Abstract
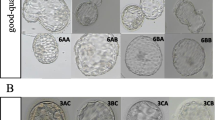
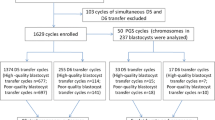
To determine whether embryo developmental stage or morphological grading can predict live birth rate (LBR) from a single blastocyst in nonbiopsied and biopsied frozen embryo transfer (FET) cycles. This retrospective study included 1336 nonbiopsied and 360 euploid FET cycles. Blastocysts were divided according to developmental stage (day 5 [D5] and day 6 [D6]) and morphology (good quality and low quality). Nonbiopsied cycles in which D5 blastocysts were transferred were associated with a significantly higher LBR than those in the D6 group (48.5 vs. 24.3%; p < 0.001), as well as in good-quality embryo transfer cycles than that in low-quality embryo cycles (52.6 vs. 25.3%; p < 0.001). Embryos reaching good-quality blastocysts on D5 yielded significantly higher LBR than those similar quality blastocysts on D6. The same trend was seen in low-quality embryos. Concerning only D5 or D6 blastocyst transfer, the LBRs of good-quality embryos were still superior to those of low-quality embryos. In the case of euploid embryo transfers, the LBR (48.9 vs. 44.9%, p = 0.444) of D5 blastocysts did not significantly differ from that of D6 blastocysts. Good-quality embryos showed a higher LBR than low-quality embryos (51.6 vs. 40.0%, p = 0.030); the adjusted odds ratio remained insignificant after controlling for confounders (aOR 1.56; 95% CI 0.99–2.45; p = 0.056). The LBRs in the same developmental stage or morphology subgroups were not statistically significant. Embryo developmental stage and morphological grade are useful predictors of LBR in nonbiopsied FET cycles. However, no association was found in euploid transfer cycles.



Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Reljic M, Knez J, Kovac V, Kovacic B. Endometrial injury, the quality of embryos, and blastocyst transfer are the most important prognostic factors for in vitro fertilization success after previous repeated unsuccessful attempts. J Assist Reprod Genet. 2017;34(6):775–9. https://doi.org/10.1007/s10815-017-0916-4.
Glujovsky D, Farquhar C, Quinteiro Retamar AM, Alvarez Sedo CR, Blake D. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. 2016;6:CD002118. https://doi.org/10.1002/14651858.CD002118.pub5.
American College of O, Gynecologists, Society for Maternal-Fetal M. ACOG Practice Bulletin No. 144: multifetal gestations: twin, triplet, and higher-order multifetal pregnancies. Obstet Gynecol. 2014;123(5):1118–32. https://doi.org/10.1097/01.AOG.0000446856.51061.3e.
Practice Committee of Society for Assisted Reproductive T, Practice Committee of American Society for Reproductive M. Elective single-embryo transfer. Fertil Steril. 2012;97(4):835–42. https://doi.org/10.1016/j.fertnstert.2011.11.050.
Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C. Clinical rationale for cryopreservation of entire embryo cohorts in lieu of fresh transfer. Fertil Steril. 2014;102(1):3–9. https://doi.org/10.1016/j.fertnstert.2014.04.018.
Ozgur K, Berkkanoglu M, Bulut H, Humaidan P, Coetzee K. Perinatal outcomes after fresh versus vitrified-warmed blastocyst transfer: retrospective analysis. Fertil Steril. 2015;104(4):899–907 e3. https://doi.org/10.1016/j.fertnstert.2015.06.031.
Franasiak JM, Forman EJ, Patounakis G, Hong KH, Werner MD, Upham KM, et al. Investigating the impact of the timing of blastulation on implantation: management of embryo-endometrial synchrony improves outcomes. Hum Reprod Open. 2018;2018(4):hoy022. https://doi.org/10.1093/hropen/hoy022.
Sunkara SK, Siozos A, Bolton VN, Khalaf Y, Braude PR, El-Toukhy T. The influence of delayed blastocyst formation on the outcome of frozen-thawed blastocyst transfer: a systematic review and meta-analysis. Hum Reprod. 2010;25(8):1906–15. https://doi.org/10.1093/humrep/deq143.
Desai N, Ploskonka S, Goodman L, Attaran M, Goldberg JM, Austin C, et al. Delayed blastulation, multinucleation, and expansion grade are independently associated with live-birth rates in frozen blastocyst transfer cycles. Fertil Steril. 2016;106(6):1370–8. https://doi.org/10.1016/j.fertnstert.2016.07.1095.
Haas J, Meriano J, Laskin C, Bentov Y, Barzilay E, Casper RF, et al. Clinical pregnancy rate following frozen embryo transfer is higher with blastocysts vitrified on day 5 than on day 6. J Assist Reprod Genet. 2016;33(12):1553–7. https://doi.org/10.1007/s10815-016-0818-x.
Yang H, Yang Q, Dai S, Li G, Jin H, Yao G, et al. Comparison of differences in development potentials between frozen-thawed D5 and D6 blastocysts and their relationship with pregnancy outcomes. J Assist Reprod Genet. 2016;33(7):865–72. https://doi.org/10.1007/s10815-016-0712-6.
Ferreux L, Bourdon M, Sallem A, Santulli P, Barraud-Lange V, Le Foll N, et al. Live birth rate following frozen-thawed blastocyst transfer is higher with blastocysts expanded on day 5 than on day 6. Hum Reprod. 2018;33(3):390–8. https://doi.org/10.1093/humrep/dey004.
Tubbing A, Shaw-Jackson C, Ameye L, Colin J, Rozenberg S, Autin C. Increased live births after day 5 versus day 6 transfers of vitrified-warmed blastocysts. J Assist Reprod Genet. 2018;35(3):417–24. https://doi.org/10.1007/s10815-017-1097-x.
Sciorio R, Thong KJ, Pickering SJ. Increased pregnancy outcome after day 5 versus day 6 transfers of human vitrified-warmed blastocysts. Zygote. 2019;27(5):279–84. https://doi.org/10.1017/S0967199419000273.
Bourdon M, Pocate-Cheriet K, Finet de Bantel A, Grzegorczyk-Martin V, Amar Hoffet A, Arbo E, et al. Day 5 versus day 6 blastocyst transfers: a systematic review and meta-analysis of clinical outcomes. Hum Reprod. 2019;34(10):1948–64. https://doi.org/10.1093/humrep/dez163.
Preimplantation Genetic Testing: ACOG Committee Opinion, Number 799. Obstet Gynecol. 2020;135(3):e133–e7. https://doi.org/10.1097/AOG.0000000000003714.
Hernandez-Nieto C, Lee JA, Slifkin R, Sandler B, Copperman AB, Flisser E. What is the reproductive potential of day 7 euploid embryos? Hum Reprod. 2019;34(9):1697–706. https://doi.org/10.1093/humrep/dez129.
Irani M, O’Neill C, Palermo GD, Xu K, Zhang C, Qin X, et al. Blastocyst development rate influences implantation and live birth rates of similarly graded euploid blastocysts. Fertil Steril. 2018;110(1):95–102 e1. https://doi.org/10.1016/j.fertnstert.2018.03.032.
Tiegs AW, Sun L, Patounakis G, Scott RT. Worth the wait? Day 7 blastocysts have lower euploidy rates but similar sustained implantation rates as day 5 and day 6 blastocysts. Hum Reprod. 2019;34(9):1632–9. https://doi.org/10.1093/humrep/dez138.
Whitney JB, Balloch K, Anderson RE, Nugent N, Schiewe MC. Day 7 blastocyst euploidy supports routine implementation for cycles using preimplantation genetic testing. JBRA Assist Reprod. 2019;23(1):45–50. https://doi.org/10.5935/1518-0557.20180089.
Chen X, Zhang J, Wu X, Cao S, Zhou L, Wang Y, et al. Trophectoderm morphology predicts outcomes of pregnancy in vitrified-warmed single-blastocyst transfer cycle in a Chinese population. J Assist Reprod Genet. 2014;31(11):1475–81. https://doi.org/10.1007/s10815-014-0317-x.
Fiorentino F, Bono S, Biricik A, Nuccitelli A, Cotroneo E, Cottone G, et al. Application of next-generation sequencing technology for comprehensive aneuploidy screening of blastocysts in clinical preimplantation genetic screening cycles. Hum Reprod. 2014;29(12):2802–13. https://doi.org/10.1093/humrep/deu277.
Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999;11(3):307–11. https://doi.org/10.1097/00001703-199906000-00013.
Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology. 2007;67(1):73–80. https://doi.org/10.1016/j.theriogenology.2006.09.014.
Makrakis E, Angeli I, Agapitou K, Pappas K, Dafereras A, Pantos K. Laser versus mechanical assisted hatching: a prospective study of clinical outcomes. Fertil Steril. 2006;86(6):1596–600. https://doi.org/10.1016/j.fertnstert.2006.05.031.
Hashimoto S, Amo A, Hama S, Ito K, Nakaoka Y, Morimoto Y. Growth retardation in human blastocysts increases the incidence of abnormal spindles and decreases implantation potential after vitrification. Hum Reprod. 2013;28(6):1528–35. https://doi.org/10.1093/humrep/det059.
Majumdar G, Majumdar A, Verma IC, Upadhyaya KC. Relationship between morphology, euploidy and implantation potential of cleavage and blastocyst stage embryos. J Hum Reprod Sci. 2017;10(1):49–57. https://doi.org/10.4103/0974-1208.204013.
Capalbo A, Rienzi L, Cimadomo D, Maggiulli R, Elliott T, Wright G, et al. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod. 2014;29(6):1173–81. https://doi.org/10.1093/humrep/deu033.
Irani M, Reichman D, Robles A, Melnick A, Davis O, Zaninovic N, et al. Morphologic grading of euploid blastocysts influences implantation and ongoing pregnancy rates. Fertil Steril. 2017;107(3):664–70. https://doi.org/10.1016/j.fertnstert.2016.11.012.
Liu XY, Fan Q, Wang J, Li R, Xu Y, Guo J, et al. Higher chromosomal abnormality rate in blastocysts from young patients with idiopathic recurrent pregnancy loss. Fertil Steril. 2020;113(4):853–64. https://doi.org/10.1016/j.fertnstert.2019.11.016.
Practice Committees of the American Society for Reproductive M, the Society for Assisted Reproductive Technology. Electronic address Aao, Practice Committees of the American Society for Reproductive M, the Society for Assisted Reproductive T. The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril. 2018;109(3):429–36. https://doi.org/10.1016/j.fertnstert.2018.01.002.
Bashiri A, Halper KI, Orvieto R. Recurrent implantation failure-update overview on etiology, diagnosis, treatment and future directions. Reprod Biol Endocrinol. 2018;16(1):121. https://doi.org/10.1186/s12958-018-0414-2.
Zhang S, Luo K, Cheng D, Tan Y, Lu C, He H, et al. Number of biopsied trophectoderm cells is likely to affect the implantation potential of blastocysts with poor trophectoderm quality. Fertil Steril. 2016;105(5):1222–7 e4. https://doi.org/10.1016/j.fertnstert.2016.01.011.
Singh S, Hobeika E, Knochenhauer ES, Traub ML. Pregnancy rates after pre-implantation genetic screening for aneuploidy are only superior when trophectoderm biopsy is performed on hatching embryos. J Assist Reprod Genet. 2019;36(4):621–8. https://doi.org/10.1007/s10815-019-01400-5.
Kang HJ, Melnick AP, Stewart JD, Xu K, Rosenwaks Z. Preimplantation genetic screening: who benefits? Fertil Steril. 2016;106(3):597–602. https://doi.org/10.1016/j.fertnstert.2016.04.027.
Kushnir VA, Darmon SK, Albertini DF, Barad DH, Gleicher N. Effectiveness of in vitro fertilization with preimplantation genetic screening: a reanalysis of United States assisted reproductive technology data 2011-2012. Fertil Steril. 2016;106(1):75–9. https://doi.org/10.1016/j.fertnstert.2016.02.026.
Harton GL, Munne S, Surrey M, Grifo J, Kaplan B, McCulloh DH, et al. Diminished effect of maternal age on implantation after preimplantation genetic diagnosis with array comparative genomic hybridization. Fertil Steril. 2013;100(6):1695–703. https://doi.org/10.1016/j.fertnstert.2013.07.2002.
Ozgur K, Berkkanoglu M, Bulut H, Yoruk GDA, Candurmaz NN, Coetzee K. Single best euploid versus single best unknown-ploidy blastocyst frozen embryo transfers: a randomized controlled trial. J Assist Reprod Genet. 2019;36(4):629–36. https://doi.org/10.1007/s10815-018-01399-1.
Funding
This study was supported by the National Natural Science Foundation of China (Grant number 81871210, 81971386, 81771536 and 81471457) and Natural Science Foundation of Jiangsu Province (BK20171126).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Nanjing Maternity and Child Health Care Hospital (NJFY-2020KY-051).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 16 kb).
Rights and permissions
About this article
Cite this article
Ji, H., Zhou, Y., Cao, S. et al. Effect of Embryo Developmental Stage, Morphological Grading, and Ploidy Status on Live Birth Rate in Frozen Cycles of Single Blastocyst Transfer. Reprod. Sci. 28, 1079–1091 (2021). https://doi.org/10.1007/s43032-020-00381-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-020-00381-6