Abstract
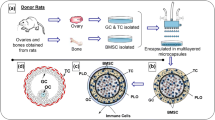
Although menopausal hormone therapy (MHT) is the most effective approach to managing the loss of ovarian activity, serious side effects have been reported. Cell-based therapy is a promising alternative for MHT. This study constructed engineered ovarian cell spheroids and investigated their endocrine function. Theca and granulosa cells were isolated from ovaries of 10-week-old rats. Two types of engineered ovarian cell spheroids were fabricated through forced aggregation in microwells, multilayered spheroids with centralized granulosa aggregates surrounded by an outer layer of theca cells and mixed ovarian spheroids lacking spatial rearrangement. The ovarian cell spheroids were encapsulated into a collagen gel. Non-aggregated ovarian cells served as controls. The endocrine function of the engineered ovarian spheroids was assessed over 30 days. The structure of the spheroids was well maintained during culture. The secretion of 17β-estradiol from both types of engineered ovarian cell spheroids was higher than in the control group and increased continuously in a time-dependent manner. Secretion of 17β-estradiol in the multi-layered ovarian cell spheroids was higher than in the non-layered constructs. Increased secretion of progesterone was detected in the multi-layered ovarian cell spheroids at day 5 of culture and was sustained during the culture period. The initial secretion level of progesterone in the non-layered ovarian cell spheroids was similar to those from the controls and increased significantly from days 21 to 30. An in vitro rat model of engineered ovarian cell spheroids was developed that was capable of secreting sex steroid hormones, indicating that the hormone secreting function of ovaries can be recapitulated ex vivo and potentially adapted for MHT.







Similar content being viewed by others
References
North American Menopause S. The 2012 hormone therapy position statement of: the North American Menopause Society. Menopause. 2012;19(3):257–71. https://doi.org/10.1097/gme.0b013e31824b970a.
Tsiligiannis S, Panay N, Stevenson JC. Premature ovarian insufficiency and long-term health consequences. Curr Vasc Pharmacol. 2019;17(6):604–9. https://doi.org/10.2174/1570161117666190122101611.
Jiao X, Zhang H, Ke H, Zhang J, Cheng L, Liu Y, et al. Premature ovarian insufficiency: phenotypic characterization within different etiologies. J Clin Endocrinol Metab. 2017;102(7):2281–90. https://doi.org/10.1210/jc.2016-3960.
Chen Y, Nguyen DT, Kokil GR, Wong YX, Dang TT. Microencapsulated islet-like microtissues with toroid geometry for enhanced cellular viability. Acta Biomater. 2019;97:260–71. https://doi.org/10.1016/j.actbio.2019.08.018.
Espona-Noguera A, Ciriza J, Canibano-Hernandez A, Orive G, Hernandez RMM, Saenz Del Burgo L, et al. Review of advanced hydrogel-based cell encapsulation systems for insulin delivery in type 1 diabetes mellitus. Pharmaceutics. 2019;11(11). https://doi.org/10.3390/pharmaceutics11110597.
Stock AA, Manzoli V, De Toni T, Abreu MM, Poh YC, Ye L, et al. Conformal coating of stem cell-derived islets for beta cell replacement in type 1 diabetes. Stem Cell Reports. 2020;14(1):91–104. https://doi.org/10.1016/j.stemcr.2019.11.004.
Ding X, Wang S, Jin W, Liu X, Chen J, Chen S. Encapsulation of a nanoporous simvastatin-chitosan composite to enhance osteointegration of hydroxyapatite-coated polyethylene terephthalate ligaments. Int J Nanomedicine. 2019;14:4881–93. https://doi.org/10.2147/ijn.S210687.
Leslie SK, Cohen DJ, Boyan BD, Schwartz Z. Production of osteogenic and angiogenic factors by microencapsulated adipose stem cells varies with culture conditions. J Biomed Mater Res B Appl Biomater. 2019;108:1857–67. https://doi.org/10.1002/jbm.b.34527.
Nadine S, Patrício SG, Correia CR, Mano JF. Dynamic microfactories co-encapsulating osteoblastic and adipose-derived stromal cells for the biofabrication of bone units. Biofabrication. 2019;12(1):015005. https://doi.org/10.1088/1758-5090/ab3e16.
Amiri A, Mousakhani-Ganjeh A, Amiri Z, Guo YG, Pratap Singh A, Esmaeilzadeh KR. Fabrication of cumin loaded-chitosan particles: characterized by molecular, morphological, thermal, antioxidant and anticancer properties as well as its utilization in food system. Food Chem. 2020;310:125821. https://doi.org/10.1016/j.foodchem.2019.125821.
Fathi M, Barar J, Erfan-Niya H, Omidi Y. Methotrexate-conjugated chitosan-grafted pH- and thermo-responsive magnetic nanoparticles for targeted therapy of ovarian cancer. Int J Biol Macromol. 2019;154:1175–84. https://doi.org/10.1016/j.ijbiomac.2019.10.272.
Gao J, Liu J, Xie F, Lu Y, Yin C, Shen X. Co-delivery of docetaxel and salinomycin to target both breast cancer cells and stem cells by PLGA/TPGS nanoparticles. Int J Nanomedicine. 2019;14:9199–216. https://doi.org/10.2147/ijn.S230376.
Yang Y, Li J, Chen F, Qiao S, Li Y, Pan W. Synthesis, formulation, and characterization of doxorubicin-loaded laponite/oligomeric hyaluronic acid-aminophenylboronic acid nanohybrids and cytological evaluation against MCF-7 breast cancer cells. AAPS PharmSciTech. 2019;21(1):5. https://doi.org/10.1208/s12249-019-1533-6.
Zhuang J, Holay M, Park JH, Fang RH, Zhang J, Zhang L. Nanoparticle delivery of immunostimulatory agents for cancer immunotherapy. Theranostics. 2019;9(25):7826–48. https://doi.org/10.7150/thno.37216.
Guo XX, Zhou JL, Xu Q, Lu X, Liang YJ, Weng J, et al. Prevention of osteoporosis in mice after ovariectomy via allograft of microencapsulated ovarian cells. Anatomical Record (Hoboken, NJ : 2007). 2010;293(2):200–7. https://doi.org/10.1002/ar.21036.
Liu C, Luan X, He Y, Xia X, Sun L, Miao W, et al. Endogenous release of female hormones from co-microencapsulated rat granulosa and theca cells. Biomed Microdevices. 2014;16(2):209–16. https://doi.org/10.1007/s10544-013-9824-2.
Sittadjody S, Enck KM, Wells A, Yoo JJ, Atala A, Saul JM, et al. Encapsulation of mesenchymal stem cells in 3D ovarian cell constructs promotes stable and long-term hormone secretion with improved physiological outcomes in a syngeneic rat model. Ann Biomed Eng. 2020;48(3):1058–70. https://doi.org/10.1007/s10439-019-02334-w.
Sittadjody S, Saul JM, Joo S, Yoo JJ, Atala A, Opara EC. Engineered multilayer ovarian tissue that secretes sex steroids and peptide hormones in response to gonadotropins. Biomaterials. 2013;34(10):2412–20. https://doi.org/10.1016/j.biomaterials.2012.11.059.
Sittadjody S, Saul JM, Opara EC. Compartmentalization of two cell types in multilayered alginate microcapsules. Methods Mol Biol. 2017;1479:225–35. https://doi.org/10.1007/978-1-4939-6364-5_18.
Pesl M, Acimovic I, Pribyl J, Hezova R, Vilotic A, Fauconnier J, et al. Forced aggregation and defined factors allow highly uniform-sized embryoid bodies and functional cardiomyocytes from human embryonic and induced pluripotent stem cells. Heart Vessel. 2014;29(6):834–46. https://doi.org/10.1007/s00380-013-0436-9.
Hummitzsch K, Ricken AM, Kloss D, Erdmann S, Nowicki MS, Rothermel A, et al. Spheroids of granulosa cells provide an in vitro model for programmed cell death coupled to steroidogenesis. Differentiation. 2009;77(1):60–9. https://doi.org/10.1016/j.diff.2008.09.002.
Ayala P, Caves J, Dai E, Siraj L, Liu L, Chaudhuri O, et al. Engineered composite fascia for stem cell therapy in tissue repair applications. Acta Biomater. 2015;26:1–12. https://doi.org/10.1016/j.actbio.2015.08.012.
Freedman BR, Mooney DJ. Biomaterials to mimic and heal connective tissues. Adv Mater. 2019;31(19):e1806695. https://doi.org/10.1002/adma.201806695.
Torrance C, Telfer E, Gosden RG. Quantitative study of the development of isolated mouse pre-antral follicles in collagen gel culture. J Reprod Fertil. 1989;87(1):367–74. https://doi.org/10.1530/jrf.0.0870367.
Hirao Y, Nagai T, Kubo M, Miyano T, Miyake M, Kato S. In vitro growth and maturation of pig oocytes. J Reprod Fertil. 1994;100(2):333–9. https://doi.org/10.1530/jrf.0.1000333.
Alm H, Katska-Ksiazkiewicz L, Ryńska B, Tuchscherer A. Survival and meiotic competence of bovine oocytes originating from early antral ovarian follicles. Theriogenology. 2006;65(7):1422–34. https://doi.org/10.1016/j.theriogenology.2005.08.014.
Abir R, Roizman P, Fisch B, Nitke S, Okon E, Orvieto R, et al. Pilot study of isolated early human follicles cultured in collagen gels for 24 hours. Hum Reprod. 1999;14(5):1299–301. https://doi.org/10.1093/humrep/14.5.1299.
Combelles CM, Fissore RA, Albertini DF, Racowsky C. In vitro maturation of human oocytes and cumulus cells using a co-culture three-dimensional collagen gel system. Hum Reprod. 2005;20(5):1349–58. https://doi.org/10.1093/humrep/deh750.
Joo S, Oh SH, Sittadjody S, Opara EC, Jackson JD, Lee SJ, et al. The effect of collagen hydrogel on 3D culture of ovarian follicles. Biomed Mater. 2016;11(6):065009. https://doi.org/10.1088/1748-6041/11/6/065009.
Magoffin DA, Erickson GF. An improved method for primary culture of ovarian androgen-producing cells in serum-free medium: effect of lipoproteins, insulin, and insulinlike growth factor-I. In Vitro Cell Dev Biol. 1988;24(9):862–70. https://doi.org/10.1007/bf02623895.
Magoffin DA, Erickson GF. Purification of ovarian theca-interstitial cells by density gradient centrifugation. Endocrinology. 1988;122(5):2345–7. https://doi.org/10.1210/endo-122-5-2345.
Lobo RA, Davis SR, De Villiers TJ, Gompel A, Henderson VW, Hodis HN, et al. Prevention of diseases after menopause. Climacteric. 2014;17(5):540–56. https://doi.org/10.3109/13697137.2014.933411.
Cesarz Z, Tamama K. Spheroid culture of mesenchymal stem cells. Stem Cells Int. 2016;2016:9176357–11. https://doi.org/10.1155/2016/9176357.
Pettinato G, Wen X, Zhang N. Formation of well-defined embryoid bodies from dissociated human induced pluripotent stem cells using microfabricated cell-repellent microwell arrays. Sci Rep. 2014;4:7402. https://doi.org/10.1038/srep07402.
Liu WF, Chen CS. Cellular and multicellular form and function. Adv Drug Deliv Rev. 2007;59(13):1319–28. https://doi.org/10.1016/j.addr.2007.08.011.
Albertini DF, Anderson E. The appearance and structure of intercellular connections during the ontogeny of the rabbit ovarian follicle with particular reference to gap junctions. J Cell Biol. 1974;63(1):234–50. https://doi.org/10.1083/jcb.63.1.234.
Vanacker J, Luyckx V, Dolmans MM, Des Rieux A, Jaeger J, Van Langendonckt A, et al. Transplantation of an alginate-matrigel matrix containing isolated ovarian cells: first step in developing a biodegradable scaffold to transplant isolated preantral follicles and ovarian cells. Biomaterials. 2012;33(26):6079–85. https://doi.org/10.1016/j.biomaterials.2012.05.015.
Amsterdam A, Plehn-Dujowich D, Suh BS. Structure-function relationships during differentiation of normal and oncogene-transformed granulosa cells. Biol Reprod. 1992;46(4):513–22. https://doi.org/10.1095/biolreprod46.4.513.
Mueller-Klieser W. Multicellular spheroids. A review on cellular aggregates in cancer research. J Cancer Res Clin Oncol. 1987;113(2):101–22. https://doi.org/10.1007/bf00391431.
Sego TJ, Kasacheuski U, Hauersperger D, Tovar A, Moldovan NI. A heuristic computational model of basic cellular processes and oxygenation during spheroid-dependent biofabrication. Biofabrication. 2017;9(2):024104. https://doi.org/10.1088/1758-5090/aa6ed4.
Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12(10):2739–46. https://doi.org/10.1089/ten.2006.12.2739.
Yalcinkaya TM, Sittadjody S, Opara EC. Scientific principles of regenerative medicine and their application in the female reproductive system. Maturitas. 2014;77(1):12–9. https://doi.org/10.1016/j.maturitas.2013.10.007.
Hernández RM, Orive G, Murua A, Pedraz JL. Microcapsules and microcarriers for in situ cell delivery. Adv Drug Deliv Rev. 2010;62(7–8):711–30. https://doi.org/10.1016/j.addr.2010.02.004.
Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210(4472):908–10. https://doi.org/10.1126/science.6776628.
Boontheekul T, Kong HJ, Mooney DJ. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials. 2005;26(15):2455–65. https://doi.org/10.1016/j.biomaterials.2004.06.044.
Mazzitelli S, Luca G, Mancuso F, Calvitti M, Calafiore R, Nastruzzi C, et al. Production and characterization of engineered alginate-based microparticles containing ECM powder for cell/tissue engineering applications. Acta Biomater. 2011;7(3):1050–62. https://doi.org/10.1016/j.actbio.2010.10.005.
Tan WH, Takeuchi S. Dynamic microarray system with gentle retrieval mechanism for cell-encapsulating hydrogel beads. Lab Chip. 2008;8(2):259–66. https://doi.org/10.1039/b714573j.
Smith AM, Hunt NC, Shelton RM, Birdi G, Grover LM. Alginate hydrogel has a negative impact on in vitro collagen 1 deposition by fibroblasts. Biomacromolecules. 2012;13(12):4032–8. https://doi.org/10.1021/bm301321d.
Lee CR, Grodzinsky AJ, Spector M. The effects of cross-linking of collagen-glycosaminoglycan scaffolds on compressive stiffness, chondrocyte-mediated contraction, proliferation and biosynthesis. Biomaterials. 2001;22(23):3145–54. https://doi.org/10.1016/s0142-9612(01)00067-9.
Meidan R, Girsh E, Blum O, Aberdam E. In vitro differentiation of bovine theca and granulosa cells into small and large luteal-like cells: morphological and functional characteristics. Biol Reprod. 1990;43(6):913–21. https://doi.org/10.1095/biolreprod43.6.913.
Luck MR. Cholinergic stimulation, through muscarinic receptors, of oxytocin and progesterone secretion from bovine granulosa cells undergoing spontaneous luteinization in serum-free culture. Endocrinology. 1990;126(2):1256–63. https://doi.org/10.1210/endo-126-2-1256.
Funding
This study was supported by Jack and Pamela Egan.
Author information
Authors and Affiliations
Contributions
Myung Jae Jeon, Young Sik Choi, Anthony Atala, James J. Yoo, and John D. Jackson contributed to study conception and design. Myung Jae Jeon, Young Sik Choi, and Il Dong Kim prepared materials, conducted experiments, and analyzed data. Myung Jae Jeon and Young Sik Choi wrote the first draft of the manuscript. Tracy Criswell, Il Dong Kim, Anthony Atala, James J. Yoo, and John D. Jackson reviewed and commented on previous versions of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Research Involving Human Participants and/or Animals
No humans or human tissues were used in this research. All animal studies were conducted with the approval of the Wake Forest University Health Sciences Animal Care and Use committee. The IACUC protocol number is A14-126.
Informed Consent
No humans or human tissue were used in this study.
Code Availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jeon, M.J., Choi, Y.S., Kim, I.D. et al. Engineering Functional Rat Ovarian Spheroids Using Granulosa and Theca Cells. Reprod. Sci. 28, 1697–1708 (2021). https://doi.org/10.1007/s43032-020-00445-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00445-7