Abstract
Prostaglandins (PGs) are profound hormones in teleost sexual behavior, especially in mating. PGs act as pheromones that affect the olfactory sensory neurons of males, inducing the initiation of a series of mating behaviors. However, the molecular mechanism by which PGs trigger mating behavior in ovoviviparous teleosts is still unclear. In the present study, we employed the ovoviviparous black rockfish (Sebastes schlegelii), an economically important marine species whose reproductive production is limited by incomplete fertilization, as a model species. The results showed that when the dose of PGE2 was higher than 10 nmol/L, a significant (P < 0.05) increase in mating behaviors was observed. Dual-fluorescence in situ hybridization indicated that PGE2 could fire specific neurons in different brain regions and receptor cells in the olfactory sac. After combining with specific neurons in the central nervous system (CNS), a series of genes related to reproduction are activated. The intracerebroventricular administration of PGE2 significantly increased lhb levels (P < 0.05) in both sexes. Moreover, steroidogenesis in gonads was also affected, inducing an increase (P < 0.05) in E2 levels in males and T levels in females. PGE2 levels were also increased significantly (P < 0.05) in both sexes. The present study revealed that PGE2 can activate mating behavior in black rockfish in both hormone and pheromone pathways, leading to variations in sex steroid levels and activation of reproductive behaviors. Our results provide not only novel insight into the onset of mating behaviors in ovoviviparous teleosts but also solutions for the incomplete fertilization caused by natural mating in cage aquaculture.
Similar content being viewed by others
Introduction
Sexual behavior is one of the most profound activities in sexually reproducing animals. Both sexes process special behavior patterns to release mature gametes for fertilization, leading to the creation of offspring. In teleosts, a series of behaviors are performed during the process of gamete release. These behaviors are named differentially depending on the pattern, such as courtship, chasing, contact, spawning, sperm release, and oviposition. They are described as “sex behavior” or “reproductive behavior”, meaning a series of behavioral acts that are performed by sexually mature females and males ultimately for the production of offspring (Munakata and Kobayashi 2010). Because the sexual behaviors of teleosts are much more derived from nature than learned, they can be performed without experience after sexual maturation. Researchers have speculated that fish might use chemicals including hormones (Munakata and Kobayashi 2010) or odorous molecules (Chung-Davidson et al. 2011), etc. to process sexual behaviors (Stacey and Sorensen 2011).
After gamete maturation, female fish usually ovulate and spawn. Meanwhile, mature males are attracted by females to start a series of mating behaviors, leading to fertilization (Kobayashi et al. 2002; Stacey et al. 2003). Hormones and neuropeptides usually play a crucial role in these processes. In general, these hormones and neuropeptides are classified into three categories. The first one is regarded as a “potentiator” (Munakata and Kobayashi 2010). A potentiating hormone has no direct effect on behavior and is not essential for the occurrence of behavior. Gonadotropin-releasing hormone (GnRH) is a typical potentiator hormone that can enhance subsequent spawning but is dispensable for sexual behavior (Volkoff and Peter 1999). The second category is characterized as a “requirement” or “primer” (Munakata and Kobayashi 2010). As the name indicates, this hormone does not activate sexual behavior, but is essential for the occurrence of the behavior. Sex steroid hormones, especially estrogen and androgens, basically function as priming agents. Studies on zebrafish (Danio rerio) have shown a correlation between high levels of both 11-ketotestosterone (11-KT) and 17-β estradiol (E2) and mating behaviors (Pradhan and Olsson 2015). In addition, when androgen is injected as a priming agent, male behavior can be triggered by other external cues (Yambe et al. 2003). The third and most important trigger is named the “physiological trigger”, which activates sexual behavior directly and rapidly when physiological and environmental conditions are appropriate (Munakata and Kobayashi 2010). One dominant trigger hormone is prostaglandins (PGs). In 1988, PGF2α and its metabolite 15-keto-PGF2α were first reported as being released from ovulated female goldfish (Carassius auratus) to initiate male courtship by activating olfactory sensory neurons (OSNs) (Sorensen et al. 1988). In oviparous medaka (Oryzias latipes) and goldfish, ovary-produced PGs can act on follicular cells to promote ovulation. Meanwhile, excess PGs are released into the environment to attract males (Appelt 1995; Fujimori et al. 2012). A study on the cichlid fish Astatotilapia burtoni showed that PGF2α injection can activate a naturalistic pattern of sexual behavior in females, which transduces signals to cells in the brain (Juntti et al. 2016). In addition, PGF2α can also act as a pheromone to trigger zebrafish reproductive behavior via the olfactory system (Yabuki et al. 2016). It is suggested that PGs play a key role in teleost mating. Apart from serving as pheromones released from females to attract males, there is also strong, robust evidence that PGs also play important roles as hormones in ovulation and spawning in teleosts (Criscuolo-Urbinati et al. 2012; Stacey et al. 2003; Takahashi et al. 2018). PG signals from the reproductive tract can communicate with the brain (Juntti et al. 2016; Saper et al. 2012), especially in the preoptic area (POA), which is thought to be related to sexual behavior across vertebrates (Goodson 2005; Wong et al. 2012). Ovariectomized peacock blenny (Salaria pavo) shows a reduction in the expression of sexual behaviors toward males, but administering PGF2α resulted in recovery of the frequency of sexual behaviors (Gonçalves et al. 2014). In goldfish, injection with PGF2α in males and females can induce typical female spawning behavior (Kobayashi et al. 2002; Saoshiro et al. 2013).
In oviparous teleosts, the mating process is always associated with female ovulation activated by PGs, and the gametes are expelled into the water environment for in vitro fertilization. However, in ovoviviparous teleosts, the gametes are always asynchronously mature (Stacey and Sorensen 2011). Early studies on ovoviviparous teleost guppies (Poecilia reticulata) showed that exposure of adult males to E2 or xenoestrogen (4-tert-octylphenol) could cause a significant decrease in the intensity and rate of sexual display (Bayley et al. 1999), and that estrogen can restore the sexual receptivity of ovariectomized females (Liley 1972). Administration of an aromatase inhibitor, fadrozole, was shown to reduce male sexual behavior in guppies (Hallgren et al. 2006). Black rockfish (Sebastes schlegelii), an economically important marine species, is an ovoviviparous teleost with long-term sperm storage. As an ovoviviparous teleost, gamete maturation in black rockfish is asynchronous. Spermatogenesis usually commences in late July and lasts until December, when mating occurs. After the mating process by the modified urogenital papillae of males, sperm are stored in the ovary cavity when oocytes are still undergoing the vitellogenesis period (Mori et al. 2003; Wang et al. 2021). When the oocytes are finally mature in late March, stored sperm activate and fuse with the oocytes. Females usually undergo an approximately 25-day pregnancy period (depending on the water temperature), and fertilized eggs develop into larvae in the ovary before final parturition (Lyu et al. 2022). In contrast to oviparity, this special reproductive strategy renders artificial reproduction difficult in black rockfish. As gamete maturation is asynchronous, fertilization is dependent on natural mating, which limits artificial insemination and the optimization of black rockfish. Artificial insemination of black rockfish invariably results in incomplete fertilization. A previous study showed that this incomplete fertilization was caused by a lack of sufficient sperm storage, and that the amount of sperm amount was related to the frequency of mating (Yao et al. 2023). Normally, a mother black rockfish could have over 50,000 fries at the same time, which means over 50,000 mature oocytes were ready for fertilization. However, if the frequency of mating is lower than normal, a proportion of the mature oocytes will not be fertilized. Mature sperm of internally fertilized black rockfish were observed swimming in female ovary fluid after mating and then stored in the crypts outside the follicular layer (Liu et al. 2019; Zhao et al. 2021). However, the female gamete is still mature during the mating season, which makes the mating initiation mechanism different from that of oviparous teleosts. There is still a lack of literature explaining the mechanism of mating initiation. In a previous study on black rockfish, COX1-2, which is a PG biosynthesis-limited enzyme, was significantly upregulated during the vitellogenesis stage when mating started, implying a potential role in mating behavior (Lyu et al. 2022). Nevertheless, a comprehensive understanding of the PG mechanism in black rockfish mating behavior is still lacking. For example, PGs function as pheromones to affect behavior alternation or as endogenic hormones that affect the hypothalamus–pituitary–gonadal axis (HPG axis) and sex steroid hormones. Prior to the present study, we tested the function of different PGs (PGF2α, PGE2 and PGD2) and steroid hormones (E2 and T) as pheromones in triggering a series of mating behaviors in black rockfish. Of these, only PGE2 altered the behavioral pattern. In the present study, we investigated the role of PGE2 in triggering mating in black rockfish. The molecular mechanism of PGE2 in the brain and gonads was further analyzed. The present study is the first to identify PGE2 as the functional pheromone for triggering mating behavior in ovoviviparous black rockfish, and mechanism by which it acts at the molecular level. Our research will provide novel information for increasing our understanding of reproduction in ovoviviparous teleosts and provide a theoretical basis for artificial reproduction in ovoviviparous teleosts.
Materials and methods
Animal collection and treatment
Black rockfish (1200 ± 300 g) were obtained from marine cages in the northern Yellow Sea, Shandong Province, China. All procedures involved in the experimental treatment of individuals were approved by the Animal Research and Ethics Committees of Ocean University of China before the initiation of the study. Animal experiments were performed in accordance to the relevant guidelines. The experimental individuals described below were anesthetized with ethyl 3-aminobenzoate methanesulfonic acid (MS-222, 200 mg/L) before sacrifice.
Observation of patterns of behavior and assay of black rockfish after PGE2 bath
Three pairs of adult male and female black rockfish were obtained in September 2020 during the mating season. One pair of fish individuals was placed into a glass tank (1 m × 1 m × 1 m, 600 L water volume, 14 ± 1 °C, photoperiod 14 L:10 D, water salinity 28) with three surveillance cameras, one each on the X axis, Y axis and Z axis. (After two days, different concentrations of PGE2 were added to the tank at 21:00. In detail, PGE2 dry powder (Shanghai Yuanye Bio-Technology, China) was dissolved in ethanol as a stock solution (108 nmol/L). A 0.6 mL aliquot of either pure ethanol (control) or of a working solution of PGE2 (107 nmol/L, or 108 nmol/L) was added to the tanks to give final concentrations of PGE2 in 0, 10 nmol/L and 100 nmol/L, respectively. Changes in behavior of individuals were observed and recorded using the surveillance cameras. These data were used to create heatmaps of the behavioral patterns at 15 min intervals.
Intracerebroventricular (ICV) administration of PGE2
Nine female and nine male black rockfish were obtained in September 2020, i.e., during the mating season, for ICV administration of PGE2. The skull of each individual was trepanned with a 1 mm2 hole approximately 1 cm above the midpoint of the two eyes. The individuals were then divided into three groups, each comprising three males and three females. 10 mg of dry PGE2 powder (Shanghai Yuanye Bio-Technology, China) was dissolved in 1 mL of ethanol. The PGE2 stock solution (10 μg/μL) was then diluted with phosphate buffered saline (PBS) and administered by ICV at concentrations of 0, 0.01 ng/g, and 0.1 ng/g wet body weight by injection through the hole. The injected PBS had the same volume of ethanol in each group. After injection, the hole was filled with dental plaster to prevent water seepage. Tissues and blood samples were collected after 6 h ICV. The sample collection protocol was as described in Sect. "Animal collection and treatment".
DNA isolation, microsatellite primers screening and PCR
Thirty pregnant individuals were sacrificed for pregnancy rate measurement. Briefly, the whole ovary was weighed before mixing the embryo within. The mixture was then sampled randomly to weigh the absolute brood amount and estimate the pregnancy rate. This process was replicated four to six times. To test the polymorphism of six microsatellite primers (KSs7, Ssc12, Ssc23, Ssc51, Ssc69, and Sra7-7), the genomic data of 230 embryos and 10 females were analyzed. DNA isolation was performed by a TIANamp Marine Animals DNA Kit (TIANGEN, China, Beijing) according to the manufacturer’s instructions.
Microsatellite primers were selected from previously published studies (An et al. 2009; Gao et al. 2018), and the 5′ end of each forward primer was labeled by ROX, FAM and HEX (Table 1). The number of alleles (Na), polymorphism information content (PIC), expected and observed heterozygosity (He, Ho), and Hardy–Weinberg equilibrium (HWE) were calculated by Cervus 3.0 (Table 1).
RNA isolation, reverse transcription, and qPCR
Ovary (O), brain region (Telencephalon, TC; Diencephalon, DC; Valvula cerebelli, VCe; Pituitary, P; Corpus cerebelli, CCe; Pons, Po; Medulla oblongata, MO) Testis (T), Urogenital papillae (UP), and olfactory sac (OS) samples from 24 individuals, including three males and three females for ptger distribution and nine males and nine females for ICV, were placed in 1 mL TRIzol solution (Vazyme, Nanjing, China) with solid-glass beads and homogenized by a high-throughput tissue lyser (DHSbio, Beijing, China). Total RNA was extracted according to the TRIzol manufacturer’s instructions (Vazyme, Nanjing, China). Qualities and concentrations of total RNA were measured by agarose gel electrophoresis and a biophotometer (OSTC, Beijing, China). Total RNA was reverse transcribed into complementary DNA (cDNA) via the HiScript III 1st Strand cDNA Synthesis Kit (Vazyme, Nanjing, China) according to the manufacturer's instructions.
qPCR was performed on a StepOnePlus Real-Time PCR System (Thermo Fisher Scientific, USA) using ChamQ SYBR Color qPCR Master Mix (Vazyme, Nanjing, China) according to the manufacturer's instructions. All primers used in the present study are listed in Table 2. After initial denaturation at 95 ℃ for 30 s, each template was amplified with 40 cycles of denaturation for 5 s at 95 ℃ and annealing for 30 s at 60 ℃. The expression level of the target gene was calculated with the 2−ΔΔct method. The expression levels were normalized against the 18S rRNA.
Colocalization of c-fos and ep2 by dual-fluorescence in situ hybridization (DISH)
DISH for ep2 and c-fos were performed to confirm the expression of EP2 on neuronal activity using previously described methods with modifications (Lyu et al. 2021). Briefly, the brain and olfactory sac were incubated in 0.1 mg/mL PGE2 (five males and five females) or solvent (five males and five females) for 5 min. Samples were then collected and fixed with buffered 4% paraformaldehyde (PFA) for approximately 24 h and embedded in paraffin. Subsequently, 7-mm thick sections were prepared for the DISH experiment. The probes for ep2 and c-fos were labeled with digoxigenin (DIG)-dUTP and biotin-dUTP (Roche Diagnostics, Mannheim, Germany), respectively. After hybridization with DIG and biotin-labeled probes and post-hybridization steps, sections were blocked with 10% goat serum (Invitrogen, Carlsbad, USA). The blocked sections were incubated with a horseradish peroxidase (HRP)-conjugated anti-DIG antibody (diluted 1:500 in the blocking reagent) and rinsed twice with sterile PBS for 5 min each time. Chromogenic reactions were then performed using a tyramide kit with Alexa Fluor 488 (Invitrogen, Carlsbad, CA, USA) for approximately 30 min. The second fluorescence detection started after the first reaction appeared to produce appropriate results. The sections were incubated with 3% hydrogen peroxide for 1 h to inactivate conjugated HRP. Sections were then incubated with HRP-conjugated streptavidin (Proteintech, Chicago, USA). The final chromogenic reaction was performed using a tyramide kit with Alexa Fluor 594 (Invitrogen, Carlsbad, CA, USA) for approximately 30 min and stopped using a stop reagent (Invitrogen, Carlsbad, USA) to detect the signal. The sections were stained with DAPI for 10 s (10 mg/mL, Solarbio, Beijing, China) and then mounted in antifade mounting medium (Beyotime, Shanghai, China). Images were captured using an Olympus BX53F fluorescence microscope (Olympus, Japan). Digital images of DISA were processed by ImageJ 1.53 software (Wayne Rasband, National Institutes of Health, Bethesda, MD, USA) (Young and Morrison 2018).
Hormone concentration measurement by radioimmunoassay and ELISA
To assess the PGE2, E2 and testosterone (T) levels after ICV treatment, blood samples from each individual were collected. PGE2 levels were measured by commercial ELISA kits (Runyu, Shanghai, China) according to the manufacturer’s instructions. RIA was performed to assay T and E2 levels by Iodine [125I] RIA kits (BNIBT, Beijing, China) according to the manufacturer’s instructions. The binding rate is highly specific with low cross-reactivity to other steroids, which was less than 0.1% for most circulating steroids.
Statistical analysis
All data are expressed as the mean ± SEM. Data analyses were performed by nonparametric T test and one-way ANOVA followed by Dunnett T3 and the LSD multiple range test. Significant differences were considered at P < 0.05. The methods of statistical analyses were chosen according to previous reports (Björnsson et al. 2018; Davis et al. 2010; Du Toit et al. 2018). All statistical analyses were performed by SPSS 19.0 software (SPSS, Chicago, USA) and GraphPad Prism 9.3.1 (GraphPad Software, USA).
Results
Estimation of pregnancy rates in black rockfish
Thirty pregnant individuals were randomly selected to measure the biological index and pregnancy rate. The average body weight was 1010.70 ± 202.64 g, body length was 36.40 ± 2.15 cm, and fatness was 2.07 ± 0.18. The average absolute brood amount was 97,720.32 ± 35,948.27, which was significantly positively correlated with average body weight (r2 = 0.7753, P < 0.05) and average body length (r2 = 0.5493, P < 0.05). Eleven out of 30 pregnant females examined were observed to have incomplete fertilization (36.67%). The lowest pregnancy rate of incompletely fertilized individuals was 37.81% (Supplementary Table 1). Figure 1A and B illustrates ovaries with complete or incomplete fertilization, respectively.
Multiple paternity analysis of black rockfish. A Dorsal ovary following complete fertilization. B Dorsal ovary following incomplete fertilization. C Comparison of paternity number in mothers with different fertilization situations. **Indicates significant difference between two groups (P < 0.01). D Proportions of paternity in different individuals. A–J represent individual mothers. M1–M8 represent the assumed male parent
Multiple paternity analysis
Genotypes at six microsatellite loci were analyzed from ten mothers and 223 embryos. As shown in Table 3, multiple paternity existed in both the incomplete and the complete fertilization groups (five mothers each). The number of sires in one female ranged from 3 to 8, with an average of 5.2 sires per brood. Furthermore, the sire numbers in the complete fertilization group were significantly higher than those in the incomplete fertilization group (P < 0.01. Figure 1C). The binomial skew index (B index) was employed to predict the existence of paternal advantage. The B index of seven out of ten broods was over 0, and four broods (C, F, G, I) were significantly skewed from equal paternal contributions (P < 0.05, Table 1). Figure 1D shows the paternity distribution of ten mother black rockfish.
PGE2 triggers a series of mating behaviors in black rockfish
To test the function of PGE2 in mating, we first observed the behavioral responses of adult male and female black rockfish under different concentrations of PGE2. The results showed that mating behavior, including chasing and contact, was elicited by 10 nmol/L PGE2 within 120 min. Furthermore, more intense behavior between males and females was observed when the concentration of PGE2 was increased to 100 nmol/L (Fig. 2A, B). Statistical analysis revealed that the percentage of contact interactions at 100 nmol/L PGE2 showed a significant difference (P < 0.05) compared with the 10 nmol/L or control groups (Fig. 2C). In addition, the percentage of contact interactions at 100 nmol/L PGE2 was significantly higher than in the 10 nmol/L (P < 0.001) or control groups (P < 0.0001) (Fig. 2D). However, no significant difference was observed in the percentage of separation and no interaction (blank) (Fig. 2E, F).
Behavior pattern analysis under PGE2 stimulation (n = 3). A Mating behavior heatmap of black rockfish. X-axis indicates the time after stimulation. Y-axis indicates PGE2 concentration (0, 10 nmol/L, 100 nmol/L). B Behavior pattern percentage of each treatment group. X-axis indicates nine experiments from three treatment groups. Y-axis indicates the percentage of each specific interaction. Blanks indicate no interaction during the experiment. C–F Statistical analysis of each specific interaction including contact, chasing, separate, and no interaction as blank. * represents P < 0.05; *** represents P < 0.001; **** represents P < 0.0001
PGE2 receptor identification in black rockfish mating behavior
Since PGE2 elicited a series of mating behaviors, subsequent studies focused on the functional receptors of PGE2. By RNA-seq and genomic database data mining, four PGE2 receptors, namely ptger EP1 (PGE2 receptor 1 subtype), EP2, EP3, and EP4, were identified. The expression levels of these four ptgers were tested by qPCR in the male and female peripheral olfactory system, central nervous system, and reproductive system in the mating season. In males, EP1 receptor mRNA was mainly detected in the DC region, followed by the CCe. The EP2 receptor was evenly distributed in different brain regions, while the highest expression level was in the OS. EP3 was mainly detected in P and TC. However, no EP4 signal was detected during mating in males (Fig. 3A–D). Meanwhile, EP1 mRNA was mainly detected in the MC + VCe region in the male brain. EP2 was significantly expressed in the OS compared with other parts of the brain and reproductive system. Similar to females, EP3 in males was also mainly expressed in P and TC. However, unlike females, EP4 was detected only in male testes (Fig. 3F–I). A heatmap demonstrated the expression patters of the four different ptgers in both sexes (Fig. 3E, J), which indicated that the potentially functional receptor in mating behavior was EP2.
Expression level of ptgers in black rockfish peripheral olfactory system and central nervous system (n = 3). A–D Expression patterns of ep1, ep2, ep3, and ep4 in females. F–I Expression patterns of ep1, ep2, ep3, and ep4 in males. E, J Heatmap of four ptger expression patterns in females and males, respectively. CCe corpus cerebelli, DC diencephalon, MC mesencephalon, MO medulla oblongata, OS olfactory sac, O ovary, P pituitary, Po pons, T testis, TC telencephalon, UP urogenital papillae, VCe valvula cerebelli
Localization of neuro cells activated by PGE2 in the brain and olfactory sac
The main functional olfactory PGE2 receptor in black rockfish mating behavior was EP2, which was also expressed in different brain regions. Together, PGE2 acts not only in the peripheral olfactory system but also in the central nervous system (CNS). To test whether PGE2 could also activate neurons in the CNS, DISH was performed to colocalize ep2 and c-fos mRNA, which is a proxy for recent neural activity. DISH results in both sexes showed that the ep2-positive signal was mainly distributed in the olfactory epithelium in areas covering the tip surface of olfactory lamellae, and a few positive signals were also observed on the ridges of lamellae. In contrast, ep2 and c-fos colocalization signals were detected only on ciliated receptor cells in the olfactory epithelium (Figs. 4C, 5C). In the TC, colocalization signals were detected in the pallium of the lateral part of the dorsal telencephalon (Dl) in both males and females. According to the qPCR results, EP2 was highly expressed in the female DC region, and the DISH results also showed a colocalization signal in the female hypothalamus (Hy). Positive signals were detected in the posterior recess in both sexes. In the male mesencephalon (MC) area, positive signals were observed in neuronal cell bodies in the optic tectum (TeO). In females, positive signals were only detected in a few small neuronal cells in the torus semicircularis (TS). The CCe area had low levels of ep2 expression, and only a few signals were detected in male Purkinje cells. In the medulla oblongata (MO), few small neuronal cells showed positive signals. Negative control by sense probes results are provided in Supplementary Fig. S1 and Supplementary Fig. S2.
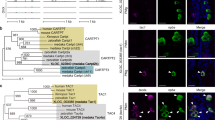
Dual-fluorescence in situ hybridization (DISH) colocalizationof c-fos (green, stained with Alexa Fluor 488) and ep2 (red, stained with Alexa Fluor 594) in male black rockfish (n = 5). A Top view, bottom view, and lateral view of male black rockfish and the sketch map showing slice positions. B Image of male black rockfish brain in lateral view. C DISH staining in peripheral olfactory system and central nervous system in treatment group (T, 0.1 mg/mL PGE2 incubation) and control group (C). Cell nucleus was stained with DAPI (blue). Scale bar (a1–a4) = 30 μm. Scale bar (h1–h4) = 250 μm. Scale bar (j1–j4) = 130 μm. Scale bar (b1–b4, c1–c4, d1–d4, e1–e4, f1–f4, g1–g4, i1–i4, k1–k4, l1–l4) = 60 μm. D Fluorescence-integrated density of c-fos signal in DISH under PGE2 treatment (0.1 mg/mL) and control. Values of control group were set to 1. Bars represent mean values ± SEM. ** represents statistically significant difference (P < 0.01); *** represents highly statistically significant difference (P < 0.001); **** represents extremely highly statistically significant difference P < 0.0001. CCe corpus cerebelli, DC diencephalon, MO medulla oblongata, MC mesencephalon, OS olfactory sac, TC telencephalon
Dual-fluorescence in situ hybridization (DISH) colocalization of c-fos (green, stained with Alexa Fluor 488) and ep2 (red, stained with Alexa Fluor 594) in female black rockfish (n = 5). A Top view, bottom view, and lateral view of female black rockfish and the sketch map showing slice positions. B Image of male black rockfish brain in lateral view. C DISH staining in peripheral olfactory system and central nervous system in treatment group (T, 0.1 mg/mL PGE2 incubation) and control group (C). Cell nucleus stained with DAPI (blue). Scale bar (a1–a4) = 30 μm. Scale bar (f1–f4, g1–g4, j1–j4) = 130 μm. Scale bar (b1–b4, c1–c4, d1–d4, e1–e4, h1–h4, i1–i4, k1–k4, l1–l4) = 60 μm. D Fluorescence-integrated density of c-fos signal in DISH under PGE2 treatment (0.1 mg/mL) and control. Values of control group were set to 1. Bars represent mean values ± SEM. ** represents statistically significant difference (P < 0.01); *** represents highly statistically significant difference (P < 0.001); **** represents extremely highly statistically significant difference P < 0.0001. CCe corpus cerebelli, DC diencephalon, MO medulla oblongata, MC mesencephalon, OS olfactory sac, TC telencephalon
Physiological and molecular changes after ICV administration of PGE2
The results of the present study implied that PGE2 can elicit mating behavior in black rockfish via the peripheral olfactory system. Furthermore, EP2 subtype receptors were expressed in different brain regions. Therefore, ICV was performed to analyze the effect of PGE2 on hormones and reproduction-related genes.
The qPCR results revealed that the mRNA levels of genes related to reproduction in the brain, including kiss1, cgnrh, and sgnrh, were not significantly different from each other (Fig. 6A–C). Gonadotropin (GtH) is a crucial factor in reproduction. In the present study, fshb and lhb showed various expression differences following PGE2 injection. The fshb mRNA level was significantly downregulated in females (P < 0.0001) and males (P < 0.05) at both PGE2 concentrations (Fig. 6D). In contrast, lhb mRNA was significantly increased in females (P < 0.001) and males (P < 0.05, Fig. 6E). Interestingly, the ep2 level was significantly (P < 0.001) upregulated in the pituitary both in males and females (Fig. 6F). In gonads, the GtH receptor also presented various expression patterns. Fshr in both sexes and lhr in males were upregulated significantly in the two injection groups (P < 0.05). However, lhr in females was downregulated significantly (P < 0.01, Fig. 6G, H). COX-2, a key synthetase of PGE2, was upregulated in both sexes. In particular, the cox2 level was upregulated only in the 0.1 ng/g group compared with the control (P < 0.01) and 0.01 ng/g groups (P < 0.01), whereas in males, levels were significantly higher (P < 0.05, P < 0.001) in both injection groups than in the controls (Fig. 6I). For a series of steroidogenesis-related enzymes, only star was significantly downregulated in both sexes (P < 0.05, Fig. 6J). Significant upregulation of cyp11a1 was detected only in males (P < 0.001, Fig. 6K). Cyp19a1a was significantly upregulated in males (P < 0.001) and downregulated in females (P < 0.05, Fig. 6L).
qPCR analysis the reproductive related genes expression pattern after ICV administration of PGE2 (0.01 ng/g, 0.1 ng/g) and control group (n = 3). A–C Expression pattern of genes in brain (cgnrh, sgnrh and kiss1). D–F Expression pattern of genes in pituitary (fshb, lhb and ep2). G–L Expression pattern of genes in gonad (fshr, lhr, cox2, star, cyp11a1, and cyp19a1a). The X-axis indicates injection with different concentrations of PGE2 in both sexes. The Y-axis indicates the relative expression normalized by 18S RNA. * represents statistic difference (P < 0.05); ** represents statistically significant difference (P < 0.01); *** represents highly statistically significant difference (P < 0.001); **** represents extremely highly statistically significant difference P < 0.0001
RIA results of ICV showed that the E2 concentration in females was significantly (P < 0.01) reduced from approximately 75 pg/mL (control group) to 13 pg/mL (0.01 ng/g or 0.1 ng/g injection group) (Fig. 7A). In contrast, in males, the serum E2 level was significantly higher than in the control group (14.56 pg/mL), and the E2 level was upregulated to 70.20 pg/mL after 0.01 ng/g injection (P < 0.01) and to 59.21 pg/mL after 0.1 ng/g injection (P < 0.05) (Fig. 7A). As the intermediate product of E2, the T concentration in females showed the opposite trend. The T level increased to 1.067 ng/mL and 3.425 ng/mL in the 0.01 ng/g and 0.1 ng/g injection groups, respectively, compared with the control group (0.397 ng/mL, P < 0.0001, Fig. 7B). However, no significant difference in T level change was observed in males (Fig. 7B). PGE2 levels were significantly increased in the 0.1 ng/g injection group in both males and females (P < 0.05, Fig. 7C). The DHP concentration was significantly induced (15.5 ng/L) in the 0.1 ng/g injection group in males compared with the control group and the 0.01 ng/g injection group (P < 0.01, Fig. 7D).
Hormone concentration after administration of PGE2 by ICV (n = 3). The X-axis indicates injection with different concentrations of PGE2 in both sexes. The Y-axis indicates the hormone concentrations of E2 (A), T (B), PGE2 (C), and DHP (D), respectively. * represents statistic difference (P < 0.05); ** represents statistically significant difference (P < 0.01); **** represents extremely highly statistically significant difference P < 0.0001
Discussion
Fecundity is one of the most important factors for aquaculture fish species. As an ovoviviparous teleost, fecundity in black rockfish is reduced in comparison with oviparous fish taxa due to its special reproductive strategy (Haldorson and Love 1991). In the present study, the absolute brood amount in female black rockfish was positively correlated with body weight and body length, and individuals weighing over 1000 g had more than 100,000 fertilized eggs. It is suggested that in aquaculture purposes, maternal fish over 1000 g would be fit for reproduction. However, 36.67% of the population investigated here exhibited incomplete fertilization, which is consistent with that in a cage-cultured population of black rockfish in the Yellow Sea near Dalian in Liaoning Province (Luo et al. 2021). Therefore, incomplete fertilization has become one of the limiting factors for artificial reproduction in black rockfish.
Multiple paternity has been confirmed in previous studies on black rockfish (Leslie and Vrijenhoek 1977; Yoshida 2001). In a study on black rockfish in the Yellow Sea near Rushan and Penglai in Shandong Province, multiple paternity was observed from ten wild females (90.9%) with 2.45 sires on average. In an aquaculture population, 11 females were observed with multiple paternity (91.7%) with 3.08 sires on average (Gao et al. 2018). In a cage-cultured population of black rockfish in the Yellow Sea near Dalian in Liaoning Province, eight out of nine individuals were detected with multiple paternity, with an average sire number of 3.56. Interestingly, one of the eight individuals was incompletely fertilized (Luo et al. 2021). In the present study of black rockfish from an aquaculture farm in Rushan, Shandong Province, all ten individuals examined exhibited multiple paternity, with an average sire number of 5.20. In addition, higher sire numbers were found in the complete fertilization group, while the sire number in the incomplete fertilization group was 3.80. This suggests that a high mating success rate might be responsible for higher sire numbers and pregnancy rates.
Previous studies have indicated that PGs are functional in mating behavior alternation. In goldfish, PGF2α, which is crucial for ovulation, is released into water as postovulatory pheromones from ovulated females to stimulate males to perform their sexual behavior (Munakata and Kobayashi 2010). Similar sexual behavior patterns were also observed in female Astatotilapia burtoni intraperitoneally injected with PGF2α. In A. burtoni, brain cells can transduce PGF2α signals to mate (Juntti et al. 2016). In zebrafish, PGF2α has been shown to activate two olfactory receptors as pheromones to induce male reproductive behaviors (Yabuki et al. 2016). Another report in zebrafish also indicated that E2 exposure can alter mating behavior (Pradhan and Olsson 2015). Moreover, a study on the Chinese black sleeper (Bostrychus sinensis) showed that PGE2-releasing tubes attract more males and females with higher spawning rates than the control group (Hong et al. 2006). The EOG response to PGE2 in the mature B. sinensis olfactory system is greater than that in immature fish (Zhang et al. 2019). Furthermore, in guppies, which like black rockfish are ovoviviparous teleosts, 30 nmol/L PGE2 can trigger courtship between female and males compared with the control group (unpublished data). In the present study, we tested the function of PGs in eliciting the mating behavior of black rockfish. The results revealed that PGE2 in the water can promote interactions between females and males. Furthermore, PGE2 was shown to activate epithelial receptor cells in the peripheral olfactory system and neurons in the central nervous system, and EP2 subtype was the main functional receptor during mating. All these findings indicate the potential function of PGE2 as a sex pheromone in teleosts.
PGs act not only as pheromones, but also as hormones that function in the HPG axis and central nervous system. PGF2α injection in a female Cichlasoma bimaculatum at any stage in the spawning cycle or parental phase induces rapid substrate cleaning and spawning behavior without egg release (Cole and Stacey 1984). In a study on Astatotilapia burtoni, the PGF2α receptor Ptgfr and c-fos mRNA were located in special cells in the POA after being allowed to spawn naturally (Juntti et al. 2016). These results indicate that PGF2α conveys to the brain of females information on the presence of ovulated oocytes in the ovary and their readiness to be oviposited (Munakata and Kobayashi 2010). In the present study, colocalization signals for ep2 and c-fos, a proxy for recent neural activity, were observed in different brain regions after PGE2 stimulation. These findings suggest that PGE2 can not only function as a pheromone to attract males, but also act as an endogenous hormone to regulate the neuroendocrine system in both males and females.
To test the direct effect of PGE2 on the neuroendocrine system, its administration by ICV was performed on male and female black rockfish during the mating season. Previous studies have mainly focused on which PG syntheses are regulated by GtH, especially LH (Piotrowska-Tomala et al. 2020; Tang et al. 2017). Less is known about how PGs participate in GtH expression and release. Injection of PGE2 and PGF2α into the third ventricle of goldfish results in significant decreases in serum GtH levels (Peter and Billard 1976). PGE2 injected into the third ventricle of rats increases LH dramatically and FSH slightly (Harms et al. 1973). These findings suggest that PGE2 may directly stimulate GtH levels. Similar to these reports, administration of PGE2 by ICV in black rockfish resulted in significant upregulation of lhb and downregulation of fshb. Generally, neuropeptides including GnRH (sGnRH and cGnRH) and kisspeptin are accepted as GtH regulators. Previous studies revealed that PGE2 from hypothalamic astrocytes and tanycytes can stimulate GnRH secretion as a gliotransmitter (Clasadonte et al. 2011). However, in the present study, sgnrh, cgnrh and kiss1 showed no difference following the administration of PGE2 by ICV, except that the sgnrh level was decreased in female black rockfish. Moreover, ep2 increased in the pituitary after ICV, which is consistent with the direct regulatory effect of PGE2 on GtH.
Following the change in GtH, steroidogenesis in the gonads also showed differences. The challenge hypothesis suggests that androgens and reproductive aggression in adult male animals are closely associated (Wingfield et al. 1990). Furthermore, androgen levels and time spent on courtship behavior are related in male blenniid fish (Rhabdoblennius nitidus), and cyproterone acetate, an antiandrogen, can shorten the time on courtship (Matsumoto et al. 2012). A study on female zebrafish showed an increase in sexuality levels and characteristic swimming patterns for mating after 30 days of treatment with a high level of T and separation from males (Liu et al. 2021). Moreover, alterations in reproductive behavior were observed when male zebrafish were exposed to E2 and female zebrafish were exposed to 11-KT (Pradhan and Olsson 2015), which implied the complexity of sex steroids on reproductive behavior patterns. Zebrafish exposed to EE2 (17α-ethinyl estradiol, a synthetic estrogen) exhibit sex reversal from male to female, and the males that do not undergo sex reversal show either unaltered male sexual behavior or reduced sexual behavior (Colman et al. 2009; Larsen et al. 2008; Nash et al. 2004). Mature male goldfish exposed to E2 exhibit severely affected reproductive behavior and physiology (Bjerselius et al. 2001). Male guppies in EE2 spend more time performing “sigmoid” displays (a term of courtship display in guppies toward the visual cues of females) (Saaristo et al. 2019). In the present study, the T level of female black rockfish significantly increased after administration of PGE2 by ICV, which is a consequence of the decrease in cyp19a1a and E2 levels, and may be responsible for behavior alternation in females. In addition, as ovoviviparous teleosts, mating and ovulation in black rockfish are dissociated. It is further indicated that steroids may also participate in sexual behavior (Stacey 1981). In males, the E2 level was significantly increased. On the one hand, E2 may act on the brain and affect mating behavior, which is consistent with the results from brain transcriptomic data after EE2 exposure in guppies (Saaristo et al. 2021). On the other hand, E2 can regulate ptger expression levels (Blesson et al. 2012), and PGE2 may have an influence on sperm mobility (Carlson et al. 2022; Kennedy et al. 2003). Further studies are required in order to elucidate this mechanism in teleosts. It is noteworthy that DHP levels also increase in males, which can not only induce spermiation, but also modulate prostaglandin receptor mRNA levels (Juntti et al. 2016; Schulz et al. 2010). Taken together, PGE2 in the water could trigger the peripheral olfactory system and central nervous system. Moreover, PGE2 functions in the brain to activate steroidogenesis by regulating GtH levels, leading to a series of steroid differences (Fig. 8) and potentially increasing the probability of mating success in black rockfish.
Conclusions
In summary, our results revealed that PGE2: (1) is a functional molecule that activates black rockfish mating behavior; (2) activates the peripheral olfactory system and CNS by binding to EP2 receptor; (3) activates lhb levels and steroidogenesis following ICV administration; (4) activates mating behavior in black rockfish via both the hormone and the pheromone pathways, leading to variation in sex steroid levels and activation of reproductive behaviors.
Data availability
The datasets generated during and/or analysed during the current study are included in this published article (and its supplementary file).
References
An HS, Park JY, Kim M-J, Lee EY, Kim KK (2009) Isolation and characterization of microsatellite markers for the heavily exploited rockfish Sebastes schlegeli, and cross-species amplification in four related Sebastes spp. Conserv Genet 10:1969–1972
Appelt CW (1995) Female goldfish appear to release pheromonally-active-prostaglandins in urinary pulses. In: Proceedings of the fifth international symposium on the reproductive physiology of fish fishsymp95, Austin
Bayley M, Nielsen JR, Baatrup E (1999) Guppy sexual behavior as an effect biomarker of estrogen mimics. Ecotoxicol Environ Saf 43:68–73
Bjerselius R, Lundstedt-Enkel K, Olsén H, Mayer I, Dimberg K (2001) Male goldfish reproductive behaviour and physiology are severely affected by exogenous exposure to 17 beta-estradiol. Aquat Toxicol 53:139–152
Björnsson BT, Einarsdóttir IE, Johansson M, Gong N (2018) The impact of initial energy reserves on growth hormone resistance and plasma growth hormone-binding protein levels in rainbow trout under feeding and fasting conditions. Front Endocrinol 9:231
Blesson CS, Büttner E, Masironi B, Sahlin L (2012) Prostaglandin receptors EP and FP are regulated by estradiol and progesterone in the uterus of ovariectomized rats. Reprod Biol Endocrinol 10:3
Carlson EJ, Georg GI, Hawkinson JE (2022) Steroidal antagonists of progesterone- and prostaglandin E(1)-induced activation of the cation channel of sperm. Mol Pharmacol 101:56–67
Chung-Davidson Y-W, Huertas M, Li W (2011) A review of research in fish pheromones. In: Breithaupt T, Thiel M (eds) Chemical communication in crustaceans. Springer, New York, pp 467–482
Clasadonte J, Sharif A, Baroncini M, Prevot V (2011) Gliotransmission by prostaglandin E(2): a prerequisite for GnRH neuronal function? Front Endocrinol 2:91
Cole KS, Stacey NE (1984) Prostaglandin induction of spawning behavior in Cichlasoma bimaculatum (Pisces cichlidae). Horm Behav 18:235–248
Colman JR, Baldwin D, Johnson LL, Scholz NL (2009) Effects of the synthetic estrogen, 17alpha-ethinylestradiol, on aggression and courtship behavior in male zebrafish (Danio rerio). Aquat Toxicol 91:346–354
Criscuolo-Urbinati E, Kuradomi RY, Urbinati EC, Batlouni SR (2012) The administration of exogenous prostaglandin may improve ovulation in pacu (Piaractus mesopotamicus). Theriogenology 78:2087–2094
Davis TL, Bott RC, Slough TL, Bruemmer JE, Niswender GD (2010) Progesterone inhibits oxytocin- and prostaglandin F2alpha-stimulated increases in intracellular calcium concentrations in small and large ovine luteal cells. Biol Reprod 82:282–288
Du Toit E, Browne L, Irving-Rodgers H, Massa HM, Fozzard N, Jennings MP, Peak IR (2018) Effect of GPR84 deletion on obesity and diabetes development in mice fed long chain or medium chain fatty acid rich diets. Eur J Nutr 57:1737–1746
Fujimori C, Ogiwara K, Hagiwara A, Takahashi T (2012) New evidence for the involvement of prostaglandin receptor EP4b in ovulation of the medaka, Oryzias latipes. Mol Cell Endocrinol 362:76–84
Gao T, Ding K, Song N, Zhang X, Han Z (2018) Comparative analysis of multiple paternity in different populations of viviparous black rockfish, Sebastes schlegelii, a fish with long-term female sperm storage. Mar Biodivers 48:2017–2024
Gonçalves D, Costa SS, Teles MC, Silva H, Inglês M, Oliveira RF (2014) Oestradiol and prostaglandin F2α regulate sexual displays in females of a sex-role reversed fish. Proc Biol Sci 281:20133070
Goodson JL (2005) The vertebrate social behavior network: evolutionary themes and variations. Horm Behav 48:11–22
Haldorson L, Love M (1991) Maturity and fecundity in the rockfishes, Sebastes spp., a review. Mar Fish Rev 53:25–31
Hallgren SL, Linderoth M, Olsén KH (2006) Inhibition of cytochrome p450 brain aromatase reduces two male specific sexual behaviours in the male Endler guppy (Poecilia reticulata). Gen Comp Endocrinol 147:323–328
Harms PG, Ojeda SR, McCann SM (1973) Prostaglandin involvement in hypothalamic control of gonadotropin and prolactin release. Science 181:760–761
Hong WS, Chen SX, Zhang QY, Zheng WY (2006) Sex organ extracts and artificial hormonal compounds as sex pheromones to attract broodfish and to induce spawning of Chinese black sleeper (Bostrichthys sinensis Lacépède). Aquac Res 37:529–534
Juntti SA, Hilliard AT, Kent KR, Kumar A, Nguyen A, Jimenez MA, Loveland JL, Mourrain P, Fernald RD (2016) A neural basis for control of cichlid female reproductive behavior by prostaglandin F2α. Curr Biol 26:943–949
Kennedy JH, Korn N, Thurston RJ (2003) Prostaglandin levels in seminal plasma and sperm extracts of the domestic turkey, and the effects of cyclooxygenase inhibitors on sperm mobility. Reprod Biol Endocrinol 1:74
Kobayashi M, Sorensen PW, Stacey NE (2002) Hormonal and pheromonal control of spawning behavior in the goldfish. Fish Physiol Biochem 26:71–84
Larsen MG, Hansen KB, Henriksen PG, Baatrup E (2008) Male zebrafish (Danio rerio) courtship behaviour resists the feminising effects of 17alpha-ethinyloestradiol–morphological sexual characteristics do not. Aquat Toxicol 87:234–244
Leslie JF, Vrijenhoek RC (1977) Genetic analysis of natural populations of Poeciliopsis monacha: allozyme inheritance and pattern of mating. J Hered 68:301–306
Liley N (1972) The effects of estrogens and other steroids on the sexual behavior of the female guppy, Poecilia reticulata. Gen Comp Endocrinol 3:542–552
Liu Q, Wang X, Xiao Y, Zhao H, Xu S, Wang Y, Wu L, Zhou L, Du T, Lv X, Li J (2019) Sequencing of the black rockfish chromosomal genome provides insight into sperm storage in the female ovary. DNA Res 26:453–464
Liu C, Yue S, Solarz J, Lee J, Li L (2021) Improving the sexual activity and reproduction of female zebrafish with high testosterone levels. Sci Rep 11:3822
Luo Z, Dong J, Zhang Z, Xu X, Zhang X (2021) Microsatellite-based parentage analysis of offspring conducted in different regions of the black rockfish (Sebastes schlegelii) ovary. JFSC 28:391–402 (In Chinese with English abstract)
Lyu LK, Li JS, Wang XJ, Yao YJ, Li JF, Li Y, Wen HS, Qi X (2021) Arg-vasotocin directly activates isotocin receptors and induces COX2 expression in ovoviviparous guppies. Front Endocrinol 12:617580
Lyu L, Wang R, Wen H, Li Y, Li J, Wang X, Yao Y, Li J, Qi X (2022) Cyclooxygenases of ovoviviparous black rockfish (Sebastes schlegelii): cloning, tissue distribution and potential role in mating and parturition. Comp Biochem Physiol B Biochem Mol Biol 257:110677
Matsumoto Y, Yabuno A, Kiros S, Soyano K, Takegaki T (2012) Changes in male courtship intensity and androgen levels during brood cycling in the blenniid fish Rhabdoblennius nitidus. J Ethol 30:387–394
Mori H, Nakagawa M, Soyano K, Koya Y (2003) Annual reproductive cycle of black rockfish Sebastes schlegeli in captivity. Fish Sci 69:910–923
Munakata A, Kobayashi M (2010) Endocrine control of sexual behavior in teleost fish. Gen Comp Endocrinol 165:456–468
Nash JP, Kime DE, Van der Ven LT, Wester PW, Brion F, Maack G, Stahlschmidt-Allner P, Tyler CR (2004) Long-term exposure to environmental concentrations of the pharmaceutical ethynylestradiol causes reproductive failure in fish. Environ Health Perspect 112:1725–1733
Peter RE, Billard R (1976) Effects of third ventricle injection of prostaglandins on gonadotropin secretion in goldfish, Carassius auratus. Gen Comp Endocrinol 30:451–456
Piotrowska-Tomala KK, Jonczyk AW, Skarzynski DJ, Szóstek-Mioduchowska AZ (2020) Luteinizing hormone and ovarian steroids affect in vitro prostaglandin production in the equine myometrium and endometrium. Theriogenology 153:1–8
Pradhan A, Olsson PE (2015) Zebrafish sexual behavior: role of sex steroid hormones and prostaglandins. Behav Brain Funct 11:23
Saaristo M, Johnstone CP, Xu K, Allinson M, Wong BBM (2019) The endocrine disruptor, 17α-ethinyl estradiol, alters male mate choice in a freshwater fish. Aquat Toxicol 208:118–125
Saaristo M, Craft JA, Tyagi S, Johnstone CP, Allinson M, Ibrahim KS, Wong BBM (2021) Transcriptome-wide changes associated with the reproductive behaviour of male guppies exposed to 17α-ethinyl estradiol. Environ Pollut 270:116286
Saoshiro S, Kawaguchi Y, Hayakawa Y, Kobayashi M (2013) Sexual bipotentiality of behavior in male and female goldfish. Gen Comp Endocrinol 181:265–270
Saper CB, Romanovsky AA, Scammell TE (2012) Neural circuitry engaged by prostaglandins during the sickness syndrome. Nat Neurosci 15:1088–1095
Schulz RW, de França LR, Lareyre JJ, Le Gac F, Chiarini-Garcia H, Nobrega RH, Miura T (2010) Spermatogenesis in fish. Gen Comp Endocrinol 165:390–411
Sorensen PW, Hara TJ, Stacey NE, Goetz FW (1988) F prostaglandins function as potent olfactory stimulants that comprise the postovulatory female sex pheromone in goldfish. Biol Reprod 39:1039–1050
Stacey NE (1981) Hormonal regulation of female reproductive behavior in fish. Am Zool 21:305–316
Stacey NE, Sorensen PW (2011) Hormonally derivedsex pheromones in fish. In: Rocha MJ, Arukwe A, Kapoor BG (eds) Fish reproduction. Science Publishers, Enfield, pp 169–1924
Stacey N, Chojnacki A, Narayanan A, Cole T, Murphy C (2003) Hormonally derived sex pheromones in fish: exogenous cues and signals from gonad to brain. Can J Physiol Pharmacol 81:329–341
Takahashi T, Hagiwara A, Ogiwara K (2018) Prostaglandins in teleost ovulation: a review of the roles with a view to comparison with prostaglandins in mammalian ovulation. Mol Cell Endocrinol 461:236–247
Tang H, Liu Y, Li J, Li G, Chen Y, Yin Y, Guo Y, Cheng CH, Liu X, Lin H (2017) LH signaling induced ptgs2a expression is required for ovulation in zebrafish. Mol Cell Endocrinol 447:125–133
Volkoff H, Peter RE (1999) Actions of two forms of gonadotropin releasing hormone and a GnRH antagonist on spawning behavior of the goldfish Carassius auratus. Gen Comp Endocrinol 116:347–355
Wang X, Wen H, Li Y, Lyu L, Song M, Zhang Y, Li J, Yao Y, Li J, Qi X (2021) Characterization of CYP11A1 and its potential role in sex asynchronous gonadal development of viviparous black rockfish Sebastes schlegelii (Sebastidae). Gen Comp Endocrinol 302:113689
Wingfield JC, Hegner RE, Dufty AM Jr, Ball GF (1990) The “challenge hypothesis”: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am Nat 136:829–846
Wong RY, Ramsey ME, Cummings ME (2012) Localizing brain regions associated with female mate preference behavior in a swordtail. PLoS ONE 7:e50355
Yabuki Y, Koide T, Miyasaka N, Wakisaka N, Masuda M, Ohkura M, Nakai J, Tsuge K, Tsuchiya S, Sugimoto Y, Yoshihara Y (2016) Olfactory receptor for prostaglandin F2α mediates male fish courtship behavior. Nat Neurosci 19:897–904
Yambe H, Munakata A, Kitamura S, Aida K, Fusetani N (2003) Methyltestosterone induces male sensitivity to both primer and releaser pheromones in the urine of ovulated female masu salmon. Fish Physiol Biochem 28:279–280
Yao Y, Wen H, Lyu L, Wang X, Li J, Xie S, Zuo C, Yan S, Wang Z, Qi X (2023) Gnih/gnihr regulates the expression of genes related to mating behavior in ovoviviparous black rockfish (Sebastes schlegelii). Acta Hydrobiol Sin 47:1–16 (in Chinese with English abstract)
Yoshida K (2001) Pedigree tracing of a hatchery-reared stock used for aquaculture and stock enhancement based on DNA markers. Fish Genet Breed Sci 30:27–35
Young K, Morrison H (2018) Quantifying microglia morphology from photomicrographs of immunohistochemistry prepared tissue using ImageJ. J vis Exp 136:57648
Zhang YT, Hong WS, Qiu HT, Wang Q, Chen SX (2019) Androgen induces olfactory expression of prostaglandin E2 receptor Ep1 in the burrow-living fish Bostrychus sinensis. J Steroid Biochem Mol Biol 188:156–165
Zhao H, Wang X, Du T, Gao G, Wu L, Xu S, Xiao Y, Wang Y, Liu Q, Li J (2021) Sperm maturation, migration, and localization before and after copulation in black rockfish (Sebastes schlegelii). Theriogenology 166:83–89
Acknowledgements
This study was supported by grants from The National Natural Science Foundation of China (41976089) and the National Key R&D Program of China (2018YFD0901204).
Author information
Authors and Affiliations
Contributions
XQ, HSW, and YL designed the study. LKL performed the experiments. LKL, SYX, XJW, YJY, and JSL participated in the sample collection. LKL performed the data analysis and wrote the manuscript and JYD participated the figure creation and improvement. XQ provided feedback on the manuscript and edited the article. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that no conflict of interest exist.
Animal and human rights statement
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee of Ocean University of China.
Additional information
Edited by Jiamei Li.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lyu, L., Yao, Y., Xie, S. et al. Mating behaviors in ovoviviparous black rockfish (Sebastes schlegelii): molecular function of prostaglandin E2 as both a hormone and pheromone. Mar Life Sci Technol 6, 15–30 (2024). https://doi.org/10.1007/s42995-023-00214-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42995-023-00214-w