. Maternal bisphenol A alters fetal endocrine system: thyroid adipokine dysfunction. Food Chem Toxicol. 2016;95:168–74.
Garcia-Marin R, Fernandez-Santos JM, Morillo-Bernal J, Gordillo-Martinez F, Vazquez-Roman V, Utrilla JC, et al. Melatonin in the thyroid gland: regulation by thyroid-stimulating hormone and role in thyroglobulin gene expression. J Physiol Pharmacol. 2015;66:643–52.
Baltaci AK, Mogulkoc R, Kul A, Bediz CS, Ugur A. Opposite effects of zinc and melatonin on thyroid hormones in rats. Toxicology. 2004;195:69–75.
Ullah A, Pirzada M, Jahan S, Ullah H, Razak S, Rauf N, et al. Prenatal BPA and its analogs BPB, BPF, and BPS exposure and reproductive axis function in the male offspring of Sprague Dawley rats. Hum Exp Toxicol. 2019;38:1344–65.
Mou D, Wang J, Liu H, Chen Y, Che L, Fang Z, et al. Maternal methyl donor supplementation during gestation counteracts bisphenol A–induced oxidative stress in sows and offspring. Nutrition. 2018;45:76–84.
El-Missiry MA, Fayed TA, El-Sawy MR, El-Sayed AA. Ameliorative effect of melatonin against gamma-irradiation-induced oxidative stress and tissue injury. Ecotoxicol Environ Saf. 2007;66:278–86.
Othman AI, Edrees GM, El-Missiry MA, Ali DA, Aboel-Nour M, Dabdoub BR. Melatonin controlled apoptosis and protected the testes and sperm quality against bisphenol A-induced oxidative toxicity. Toxicol Ind Health. 2016;32:1537–49.
Peyrot F, Ducrocq C. Potential role of tryptophan derivatives in stress responses characterized by the generation of reactive oxygen and nitrogen species. J Pineal Res. 2008;45:235–46.
Fischer TW, Kleszczyński K, Hardkop LH, Kruse N, Zillikens D. Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2′-deoxyguanosine) in ex vivo human skin. J Pineal Res. 2013;54:303–12.
Newbold RR, Jefferson WN, Padilla-Banks E. Prenatal exposure to bisphenol a at environmentally relevant doses adversely affects the murine female reproductive tract later in life. Environ Health Perspect. 2009;117:879–85.
Fernández M, Bianchi M, Lux-Lantos V, Libertun C. Neonatal exposure to bisphenol a alters reproductive parameters and gonadotropin releasing hormone signaling in female rats. Environ Health Perspect. 2009;117:757–62.
Chuffa LGA, Amorim JPA, Teixeira GR, Mendes LO, Fioruci BA, Pinheiro PFF, et al. Long-term melatonin treatment reduces ovarian mass and enhances tissue antioxidant defenses during ovulation in the rat. Braz J Med Biol Res. 2011;44:217–23.
Mohammadghasemi F, Jahromi SK, Hajizadeh H, Homafar MA, Saadat N. The protective effects of exogenous melatonin on nicotine-induced changes in mouse ovarian follicles. J Reprod Infertil. 2012;13:143.
Hanson M, Godfrey KM, Lillycrop KA, Burdge GC, Gluckman PD. Developmental plasticity and developmental origins of non-communicable disease: theoretical considerations and epigenetic mechanisms. Prog Biophys Mol Biol. 2011;106:272–80.
Santangeli S, Consales C, Pacchierotti F, Habibi HR, Carnevali O. Transgenerational effects of BPA on female reproduction. Sci Total Environ. 2019;685:1294–305.
Wang J, Xiao X, Zhang Y, Shi D, Chen W, Fu L, et al. Simultaneous modulation of COX-2, p300, Akt, and Apaf-1 signaling by melatonin to inhibit proliferation and induce apoptosis in breast cancer cells. J Pineal Res. 2012;53:77–90.
Author information
Authors and Affiliations
Contributions
AA, KH, SI, DE, and AHA contributed equally to this work. Methodology, analysis of data, and writing of the manuscript were done mostly by all authors. AA and KH finished the final version of the manuscript and approved the final form.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Statement
All animal procedures were applied in accordance with the guidelines of the local Institutional Animal Care and Use Committee for Faculty of Veterinary Medicine, Minia University, Egypt.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdel-Wahab, A., Hassanin, K.M.A., Ibrahim, S.S. et al. Developmental Programming: Physiological Impacts of Prenatal Melatonin Administration on Reproductive Capacity and Serum Triiodothyronine of Adult Female Offspring Rat Born to Moms Exposed to Bisphenol A During Pregnancy. Reprod. Sci. 28, 1956–1966 (2021). https://doi.org/10.1007/s43032-020-00452-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00452-8