Abstract
Metabolites of microorganisms have long been considered as potential sources for drug discovery. In this study, five new depsidone derivatives, talaronins A-E (1–5) and three new xanthone derivatives, talaronins F–H (6–8), together with 16 known compounds (9–24), were isolated from the ethyl acetate extract of the mangrove-derived fungus Talaromyces species WHUF0362. The structures were elucidated by analysis of spectroscopic data and chemical methods including alkaline hydrolysis and Mosher’s method. Compounds 1 and 2 each attached a dimethyl acetal group at the aromatic ring. A putative biogenetic relationship of the isolated metabolites was presented and suggested that the depsidones and the xanthones probably had the same biosynthetic precursors such as chrysophanol or rheochrysidin. The antimicrobial activity assay indicated that compounds 5, 9, 10, and 14 showed potent activity against Helicobacter pylori with minimum inhibitory concentration (MIC) values in the range of 2.42–36.04 μmol/L. While secalonic acid D (19) demonstrated significant antimicrobial activity against four strains of H. pylori with MIC values in the range of 0.20 to 1.57 μmol/L. Furthermore, secalonic acid D (19) exhibited cytotoxicity against cancer cell lines Bel-7402 and HCT-116 with IC50 values of 0.15 and 0.19 μmol/L, respectively. The structure–activity relationship of depsidone derivatives revealed that the presence of the lactone ring and the hydroxyl at C-10 was crucial to the antimicrobial activity against H. pylori. The depsidone derivatives are promising leads to inhibit H. pylori and provide an avenue for further development of novel antibiotics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microbial secondary metabolites have received great attention as a potential resource of lead drugs owing to their productive biological activities and massive chemical diversity (Hai et al. 2021; Xu et al. 2022). Due to the special mangrove environment, including high salinity, low oxygen, nutrient limitation, and drought, mangrove-derived fungi have the biosynthetic potential to produce a variety of unique secondary metabolites (Liang et al. 2019; Nathan et al. 2020; Xu et al. 2014; Zhang et al. 2021). The genus Talaromyces (Trichocomaceae) is a sexual state of Penicillium, and has the potential to produce depsidones (Zhao et al. 2015; Wu et al. 2015). In the viewpoint of ecologies, the occurrence of Talaromyces makes these fungi increasingly regarded as a source of interesting bioactive compounds, leading to the discovery of drugs, such as penicillin, compactin, anti-mycotoxins, and miscellaneous antitumor products (Nicoletti and Trincone 2016; Nicoletti et al. 2018).
The depsidones were a series of compounds derived from depsides by a loss of hydrone in an oxidative cyclization and aroused great pharmacological interest as antimicrobial and cytotoxic agents (Hong et al. 2018; Ureña-Vacas et al. 2022; Yilmaz et al. 2004). Some depsidones act as RecA protein inhibitors by increasing bactericidal activity and reducing antibiotic resistance. Furthermore, depsidones have also targeted the protein FabZ of the bacterial system for fatty acid biosynthesis (FAS) (Alam et al. 2016; McGillick et al. 2016). Depsidones can attenuate cell tumor growth by acting as selective inhibitors of Plk1 activity or directly target antiapoptotic Bcl-2 family proteins (Hong et al. 2018; WilliaNms et al. 2011).
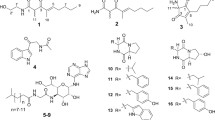
In the current study, the fungus Talaromyces sp. WHUF0362, isolated from the mangrove soil sample collected from Yalog Bay, at Sanya, Haian, China, showed potent antimicrobial activities against Escherichia coli and H. pylori G27. During our search for active secondary metabolites from the marine-derived fungi, the chemical investigation of secondary metabolites of Talaromyces sp. WHUF0362 was performed. This work resulted in the purification and identification of five new depsidones, talaronins A-E (1–5), and three new xanthone derivatives, talaronins F–H (6–8), together with 16 known compounds (9–24) (Fig. 1). In addition, the isolated compounds were evaluated for antimicrobial activity (ten Gram-negative bacteria, seven Gram-positive bacteria, a Mycobacterium, and two fungi) and cytotoxic activity (Bel-7402, HCT-116, and A549).
Results and discussion
The strain Talaromyces sp. WHUF0362 was isolated from the mangrove soil sample collected from Yalog Bay, at Sanya, Haian, China. A crude extract of Talaromyces sp. WHUF0362 cultivated in the PDA medium exhibited antimicrobial activities against Escherichia coli and H. pylori G27. A further chemical investigation of the rice fermentation products of Talaromyces sp. WHUF0362 was carried out and led to isolation and identification of 24 polyketide derivatives, eight (1–8) of which were determined as new by comprehensive analysis of spectroscopic data (1D and 2D NMR, HRESIMS, IR, and UV) and chemical methods including alkaline hydrolysis and Mosher’s method.
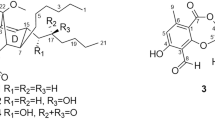
Talaronin A (1) was obtained as a colorless oil. Its molecular formula was deduced to be C23H28O7 based on high resolution electrospray ionization mass spectroscopy (HRESIMS) data (m/z: 439.1735 [M + Na]+, calcd. for C23H28O7Na: m/z 439.1727), indicating ten degrees of unsaturation. The 1H and 13C NMR (Table 1) data indicated that the presence of two aromatic rings [δH 7.56 (d, J = 8.6 Hz), 7.19 (d, J = 2.1 Hz), 7.08 (d, J = 8.6 Hz), 7.04 (d, J = 2.1 Hz), δC 115.5, 131.9, 135.8, 158.9, 114.6, 143.9, 121.6, 136.0, 124.8, 130.7, 146.6, and 161.0], three methoxy groups (δH 3.88, 3.37, and 3.35, δC 63.0, 53.6, and 53.5), an ester carbonyl group (δC 161.8), three aliphatic methyls [δH 2.32 (s), 0.97 (d, J = 6.5 Hz), 0.94 (d, J = 6.7 Hz), δC 21.1, 21.9, and 23.6], three methines [δH 5.81 (s), 5.07 (dd, J = 9.2, 4.0 Hz), 1.78 (m), δC 98.8, 67.0, 25.1], and a methylene [δH 1.64 (ddd, J = 14.2, 9.2, 5.1 Hz), 1.44 (ddd, J = 14.2, 8.8, 4.0 Hz), δC 47.6] by comprehensive analysis of 1H and 13C nuclear magnetic resonance (NMR) and heteronuclear single quantum coherence (HSQC). The aforementioned 1H and 13C NMR (Table 1) data which were similar to those of purpactin C′ (11) revealed that 1 could be a depsidone derivative (Chen et al. 2015). The main differences were the absence of the signals of an aldehyde and an acetyl in 1. Instead, it was found to be the presence of two methoxyls and a methine. The heteronuclear multiple bond connectivity (HMBC) correlations from H3-13 (δH 3.37) and H3-14 (δH 3.35) to C-10a (δC 98.8) confirmed the presence of a dimethyl acetal unit in 1 and the HMBC correlations from H-10a (δH 5.81) to C-5 (δC 124.8) and C-8a (δC 146.6) indicated the dimethyl acetal unit was at C-10a. The 1H-1H correlation spectroscopy (COSY) displayed one isolated proton spin system for the isoamyl group as shown in Fig. 2. The chemical shift of H-1′ [δH 5.05 (dd, J = 9.2, 4.0 Hz)] in 1 implied the presence of a hydroxyl at C-1′ instead of 1′-acetyl group by combined analysis of its HRESIMS data. The configuration of C-1′ was determined on the basis of the Mosher’s method (Zhang et al. 2013). Treatment of 1 with (R)- and (S)-α-methoxy-α-(trifluoromethyl) phenylacetyl chloride (MTPA-Cl) gave the (S)- and (R)-MTPA esters (1a and 1b), respectively. The 1H NMR signals of two MTPA esters were assigned. The absolute configuration was determined to be 1′S by the calculation of Δδ(S-R) values (Fig. 3A). Thus, the structure of 1 was assigned as shown and it was named as talaronin A.
Talaronin B (2) was obtained as a yellow oil and possessed the molecular formula C25H30O8 determined by HRESIMS ion at m/z 481.1844 [M + Na]+ (calcd. for C25H30NaO8: m/z 481.1833), indicating a 42 mass unit more than 1. Comparison of the 1H and 13C NMR data (Table 1) of 2 with those of 1 revealed that additional acetyl signals (δH 2.04, δC 21.3 and 170.3) appeared in 2. The downfield shift of H-1′ [δH 6.12 (dd, J = 9.4, 4.3 Hz)] in 2 indicated the presence of an acetyl group at C-1′ instead of the 1′-hydroxyl. The HMBC correlation from H-1′ (δH 6.12) to a carbonyl group at δC 170.3 confirmed the above hypothesis (Fig. 2). Compound 2 was hydrolyzed by LiOH in methanol to obtain the hydrolysis product 2a (Fig. 3B). Compound 2a was reacted with (R)- and (S)-MTPA-Cl to yield (S)- and (R)-MTPA esters (2a-a and 2a-b), respectively. The absolute configuration of 2 was deduced to be 1′S by the calculation of Δδ(S-R) values of the MTPA esters (Fig. 3A). Therefore, compound 2 was determined and named talaronin B.
Talaronin C (3) was obtained as a colorless oil with the molecular formula C25H28O8 inferred from its HRESIMS (m/z 474.2139 [M + NH4]+, calcd. for C25H32NO8: m/z 474.2122), accounting for twelve degrees of unsaturation. The general features of the 1H and 13C NMR spectra resembled those of talaromyone B (12) (Cai et al. 2017) except for the presence of an additional acetyl moiety. The significant downfield shift of H-10 and the key correlations from H-10 (δH 5.35, 5.32) to a carbonyl group at δC 172.1 suggested the acetyl group should be located at C-10. Thus, the structure of 3 was determined as shown and given the name talaronin C.
Talaronin D (4) was isolated as a yellow oil with the molecular formula of C21H22O6 based on its HRESIMS data (m/z: 369.1337 [M-H]–, calcd. for C21H22O6: m/z 369.1344), accounting for eleven degrees of unsaturation. Analyses of the 1H and 13C NMR (Table 1) signals of 4 indicated the presence of an isoamyl (δH 5.07, 1.63, 1.43, 1.78, 0.97, 0.94; δC 66.9, 47.6, 25.1, 21.9, 23.5), an aldehyde group (δH 10.61, δC 187.8), and a methoxyl (δH 3.91, δC 63.2). The 1D NMR spectroscopic data of 4 were similar to those obtained from 1 beyond the absence of a dimethyl acetal unit and the presence of an aldehyde group in 4. The aldehyde group was determined to be at C-10 as evident by HMBC correlations from H-10 (δH 10.61) to C-5 (δC 125.4) and C-10 (δC 128.6). Unambiguously, the structure of 4 was assigned as shown and named to be talaronin D.
Talaronin E (5) was isolated as a colorless oil and assigned a molecular formula of C25H30O8 based on the HRESIMS data (m/z: 457.1879 [M-H]–, calcd. for C25H29O8: m/z 457.1868), implying eleven degrees of unsaturation. Analyses of the 1H NMR data with the aid of HSQC and 13C NMR spectra revealed the existence of an aldehyde group (δH 10.20, δC 189.5), two aromatic rings [δH 7.27 (d, J = 8.8 Hz), 7.26 (d, J = 2.1 Hz), 7.10 (d, J = 2.1 Hz), 6.49 (d, J = 8.7 Hz)], an ester group (δC 167.5) and a acetyl (δH 2.03, δC 21.3, δC 170.4). These groups were attributed to the eleven degrees of unsaturation given by HRESIMS, which indicated that there was no lactonic ring in 5 compared to the depsidone. 1D NMR spectra of 5 showed a close similarity with those of secopenicillide B (14) (Komai et al. 2006) with exceptions of the absence of the methoxyl group and the presence of an ethoxyl [δH 4.53 (q, J = 7.1 Hz) 1.47 (t, J = 7.2 Hz)]. The HMBC correlation from δH 4.53 to C-9 (δC 167.5) suggested the ethoxyl moiety was bonded to the C-9 carbonyl. Therefore, compound 5 was named as talaronin E.
Talaronin F (6) was isolated as a brown oil with a molecular formula of C16H14O8 revealed by analysis of its HRESIMS (m/z 333.0635 [M-H]–, calcd. for C16H13O8: m/z 333.0616), implying ten degrees of unsaturation in 6. Comparison of the 1H NMR and 13C NMR data of 6 with those of ergochrome E (Yong et al. 2021) hinted 6 should be an xanthone analogue of ergochrome E. The 1D NMR data (Table 2) were fully assigned by detailed analysis of the HSQC and HMBC spectra. The presence of three aromatic protons [δH 7.50 (t, J = 8.3 Hz), 6.63 (d, J = 8.2 Hz), 6.61 (d, J = 8.3 Hz)] indicated an AMX-spin system in 6. The HMBC correlations from H3-11 (δH 2.17) and H-5 (δH 4.65) to C-6, 7 (δC 156.5, 128.4) denoted the aliphatic carbons of C-5 and C-6 in ergochrome E were replaced by two olefinic carbons in 6. The chemical shift of δC 191.2 in 6 implies an α, β-unsaturated ketone by analysis of the chemical shift of C-6 and C-7 combined with the weak HMBC correlation of H3-11/C-8. The HMBC correlation from H-7 to C-8a signified a hydroxyl at C-8a. Thus, compound 6 was named talaronin F.
Talaronin G (7) was obtained as a yellow solid. The molecular formula was determined to be C16H12O8 based on an ion at m/z 331.0458 [M-H]– by the HRESIMS, indicating eleven degrees of unsaturation. The 1H NMR (Table 2) spectrum exhibited an AMX-spin system [δH 7.50 (dd, J = 7.8, 0.9 Hz), 7.30 (t, J = 8.0 Hz), 7.06 (dd, J = 7.8, 0.9 Hz)], two overlapping aromatic proton signals (δH 6.90), and a methoxyl (δH 3.72). The 13C NMR data revealed the presence of three carboxyl groups, two aromatic rings, and a methoxyl by taking the 1H NMR and HSQC spectroscopic data into account. The general features of the 1H and 13C NMR spectra of 7 were similar to those of the known compound methyl peniphenone (Liu et al. 2016). The main difference was that there were two overlapping aromatic proton signals in 7 instead of the AMX-spin system, which was in accordance with a symmetrically substituted aromatic ring. The HMBC correlation from H-5, 7 (δH 6.90) to a carbonyl resonance (δC 175.5) suggested the carboxyl was located at C-6 combined with the analysis of HRESIMS data. Hence, the structure of 7 was established as shown and assigned the name talaronin G.
Talaronin H (8), a yellow solid, was found to possess a molecular formula of C17H14O9 from HRESIMS ion at m/z 361.0547 [M-H]– (Calcd. for C17H13O9, 361.0565). The 1H and 13C NMR data (Table 2) suggested the presence of a characteristic ketone carboxyl, two aromatic rings, and two methoxy groups. 1D NMR spectrum of 8 was also in accordance with 7 except for an additional methoxyl and the absence of the AMX-spin system. The HMBC correlations between H-3-Me (δH 3.85) and C-3 (δC 162.2), H-4 (δH 6.98)/H-2 (δH 6.63) and C-3 (δC 162.2) indicated the methoxy group was placed at C-3. The structure of 8 was identified as shown and named talaronin H.
The known compounds were identified as purpactin A (9) (Sy-Cordero et al. 2015), talaromyone A (10) (Cai et al. 2017), purpactin C′ (11) (Chen et al. 2015), talaromyone B (12) (Cai et al. 2017), secopenicillide A (13) (Komai et al. 2006), secopenicillide B (14) (Komai et al. 2006), talaromycin C (15) (Chen et al. 2015), deacetyl talaromycin C (16) (Wu et al. 2016), tenellic acid C (17) (Cai et al. 2017), alternaphenol B (18) (Shen et al. 2017), secalonic acid D (19) (Hong 2011), bacillisporin C (20) (Dramae et al. 2020), 4-hydroxy-3,6-dimethyl-2-pyrone (21) (Smetanina et al. 2017), 3,4-dihydroxybenzaldehyde (22), 4,6-dihydroxymellein (23) (Takenaka et al. 2011), and similanpyrone B (24) (Prompanya et al. 2014) based on their NMR and MS data as well as comparison of their spectroscopic data with those published before.
Compared with the reported depsidone derivatives, the main differences of the five new depsidone derivatives are in the different substituents of C-10, C-9, and C-1′. The known depsidones usually have an aldehyde group at C-10. However, compounds 1 and 2 each contain a dimethyl acetal group at C-10 and the aldehyde group in compound 3 was reduced to a hydroxymethyl group. This provided clues for us to speculate on the biosynthetic pathway of the depsidone. The skeleton of depsidones was produced from acetyl- and malonyl-coenzyme A by nonreducing polyketide synthase (PKS) (Cox 2007; Xu et al. 2014). It was accepted that the depsidones involved the oxidative coupling of benzophenone to give spirobenzofuran-1,2′-cyclohexa-3′,5′-diene-2′,3-dione as an intermediate, which in turn rearranged to the depsidone (Nishida et al. 1992; Xu et al. 2014). Most of the isolates herein we obtained could be divided into two series, the depsidone derivatives (1–5 and 9–18) and the xanthone derivatives (6–8 and 19). Both groups probably had the same biosynthetic precursors such as chrysophanol or rheochrysidin. The oxidation product of chrysophanol/rheochrysidin was methylated to offer compounds 6 and 7 (Wei and Matsuda 2020). Cyclization of the oxidation product gave compounds 6 and 18 (Frisvad et al. 2020) or under the action of dimerase to obtain compound 19 (Wei et al. 2021). In addition, the intermediate of spirane was obtained by PKS (Xu et al. 2014), and the rearrangement occurred due to the instability of the spiroane structure (Nishida et al. 1991). The isoprenylation of the rearrangement product was then methylated and/or acetylated to give compounds 1–3 and 10–12 or directly methylated and/or acetylated to yield compounds 5 and 13–17 (Kikuchi et al. 2012; Masters and Bräse 2012). The proposed biogenetic relationship of the isolated metabolites was shown in Supplementary Fig. S68.
The biosynthetic pathways of 3–5 were expected to be the same as that of 1. Thus, the absolute configuration of C-1′ in 3–5 was proposed to be S.
All of the isolates with adequate amount were evaluated for their antimicrobial activities and cytotoxicity activities (Supplementary Table S1, Table S2, and Table S3). Compounds 5, 9, 10, and 14 showed antibacterial activity against H. pylori with MIC values in the range from 2.42 to 36.04 μmol/L, with amoxicillin as positive control with MIC values of 0.14 to 38.14 μmol/L. Compound 11 showed antibacterial activity against Staphylococcus aureus NEWMAN with an MIC value of 38.83 μmol/L (Table 3). Particularly, compound 19 showed significant antimicrobial activity against H. pylori with MIC values of 0.20 to 1.57 μmol/L. In addition, compound 19 preeminently inhibited cancer cell lines Bel-7402 and HCT-116 with IC50 values of 0.15 and 0.19 μmol/L compared with 5-fluorouracil as a positive control with IC50 values of 13.69 and 12.23 μmol/L, respectively. According to their structural characteristics, the isolated depsidone derivatives could be divided into two categories: with lactone ring and without lactone ring. Interestingly, 5 and 13–17 without lactone ring exhibited the weak anti-H. pylori activity with MIC values higher than 32.65 μmol/L. While 9 and 10 possessed the lactone ring showed anti-H. pylori activity with MIC values of 2.41 to 10.75 μmol/L. It was suggested that the presence of the lactone ring in depsidone derivatives was related to the anti-H. pylori activity. Furthermore, 4 displayed no activity against H. pylori; whereas 10, reduzate of 4, had better anti-H. pylori activity with MIC values of 5.38 to 10.75 μmol/L. Additionally, the substituted group at C-10 in 9 was a hydroxyl which made it exhibit better anti-H. pylori activity with MIC values of 2.41 to 4.83 μmol/L. The above evidence indicated that the presence of the lactone ring and the hydroxyl at C-10 played an important role for antimicrobial activity against H. pylori. A previous study (Cai et al. 2017) indicated talaromyone B (12) possessed inhibitory activity against Bacillus subtilis. This work first presented the inhibitory activity against H. pylori of the depsidone analogues and provided an avenue for the further development of novel antibiotics.
Conclusions
In summary, five new depsidones, talaronins A-E (1–5), and three new xanthone derivatives, talaronins F–H (6–8), together with 16 known compounds were isolated from the culture of the mangrove-derived fungus Talaromyces sp. WHUF0362. Most of the isolates, could be divided into two series of compounds, the depsidone derivatives (1–5 and 9–17) and the xanthone derivatives (6–8, 18 and 19), and all of them probably had the same biosynthetic precursors, chrysophanol or rheochrysidin. In the bioactivity assays, secalonic acid D (19) demonstrated promising inhibitory activity against the cancer cell lines Bel-7402 and HCT-116 with IC50 0.15 and 0.19 μmol/L, respectively. In additional, secalonic acid D (19) showed significant antimicrobial activity against four strains of H. pylori with MIC values of 0.20 to 1.57 μmol/L. In addition, the investigated isolates 5, 9, 10, and 14 showed potential activity against H. pylori with MIC values of 2.42 to 36.04 μmol/L. The structure–activity relationship of depsidones revealed that the presence of the lactone ring and the hydroxyl at C-10 was crucial to the antimicrobial activity against H. pylori. These promising biological findings could provide an optimistic direction for finding new drugs against H. pylori.
Materials and methods
General Experimental Procedure
All the 1D and 2D NMR spectra were obtained by a Bruker AVANCE III 600 MHZ spectrometer with TMS as an internal standard (Bruker company, Switzerland). The HRESIMS data were obtained on an Agilent 6210 TOF MS system (Agilent Technologies, Santa Clara, CA, USA) or AB SCIEX Triple TOF 5600+ (AB SCIEX, USA). Optical rotations were measured by a JASCO P-1020 polarimeter (Jasco Tokyo Japan). UV spectra were performed in MeOH by using a Shimadzu UV spectrometer-1800 (Shimadzu Corp., Kyoto, Japan). IR spectra (KBr) were obtained on a Nicolet 6700 FT-IR spectrometer (Thermo Electric Nicoli, United States). Semipreparative high performance liquid chromatography (HPLC) was performed by an Agilent 1260 separation system with an Aglient ZORBAC SB-C18 column (5 μm, 250 mm × 9.4 mm, 3 mL/min). Sephadex LH-20 gel (GE Healthcare, Uppsala, Sweden) and MCI gel (Mitsubishi Chemical Corp., Japan) were used in column chromatography. And silica gel (200–300 mesh for column chromatography, GF254 for TLC) was supplied by the Yantai Zhifu Huanwu Silicone Factory, Yantai, China.
Fungal material
The fungal strain Talaromyces sp. WHUF0362 was isolated from a mangrove soil sample collected from Yalog Bay, at Sanya, Haian, China, in Dec, 2018. The strain was selected by strong and selective activity against microbial pathogens during assays against E. coli CCTCC AB 93,154, S. aureus CCTCC AB 91,093 and Candida albus CCTCC AY 206,001, and presented serious peaks at UV absorption of 200 nm, 254 nm, and 380 nm. The fungus was identified as Talaromyces sp. according to its morphological characteristics and ITS gene sequences (NCBI accession: NR_147424.1). A reference culture of Talaromyces sp. WHUF0362 maintained at − 80 °C is stored in Wuhan University, China.
Fermentation
The fungi were cultured in liquid medium (soluble starch 15 g, glucose 5 g, peptone 5 g, yeast extract 5 g, (NH4)2SO4 0.5 g, K2HPO4 0.5 g, NaCl 0.5 g, MgSO4 0.5 g, CaCO3 1 g, water 1 L, pH 7.5) as a seed solution, and then 5 mL seed solution was inoculated into 60 × 1000 mL glass culture flasks, each containing solid rice medium (rice 80 g, distilled water 120 mL). The fungi were statically fermented for 15 days in room temperature.
Extraction and Isolation
Fermentation products were extracted three times with EtOAc by soaking overnight. The crude extraction (83.77 g) was obtained by vacuum distillation. This extract was fractionated by silica gel column chromatography using the PE (petroleum ether, 60–90 °C) and the EtOAc gradient system (1:0 to 0:1, v/v) to give 7 fractions (A–G). Fraction D was applied to the silica gel column chromatography eluting with a step gradient of petroleum ether (PE):EtOAc (10:1 to 0:1, v/v) to obtain 9 fractions (D1–D9). Fraction D6 was purified by semipreparative HPLC (MeOH-H2O, 45:55, v/v) to yield 7 (5.8 mg) and 22 (2.1 mg). Fraction D2 was submitted to silica gel column chromatography eluting with PE:EtOAc (15:1 to 1:1, v/v) and purified by HPLC (MeOH-H2O, 68:32, v/v) to afford 9 (25.6 mg), 13 (5.8 mg), 19 (33.7 mg) and a mixture which was further purified by semipreparative HPLC (MeOH-H2O, 35:65, v/v) to afford 6 (10.5 mg). Fraction D3 was fractionated further by the silica gel column chromatography into two main subfractions (D3a and D3b) eluting with the gradient CH2Cl2:MeOH (40:1 to 5:1, v/v). Fraction D3a was purified by semipreparative HPLC (MeOH-H2O, 55:45, v/v) to yield 10 (8.5 mg). Fraction D3b was applied to silica gel column chromatography eluting with CH2Cl2:MeOH (70:1 to 20:1, v/v) and further purified by semipreparative HPLC (MeOH-H2O, 25:75, v/v) to yield 21 (2.6 mg). Fraction D4 was passed over a Sephadex LH-20 column, which was eluted with MeOH, and further purified by semipreparative HPLC (MeOH-H2O, 30:70, v/v) to give 23 (3.1 mg). Fraction D5 was applied to a Sephadex LH-20 column eluting with MeOH and further purified by semipreparative HPLC (MeOH-H2O, 37:63, v/v) to afford 8 (5.9 mg). Fraction C was separated into ten fractions (C1 to C10) by column chromatography on MCI gel eluting with a step gradient of MeOH-H2O (20:80 to 100:0, v/v). Fraction C10 was applied to a silica gel column eluting with PE:EtOAc (10:1 to 5:1, v/v) and further purified by semipreparative HPLC (MeOH-H2O, 80:20, v/v) to yield 2 (13.4 mg) and 11 (14.0 mg). Fraction C10-2 was applied to the semipreparative HPLC (MeOH-H2O, 80:20, v/v) to afford 3 (4.0 mg). Fraction C9 was fractionated by silica gel column chromatography eluting with PE:EtOAc (10:1 to 5:1, v/v) to give eight subfractions (C9-1 to C9-8). Compound 5 (9.8 mg) was obtained by semipreparative HPLC (MeOH-H2O, 69:31, v/v) from fraction C9-3. Fraction C9-4 was submitted to Sephadex LH-20 column eluting with MeOH and further purified by semipreparative HPLC (CH3CN-H2O, 65:35, v/v) to yield 15 (13.0 mg). Fraction C9-5 was applied to semipreparative HPLC (MeOH-H2O, 71:29, v/v) to afford 1 (7.0 mg). A precipitate from fraction C9-7 was washed with chloroform to give 20 (200.0 mg). Fraction C8 was applied to the silica gel column eluting with PE:EtOAc (15:1 to 3:1, v/v) to give 14 (3.0 g) and fraction C8-1. Fraction C8-1 was performed on the Sephadex LH-20 column eluting with MeOH and subsequently purified by semipreparative HPLC (MeOH-H2O, 65:35, v/v) to yield 4 (5.6 mg), 12 (4.4 mg) and 16 (6.9 mg). Fraction C7 was applied to a silica gel column eluting with a gradient of PE:EtOAc (5:1 to 3:1, v/v) to obtain 17 (933.6 mg) and a mixture containing 24 (2.0 mg) which was further isolated by semipreparative HPLC (MeOH-H2O, 72:28, v/v). Fraction C3 was separated by semipreparative HPLC (MeOH-H2O-formic acid, 70:30:0.1, v/v) to give 18 (7.9 mg).
Talaronin A (1): colorless oil: [α]20 D – 22.2 (c 0.14, CHCl3); UV (MeOH) λmax (log ε) 210 (4.38), 279 (3.41) nm; IR (KBr) vmax 3445, 2955, 2932, 1748, 1596, 1470, 1275, 1053 cm−1; HRESIMS m/z 439.1735 [M + Na]+ (calcd. for C23H28O7Na,439.1727); 1H NMR and 13C NMR data in Table 1.
Talaronin B (2): yellow oil; [α]20 D + 8.8 (c 0.11, CHCl3); UV (MeOH) λmax (log ε) 210 (4.44), 299 (3.38) nm; IR (KBr) vmax 2957, 2917, 2849, 1747, 1471, 1279, 1234, 1050 cm−1; HRESIMS m/z 481.1844 [M + Na]+ (calcd. for C25H30O8Na, 481.1833); 1H NMR and 13C NMR data in Table 1.
Talaronin C (3): colorless oil: [α]20 D − 8.8 (c 0.07, CH3OH); UV (MeOH) λmax (log ε) 210 (4.53), 280 (3.55) nm; IR (KBr) vmax 2958, 1743, 1597, 1471, 1235, 1050 cm−1; HRESIMS m/z 474.2139 [M + NH4]+ (calcd. for C25H32NO8, 474.2122); 1H NMR and 13C NMR data in Table 1.
Talaronin D (4): yellow oil; [α]20 D − 20.4 (c 0.11, CHCl3); UV (MeOH) λmax (log ε) 208 (4.27), 278 (3.29) nm; IR (KBr) vmax 3445, 2955, 2919, 1697, 1747, 1595, 1469, 1307, 1276, 1050 cm−1; HRESIMS m/z 369.1337 [M-H]− (calcd. for C21H21O6, 369.1344); 1H NMR and 13C NMR data in Table 1.
Talaronin E (5): colorless oil: [α]20 D − 30.4 (c 0.17, CHCl3); UV (MeOH) λmax (log ε) 210 (4.56), 268 (3.84) nm; IR (KBr) vmax 2957, 1738, 1694, 1475, 1279, 1236, 1049 cm−1; HRESIMS m/z 457.1879 [M-H]– (calcd. for C25H29O8, 457.1868); 1H NMR and 13C NMR data in Table 2.
Talaronin F (6): brown oil; [α]20 D + 62.7 (c 0.10, CH3OH); UV (MeOH) λmax (log ε) 210 (3.99), 276 (3.55), 354 (3.13) nm; IR (KBr) vmax 3390, 2918, 2849, 1730, 1698, 1626, 1581, 1461, 1383,1030,816 cm−1; HRESIMS m/z 333.0635 [M-H]– (calcd. for C16H13O8, 333.0616), 1H NMR and 13C NMR data in Table 2.
Talaronin G (7): yellow solid; UV (MeOH) λmax (log ε) 234 (3.78), 288 (3.83) nm; IR (KBr) vmax 3390, 2922, 1722, 1600, 1558, 1488, 1405, 1385, 1292, 1197, 1015, 790, 761 cm−1; HRESIMS m/z 331.0459 [M-H]– (calcd. for C16H11O8, 331.0448); 1H NMR and 13C NMR data in Table 2.
Talaronin H (8): yellow solid; UV (MeOH) λmax (log ε) 231 (3.82), 289 (3.86) nm; IR (KBr) vmax 3424.79, 2919, 2850, 1721, 1600, 1558, 1489, 1385, 1293, 1197, 1079, 1015, 790, 761 cm−1; HRESIMS m/z 361.0547 [M-H]– (calcd. for C17H13O9, 361.0565); 1H NMR and 13C NMR data in Table 2.
Antimicrobial assays
Ten Gram-negative bacteria: Salmonella typhimurium 14028 s, Shigella dysenteriae, H. pylori 26,695, H. pylori G27, H. pylori 159, H. pylori 129, E. coli MG1655, Pseudomonas aeruginosa PAO1, Acinetobacter baumannii (ATCC 19,606), and Klebsiella neumoniae (ATCC 35,657); seven Gram-positive bacteria: S. aureus (ATCC 25,923), S. aureus NEWMAN, S. aureus USA300, S. aureus NRS 271, Enterococcus faecalis FA2-2, Enterococcus Faecium (ATCC 19,434), and Bacillus subtilis 168; two fungi: Candidia albicans (ATCC SC5314) and Candidia albicans clinical isolates YY-1–4, and a Mycobacterium: Mycolicibacterium smegmatis (ATCC 607) were used for the antimicrobial assay. The antimicrobial assay and the determination of the MIC values were performed following the broth microdilution method in 96-well plates which was an established protocol (Lv et al. 2021). A detailed protocol can be found in the supporting information.
Cytotoxicity assay
Compounds were tested for cytotoxicity against human lung epithelial carcinoma (A-549), human hepatocellular carcinoma (Bel-7402), and human colon cancer (HCT-116) cell lines by using the SRB assay. All of the cancer cell lines were purchased from American Type Culture Collection (ATCC). The inhibitory rates of cell proliferation (%) were calculated as [1-(Atreated/Acontrol)] × 100%, and three independent repeated trials were conducted for each compound (n = 3). The IC50 values were determined with the Logit method from the results of six concentrations of each compound (Wu et al. 2021). A detailed protocol can be found in the supporting information.
Data availability
The data that supports the findings of this study are included in this published article (and its supplementary information files).
References
Alam MK, Alhhazmi A, DeCoteau JF, Luo Y, Geyer CR (2016) RecA inhibitors potentiate antibiotic activity and block evolution of antibiotic resistance. Cell Chem Biol 23:381–391
Cai R, Chen S, Long Y, Li C, Huang X, She Z (2017) Depsidones from Talaromyces stipitatus SK-4, an endophytic fungus of the mangrove plant Acanthus ilicifolius. Phytochem Lett 20:196–199
Chen M, Han L, Shao CL, She ZG, Wang CY (2015) Bioactive diphenyl ether derivatives from a gorgonian-derived fungus Talaromyces sp. Chem Biodivers 12:443–450
Cox RJ (2007) Polyketides, proteins and genes in fungi: programmed nano-machines begin to reveal their secrets. Org Biomol Chem 5:2010–2026
Dramae A, Intaraudom C, Bunbamrung N, Saortep W, Srichomthong K, Pittayakhajonwut P (2020) Heptacyclic oligophenalenones from the soil fungus Talaromyces bacillisporus BCC17645. Tetrahedron 76:130980
Frisvad JC, Isbrandt T, Larsen TO (2020) Fungal partially reducing polyketides and related natural products from Aspergillus, Penicillium, and Talaromyces. Compr Nat Prod III 1:313–332
Hai Y, Wei MY, Wang CY, Gu YC, Shao CL (2021) The intriguing chemistry and biology of sulfur-containing natural products from marine microorganisms (1987–2020). Mar Life Sci Tech 3:488–518
Hong R (2011) Secalonic acid D as a novel DNA topoisomerase I inhibitor from marine lichen-derived fungus Gliocladium sp. T31. Pharm Biol 49:796–799
Hong JM, Suh SS, Kim TK, Kim JE, Han SJ, Youn UJ, Yim JH, Kim IC (2018) Anti-cancer activity of lobaric acid and lobarstin extracted from the Antarctic lichen Stereocaulon alpnum. Molecules 23:658
Kikuchi H, Isobe M, Kurata S, Oshima Y (2012) New dimeric and monomeric chromanones, gonytolides DeG, isolated from the fungus Gonytrichum sp. Tetrahedron 68:6218–6223
Komai S, Hosoe T, Itabashi T, Nozawa K, Yaguchi T, Fukushima K, Kawai K (2006) New penicillide derivatives isolated from Penicillium simplicissimum. J Nat Med 60:185–190
Liang ZY, Shen NX, Zhang YY, Wu JT, Miao L, Fu XM, Chen M, Wang CY (2019) Two new unsaturated fatty acids from the mangrove rhizosphere soil-derived from fungus Penicillin javanicum HK1-22. Bioorg Chem 93:103331
Liu H, Chen S, Liu W, Liu Y, Huang X, She Z (2016) Polyketides with immunosuppressive activities from mangrove endophytic fungus Penicillium sp. ZJ-SY2. Mar Drugs 14:217
Lv H, Wang K, Xue Y, Chen J, Su H, Zhang J, Wu Y, Jia J, Bi H, Wang H, Hong K, Li X (2021) Three new metabolites from the marine-derived fungus Aspergillus sp. WHUF03110. Nat Prod Commun 16:1–7
Masters KS, Bräse S (2012) Xanthones from fungi, lichens, and bacteria: the natural products and their synthesis. Chem Rev 112:3717–3776
McGillick BE, Kumaran D, Vieni C, Swaminathan S (2016) β-Hydroxyacyl-acyl carrier protein dehydratase (FabZ) from Francisella tularensis and Yersinia pestis: structure determination, enzymatic characterization, and crossinhibition studies. Biochemistry 55:1091–1099
Nathan VK, Vijayan J, Parvathi A (2020) Optimization of urease production by Bacillus halodurans PO15: a mangrove bacterium from Poovar mangroves, India. Mar Life Sci Tech 2:194–202
Nicoletti R, Trincone A (2016) Bioactive compounds produced by strains of Penicillium and Talaromyces of marine origin. Mar Drugs 14:37
Nicoletti R, Salvatore MM, Andolfi A (2018) secondary metabolites of mangrove-associated strains of Talaromvces. Mar Drugs 16:12
Nishida H, Tomoda H, Cao J, Okuda S, Õmura S (1991) Purpactins, new inhibitors of acyl-CoA:cholesterol acyltransferase produced by Penicillium purpurogenum II. sturcture elucidation of purpactin A, B and C. J Antibiot 44:144–151
Nishida H, Tomoda H, Okuda S, Õmura S (1992) Biosynthesis of purpactin A. J Org Chem 57:1271–1274
Prompanya C, Dethoup T, Bessa LJ, Pinto MMM, Gales L, Costa PM, Silva AMS, Kijjoa A (2014) New isocoumarin derivatives and meroterpenoids from the marine sponge-associated fungus Aspergillus similanensis sp. nov. KUFA 0013. Mar Drugs 12:5160–5173
Shen Y, Xu QL, Cheng P, Liu CL, Lu ZY, Li W, Wang TT, Lu YH, Tan RX, Ge HM, Jiao RH (2017) Aromatic polyketides from a caterpillar associated Alternaria sp. Tetrahedron Lett 58:3069–3072
Smetanina OF, Yurchenko AN, Ivanets EV, Gerasimenko AV, Trinh PTH, Ly BM, Nhut ND, Van TTT, Yurchenko EA, Afiyatullov SS (2017) Aromatic metabolites of marine fungus Penicillium sp. KMM 4672 associated with a brown alga Padina sp. Chem Nat Compd 53:600–602
Sy-Cordero AA, Figueroa M, Raja HA, Aviña MEM, Croatt MP, Adcock AF, Kroll DJ, Wani MC, Pearce CJ, Oberlies NH (2015) Spiroscytalin, a new tetramic acid and other metabolites of mixed biogenesis from Scytalidium cuboideum. Tetrahedron 71:8899–8904
Takenaka Y, Morimoto N, Hamada N, Tanahashi T (2011) Phenolic compounds from the cultured mycobionts of graphis proserpens. Phytochemistry 72:1431–1435
Ureña-Vacas I, González-Burgos E, Divakar PK, Gómez-Serranillos MP (2022) Lichen depsidones with biological interest. Planta Med 88:855–880
Wei X, Matsuda Y (2020) Unraveling the fungal strategy for tetrahydroxanthone biosynthesis and diversification. Org Lett 22:1919–1923
Wei X, Chen X, Chen L, Yan D, Wang WG, Matsuda Y (2021) Heterologous biosynthesis of tetrahydroxanthone dimers: determination of key factors for selective or divergent synthesis. J Nat Prod 84:1544–1549
Willianms D, Loganzo F, Whitney L, Togias J, Harrison R, Singh M, McDonald L, Chelvendran S, Andersen R (2011) Depsides isolated from the sri lankan lichen Parmotrema sp. exhibit selective Plk1 inhibitory activity. Pharm Biol 49:296–301
Wu B, Ohlendorf B, Oesker V, Wiese J, Malien S, Schmaljohann R, Imhoff JF (2015) Acetylcholinesterase inhibitors from a marine fungus Talaromyces sp. strain LF458. Mar Biotechnol 17:110–119
Wu C, Zhao Y, Chen R, Liu D, Liu M, Proksch P, Guo P, Lin W (2016) Phenolic metabolites from mangrove-associated Penicillium pinophilum fungus with lipid-lowering effects. RSC Adv 6:21969
Wu J, Zhang H, He LM, Xue YQ, Jia J, Wang SB, Zhu KK, Hong K, Cai YS (2021) A new fusicoccane-type norditerpene and a new indone from the marine-derived fungus Aspergillus aculeatinus WHUF0198. Chem Biodivers 18:e2100562
Xu J (2015) Bioactive natural products derived from mangrove-associated microbes. RSC Adv 5:841
Xu X, Liu L, Zhang F, Wang W, Li J, Guo L, Che Y, Liu G (2014) Identification of the first diphenyl ether gene cluster for pestheic acid biosynthesis in plant endophyte Pestalotiopsis fici. ChemBioChem 15:284–292
Xu WF, Wu NN, Wu YW, Qi YX, Wei MY, Pineda LM, Ng MG, Spadafora C, Zheng JY, Lu L, Wang CY, Gu YC, Shao CL (2022) Structure modification, antialgal, antiplasmodial, and toxic evaluations of a series of new marine-derived 14-membered resorcylic acid lactone derivatives. Mar Life Sci Tech 4:88–97
Yilmaz M, Türk AÖ, Tay T, Kıvanc M (2004) The antimicrobial activity of extracts of the lichen Cladonia foliacea and its (-)-usnic acid, atranorin, and fumarprotocetraric acid constituents. Z Naturforsch C 59:249–254
Yong K, Kaleem S, Yi W, Wu B, Zhang Z (2021) New polyhydroxanthones from the marine-associated fungus Penicillium sp. ZZ1750. Tetrahedron Lett 81:153354
Zhang Y, Wang JS, Wei DD, Gu YC, Wang XB, Kong LY (2013) Bioactive terpenoids from the fruits of Aphanamixis grandifolia. J Nat Prod 76:1191–1195
Zhang CJ, Chen YL, Sun YH, Pan J, Cai MW, Li M (2021) Diversity, metabolism and cultivation of archaea in mangrove ecosystems. Mar Life Sci Tech 3:252–262
Zhao DL, Shao CL, Zhang Q, Wang KL, Guan FF, Shi T, Wang CY (2015) Azaphilone and diphenyl ether derivatives from a gorgonian derived strain of the fungus Penicillium pinophilum. J Nat Prod 78:2310–2314
Acknowledgements
This research was funded by grants from National Key Research and Development Program of China (2018YFC0311002) and High-Level Talent Special Support Plan of Zhejiang Province (2019R52009).
Author information
Authors and Affiliations
Contributions
LH and SH performed the separative experiments and structure identification; XY carried out strain isolation, evaluation and identification. JJ, BH and WS carried out the antimicrobial and antitumor bioassays. ZhM and ZJ carried out the mass experiments. EM revised the manuscript. LX, HK and WH designed the experiment and revised the manuscript. All authors discussed the experimental results and contributed to the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Animal and human rights statement
This article does not contain any studies with human participants or animals performed by the authors.
Additional information
Edited by Chengchao Chen.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lv, H., Su, H., Xue, Y. et al. Polyketides with potential bioactivities from the mangrove-derived fungus Talaromyces sp. WHUF0362. Mar Life Sci Technol 5, 232–241 (2023). https://doi.org/10.1007/s42995-023-00170-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42995-023-00170-5