Abstract
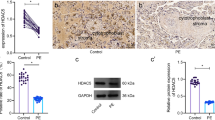
Reduced activity of trophoblast cells is well-recognized to lead to preeclampsia (PE) progression. This study aims to evaluate the roles of histone deacetylase sirtuin 2 (SIRT2) in activity of trophoblast cells and the molecules involved. Differentially expressed genes in placental tissues between PE patients and healthy individuals were screened using microarray analyses. SIRT2 and atypical chemokine receptor 2 (ACKR2) were downregulated while miR-146a was upregulated in PE patients. SIRT2 was localized in placental syncytiotrophoblasts. Upregulation of SIRT2 enhanced viability, migration and invasion, while reduced apoptosis of HTR-8/SVneo cells. SIRT2 was found to trigger p65 deacetylation level and suppress miR-146a expression according to the luciferase and ChIP assays, whereas miR-146a was found to target ACKR2. Downregulation of p65 promoted migration and invasion of cells. Overexpression of miR-146a inhibited cell viability and blocked the function of SIRT2. ACKR2 was downregulated in tissues from PE women and its upregulation blocked the role of miR-146a. To conclude, SIRT2 promotes p65 deacetylation to suppress miR-146a expression and upregulates ACKR2 expression, therefore enhancing proliferation, migration, and invasion of HTR-8/SVneo cells. This study may offer novel thoughts into the management of PE.








Similar content being viewed by others
References
Huppertz B. Traditional and new routes of trophoblast invasion and their implications for pregnancy diseases. Int J Mol Sci. 2019;21(1):289.
Lala PK, Nandi P. Mechanisms of trophoblast migration, endometrial angiogenesis in preeclampsia: the role of decorin. Cell Adhes Migr. 2016;10(1–2):111–25.
Lyall F, Robson SC, Bulmer JN. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: relationship to clinical outcome. Hypertension. 2013;62(6):1046–54.
Cierna Z, Varga I, Danihel L Jr, Kuracinova K, Janegova A, Danihel L. Intermediate trophoblast--a distinctive, unique and often unrecognized population of trophoblastic cells. Ann Anat. 2016;204:45–50.
Huppertz B. The critical role of abnormal trophoblast development in the etiology of preeclampsia. Curr Pharm Biotechnol. 2018;19(10):771–80.
Sang C, Wang S, Zhang Z, Lu J. Characteristics and outcome of severe preeclampsia/eclampsia concurrent with or complicated by acute pancreatitis: a report of five cases and literature review. J Matern Fetal Neonatal Med. 2019;32(4):633–40.
Staff AC. The two-stage placental model of preeclampsia: an update. J Reprod Immunol. 2019;134–135:1–10.
Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. 2019;124(7):1094–112.
Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–95.
Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404(1):1–13.
Yang T, Sauve AA. NAD metabolism and sirtuins: metabolic regulation of protein deacetylation in stress and toxicity. AAPS J. 2006;8(4):E632–43.
Lappas M, Mitton A, Lim R, Barker G, Riley C, Permezel M. SIRT1 is a novel regulator of key pathways of human labor. Biol Reprod. 2011;84(1):167–78.
Hannan NJ, Beard S, Binder NK, Onda K, Kaitu'u-Lino T'J, Chen Q, et al. Key players of the necroptosis pathway RIPK1 and SIRT2 are altered in placenta from preeclampsia and fetal growth restriction. Placenta. 2017;51:1–9.
Li Y, Dai D, Lu Q, Fei M, Li M, Wu X. Sirt2 suppresses glioma cell growth through targeting NF-kappaB-miR-21 axis. Biochem Biophys Res Commun. 2013;441(3):661–7.
Pais TF, Szego EM, Marques O, et al. The NAD-dependent deacetylase sirtuin 2 is a suppressor of microglial activation and brain inflammation. EMBO J. 2013;32(19):2603–16.
Arthurs AL, Lumbers ER, Pringle KG. MicroRNA mimics that target the placental renin-angiotensin system inhibit trophoblast proliferation. Mol Hum Reprod. 2019;25(4):218–27.
Pacifico F, Lepore A, Mellone S, Sanguigno L, Federico G, Greco A, et al. The chemokine scavenging receptor D6/ACKR2 is a target of miR-146a in thyroid cancer. Genes Cancer. 2017;8(5–6):577–88.
Tian S, Yu J, Zhang Y, Bian Y, Ma J, Yan J. Overexpression of PTEN regulated by miR-19b and miR-494 in the villous of recurrent spontaneous abortion patients. J Reprod Immunol. 2020;140:103133.
Yan S, Cui S, Zhang L, Yang B, Yuan Y, Lv X, et al. Expression of ACKR2 in placentas from different types of preeclampsia. Placenta. 2020;90:121–7.
Ayuk SM, Abrahamse H, Houreld NN. The role of photobiomodulation on gene expression of cell adhesion molecules in diabetic wounded fibroblasts in vitro. J Photochem Photobiol B. 2016;161:368–74.
Ning H, Albersen M, Lin G, Lue TF, Lin CS. Effects of EdU labeling on mesenchymal stem cells. Cytotherapy. 2013;15(1):57–63.
Diaz D, Prieto A, Reyes E, Barcenilla H, Monserrat J, Alvarez-Mon M. Flow cytometry enumeration of apoptotic cancer cells by apoptotic rate. Methods Mol Biol. 2015;1219:11–20.
Ahmed A, Rezai H, Broadway-Stringer S. Evidence-based revised view of the pathophysiology of preeclampsia. Adv Exp Med Biol. 2017;956:355–74.
Li Q, Zhang J, Su DM, et al. lncRNA TUG1 modulates proliferation, apoptosis, invasion, and angiogenesis via targeting miR-29b in trophoblast cells. Hum Genomics. 2019;13(1):50.
Shan N, Zhang X, Xiao X, Zhang H, Tong C, Luo X, et al. Laminin alpha4 (LAMA4) expression promotes trophoblast cell invasion, migration, and angiogenesis, and is lowered in preeclamptic placentas. Placenta. 2015;36(8):809–20.
Wang Y, Yang J, Hong T, Chen X, Cui L. SIRT2: controversy and multiple roles in disease and physiology. Ageing Res Rev. 2019;55:100961.
Harting K, Knoll B. SIRT2-mediated protein deacetylation: an emerging key regulator in brain physiology and pathology. Eur J Cell Biol. 2010;89(2–3):262–9.
Rothgiesser KM, Erener S, Waibel S, Luscher B, Hottiger MO. SIRT2 regulates NF-kappaB dependent gene expression through deacetylation of p65 Lys310. J Cell Sci. 2010;123(Pt 24):4251–8.
Yuan F, Xu ZM, Lu LY, Nie H, Ding J, Ying WH, et al. SIRT2 inhibition exacerbates neuroinflammation and blood-brain barrier disruption in experimental traumatic brain injury by enhancing NF-kappaB p65 acetylation and activation. J Neurochem. 2016;136(3):581–93.
Xiao C, Rui Y, Zhou S, Huang Y, Wei Y, Wang Z. TNF-related apoptosis-inducing ligand (TRAIL) promotes trophoblast cell invasion via miR-146a-EGFR/CXCR4 axis: a novel mechanism for preeclampsia? Placenta. 2020;93:8–16.
Teoh PJ, Menzies FM, Hansell CA, et al. Atypical chemokine receptor ACKR2 mediates chemokine scavenging by primary human trophoblasts and can regulate fetal growth, placental structure, and neonatal mortality in mice. J Immunol. 2014;193(10):5218–28.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Consents
This research was carried out with the approval and supervision of the Ethics Committee of
Zibo Maternal and Child Health Hospital and in line with the guidelines in Declaration of Helsinki.
Written confirmed consent was acquired from each respondent.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, Y., An, X. & Fan, D. Histone Deacetylase Sirtuin 2 Enhances Viability of Trophoblasts Through p65-Mediated MicroRNA-146a/ACKR2 Axis. Reprod. Sci. 28, 1370–1381 (2021). https://doi.org/10.1007/s43032-020-00398-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00398-x