Abstract
Spatial neophobia and exploration are often assessed in nonhuman animals by measuring behavioral responses to novel environments. These traits may especially affect the performance of individuals translocated to novel environments for conservation purposes. Here, we present methods to administer and analyze a minimally invasive novel environment test that accounts for the social context of focal individuals. We used an aerial platform to capture video footage of a captive herd of scimitar-horned oryx (Oryx dammah) entering an unfamiliar enclosure. We analyzed footage for seven individually identifiable oryx, scoring their behavioral responses (i.e., latency to enter the enclosure, and movement and posture after entering the enclosure) and social context (i.e., relative position and number of nearby animals). We performed a principal components analysis (PCA) to explore individual traits and responses, and used generalized linear mixed models (GLMMs) to determine the effect of individual traits and social context on individual posture and movement behaviors. Both PCA and GLMMs supported our expectation that social context affects individual behavior: high neighbor density and relative position were negatively related to individual movement, and variation in social context was positively related with head-up postures and movement. Oryx were well differentiated along two principal components that reflected (1) vigilance or caution, and (2) changing social context and age. These methods provide a framework for assessing individual responses to a novel environment in a group setting, which can inform reintroduction and wildlife management efforts, while minimizing interference with animal behavior and management operations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Consistency in behavior across time and contexts (i.e., animal personality; Réale et al. 2007) influences key aspects of survival and reproduction for wildlife, such as foraging style (Traisnel and Pichegru 2019), resource guarding (Maskrey et al. 2018), and mate selection (Martin-Wintle et al. 2017). It is, thus, an important area of research with applications across disciplines (Sih et al. 2004; Fogarty et al. 2011; Aplin et al. 2014; Spiegel et al. 2017). Studies of neophobia (aversion to novelty) have been particularly important to the developing field of animal personality, because they often rely on simple experimental manipulations while maintaining ecological relevance (i.e., animals encountering new circumstances through, e.g., dispersal, habitat fragmentation, or anthropogenic features; Greenberg and Mettke-Hofmann 2001).
In a neophobia assessment, animals are introduced to an unfamiliar object or environment while observers record their behavioral responses such as latency to approach, spatial exploration, and level of activity (Greggor et al. 2015). This approach has yielded valuable insights into the fitness consequences of personality, revealing, for example, that swift foxes (Vulpes velox) with bolder responses toward a novel object have lower survival rates after reintroduction (Bremner-Harrison et al. 2004), that exploratory behavior in a novel environment is correlated with nest success and fledgling size in great tits (Parus major; Both et al. 2005), and that Siberian chipmunk (Eutamias sibiricus) tick load increases with space use in a novel environment (Boyer et al. 2010). Together, these and other studies demonstrate that animal responses to novelty can be reliably measured, and may be correlated with fitness-relevant outcomes across a range of taxa.
Neophobia tests typically control for external influences such as the presence of conspecifics by isolating individuals during experimental trials (Webster and Ward 2011; Dall and Griffith 2014; Perals et al. 2017). However, the behavior of conspecifics is likely to be an important factor in an individual’s response to novelty (Haage et al. 2013). For example, the mating activity of groups of water striders (Aquarius remigis) is affected by the presence of hyper-aggressive males (Sih and Watters 2005; Spiegel et al. 2017), while zebra finches (Taeniopygia guttata) are more similar in exploratory behavior when measured in pairs than when measured in isolation, with important implications for foraging efficiency (Schuett and Dall 2009). Moreover, social animals are rarely isolated from conspecifics in nature (van Oers et al. 2005; Sibbald et al. 2009; Mainwaring et al. 2011) and their decisions are informed by the actions of nearby animals (Webster and Ward 2011; Dall and Griffith 2014). Previous studies have measured individual responses to novelty in group settings and used generalized social metrics (e.g., dominance status, mean number of nearby animals) to explain individual variation in response to novelty (Sibbald et al. 2009; Benson-Amram and Holekamp 2012; Massen et al. 2013a, b). However, social context should arguably be considered more explicitly (Veissier and le Neindre 1992; Webster and Ward 2011; Dall and Griffith 2014), and there is a need for neophobia research that accounts for the often rapidly changing social environment of subjects while they are exposed to novelty.
Accounting for social context may be especially relevant to species reintroductions and other conservation translocations, where a cohort of animals is released into a new environment at the same time. How animals behave in a novel environment after translocation may directly translate to their ability to avoid predation (Quinn and Cresswell 2005), reproduce successfully (Martin-Wintle et al. 2017), and locate essential resources (Stamps and Swaisgood 2007). In addition, individual differences in ability to cope with the stress of capture and handling may also impact post-release performance (Archard and Braithwaite 2010; May et al. 2016; Merrick and Koprowski 2017). For example, more stressed individuals may be less efficient while searching for resources (Teixeira et al. 2007) and adapt more slowly to a novel environment (Mendl 1999). Further, reintroduced populations are often sourced from a limited population of threatened or endangered species, and represent considerable financial and time investments (Cheyne 2009). Thus, protocols that maximize the chances of success for released animals are crucial. Assessments that account for behavior in group settings may be particularly insightful, as group members can elicit behaviors that may be advantageous or disadvantageous after release (Webster and Ward 2011; Goldenberg et al. 2019).
In addition to the biological relevance of quantifying response to novelty in the presence of conspecifics, there are many practical reasons for such an approach. For example, managers of captive populations must prioritize animal welfare (e.g., minimizing the potential stress of isolation or handling) with limited staffing resources. Thus, approaches to assess neophobia that leverage existing management routines, avoid disrupting existing social groups, and yield robust individual-level measurements are highly desirable in a captive management context (Foris et al. 2018). The increasingly affordable and accessible approach of using remote aerial platforms to monitor animals presents an exciting opportunity to integrate these ideas. Remote monitoring platforms offer the capacity to observe animals entering novel environments while avoiding disruptions to existing social groups and minimizing direct human–animal interactions.
Here, we present a minimally invasive approach to assessing spatial neophobia and exploration in a herd of captive scimitar-horned oryx (Oryx dammah; hereafter “oryx”, Appendix Fig. A1) that can be applied to groups of animals during preparations for release into the wild. Such information is urgently needed to inform ongoing efforts to reintroduce this species to its former range (Chuven et al. 2018; Mertes et al. 2019). We used a passive aerial platform (an aerostat) to monitor the herd as they entered and moved within a, enclosure they had not accessed in several months. We documented behaviors commonly recorded in novel environment tests, including latency to enter (representing spatial neophobia), and movement and head posture (representing spatial exploration; Mettke-Hofmann et al. 2009).
In addition, we measured the fine-scale social context of individuals using (1) their relative position in the herd and (2) the number of nearby animals. We expected social context to influence individual behavior, and thus predicted that social context variables would significantly affect the behavioral responses recorded after oryx entered the enclosure. This study presents a single test, and thus is not adequate to characterize the personality traits of individual oryx. However, the methodological framework presented here provides a promising avenue to characterize behavioral metrics within a group, without the need to handle animals or disrupt their social environment, and may be used to assess animal personality dimensions when implemented as multiple tests. We discuss the implications of this method for advancing the study of animal personality, and describe valuable potential applications for species conservation and wildlife management.
Methods
Study site and animals
We conducted a novel environment test at the Smithsonian Conservation Biology Institute (SCBI) on November 14, 2019. SCBI is a 1300-hectare research and captive animal institution located approximately 5 km south of Front Royal, Virginia (38°54′ N, 78°09′ W). The primary vegetation communities at SCBI are Appalachian oak forest and other mixed deciduous forests typical of the mid-Atlantic region of eastern North America (Bourg et al. 2013), as well as pastures managed for animal husbandry purposes.
We implemented this test using a female herd of oryx resident at SCBI. Scimitar-horned oryx are large African antelope adapted to the arid, seasonal grasslands surrounding the Sahara Desert (Newby 1978, 1980; Morrow et al. 2013). The species once numbered in the hundreds of thousands and ranged across the Sahel from Mauritania to Sudan (Newby 1978; Harris et al. 2009). However, overhunting, regional conflicts, habitat degradation and fragmentation, and competition with domestic livestock led to the species’ decline (Dixon et al. 1991). The last sightings of wild oryx occurred in the late 1980s (Newby 1988; Millington et al. 1991), and the species has been classified as Extinct in the Wild since 2000 (Mallon and Kingswood 2000). Fortunately, large numbers of oryx survived in private collections and zoological institutions, which have functioned as source populations for restoration efforts in Tunisia, Morocco, and Sengal. More recently, the Environment Agency—Abu Dhabi, in partnership with the Chadian Ministère de l’Environnement, de l’Eau, et du Developpement Durable, and implemented on the ground by SaharaConservation, began releasing captive-born oryx into the Réserve de Faune du Ouadi Rimé-Ouadi Achim (RFOROA) in central Chad in 2016.
All members of the SCBI oryx herd were captive-born animals acclimated to human management. The herd was comprised of 18 females and 5 dependent calves (~ 5 months old), ranging from 1.4 to 14.5 years old. To control reproduction and minimize injuries due to agonistic interactions, female oryx are housed and managed together as one social group in a ~ 1.5 ha enclosure, and were isolated from male oryx since August 2019. Seven oryx in the herd were born at SCBI, including one hand-raised individual (“Esmeralda”); the remaining adults (n = 11) were transferred to SCBI as adults from several US institutions as part of the oryx species survival plan (SSP). For females not born at SCBI, time since accession ranged from ~ 6 months to 10.4 years. Social ranking within the herd was determined based on 10 h of video recordings of agonistic interactions, recorded across 10 days (Moraes et al. in prep). We used the BORIS software (Friard and Gamba 2016) to identify and count dyadic interactions (Langbein and Puppe 2004), and used the win–loss matrix to calculate David’s scores and the best rank order (De Vries et al. 2006).
Novel environment
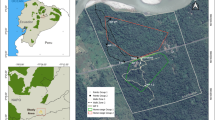
No oryx in the herd had access to the enclosure where the experiment was conducted (Fig. 1, Pasture B) for approximately 3 months. The experimental enclosure (~ 1.7 ha) was separated from the herd’s previous enclosure (Fig. 1, Pasture A) by a 6’ chain-link fence with brush growing through many links, such that visibility into either pasture from the other is substantially obscured. The experimental enclosure is also long and thin and declines steeply after the first ca. 150 m, such that the majority of its area is not visible from either the herd’s previous enclosure, or the gate from which the herd entered (Fig. 1, red line).
Aerial video footage was recorded using a DJI Osmo Pocket camera (UHD 4 k; 30 frames/second) suspended from a tethered 2 m3 Skyshot Helikite (Allsopp Helikites® Ltd., Hampshire, England). A Helikite is a non-motorized aerial platform consisting of a helium balloon with kite fins, providing greater stability and increased payloads compared with traditional aerostats (Verhoeven et al. 2009). Helikites are a relatively affordable, noise-free, and minimally regulated option for capturing aerial footage, but a wide range of non-motorized and conventional unmanned aerial vehicles (UAVs) could obtain comparable footage (Hughey et al. 2017).
Prior to the novel environment test, seven flights were conducted to acclimate the oryx to the presence of the Helikite. Acclimation flights were flown at heights between 25 and 30 m in enclosures that the oryx had access to during the month prior to the test (Fig. 1, Pasture A). Each flight was piloted from a blind (i.e., a camouflaged hide or tent approximately 150 cm × 150 cm × 150 cm in size) and lasted approximately 5 h, allowing the oryx to become accustomed to the presence of both the Helikite and the observer-occupied blind. We used behavioral indicators (e.g., non-avoidance of areas near the helikite, observation of routine behaviors before, during, and after flights) to determine that oryx were sufficiently acclimated.
On the day of the novel environment test, the Helikite was flown at a height of 25 m, with the DJI Osmo Pocket remotely operated by an observer located in a blind next to the Helikite anchor (Fig. 1, white oval; Fig. 2). Animal management personnel entered through the novel pasture (Fig. 1, Pasture B), opened the gate separating the two pastures without calling or otherwise alerting the animals, fixed the gate in an open position, and exited through the novel pasture. Management personnel were visible to the oryx briefly when opening the gate, but not thereafter. The oryx were fed prior to the novel environment test, supplementary food was not stationed within the novel pasture, and available forage was consistent across pastures. Thus, food was available ad libitum and oryx were free to enter the novel environment at will (Greggor et al. 2015). Aerial footage was continuously recorded before, during, and after the herd was given access to the experimental pasture, to record animal behaviors (Table 1). The novel environment test began when management personnel exited the open gate and left through the experimental pasture. Latency to enter was measured from this time, and behavioral observations for each individually identifiable oryx began as the individual entered the novel environment. The duration of behavior observations was defined by the camera field of view, which was limited by the position of the Helikite (because the Helikite is tethered, and thus cannot be maneuvered). Observations ended when no oryx could be individually distinguished in the aerial footage.
During the novel environment test, video footage of the oryx was also recorded from the blind using a Nikon D3500 camera. Identifying information, such as horn shape and ear tag color combinations, were noted by observers inside the blind as individual oryx entered the novel enclosure. Ground and aerial recordings were subsequently paired to confirm individual identification. However, identifying animals solely from aerial footage is possible if individuals have unique markings that can be distinguished in video footage (i.e., natural markings or ear tags, collars, horn tape, or other items used for management or research purposes).
Data analysis
We annotated aerial footage for seven female oryx that could be consistently identified in the video recorded from the Helikite (Table 1). We used the BORIS software (Friard and Gamba 2016) to record the timing and duration of head-up postures, head-down postures, moving behaviors, and resting behaviors (Appendix Table A1). These behavior categories were reliably separable in our aerial footage and capture biologically meaningful behaviors in group-living ungulates.
Head-up posture is often used as a proxy for vigilance in ungulates, especially in studies of group foraging behavior (Hunter and Skinner 1998). The frequency or total time that an individual holds its head raised is generally interpreted as its assessment of risk (e.g., from predation or conspecifics; Peterson and Weckerly 2018). Because grazers cannot feed when performing head-up postures, increasing vigilance or scanning for risk typically reduces the ability of an individual to forage (Creel et al. 2014). Likewise, movement is frequently considered an aspect of exploration in novelty tests, because it may be correlated with approach to novel objects, movement through a novel environment, or flight from a novel stimulus (Christensen et al. 2005; Olsen and Klemetsdal 2019). Importantly, grazers also cannot feed when locomoting. Thus, the response variables of head posture and movement represent biologically meaningful trade-offs between acquiring resources and performing other activities (vigilance, exploration) related to individual fitness.
We also used BORIS to measure the timing and duration of two variables we developed to capture the effect of social context on individual responses to novelty: (1) ‘relative position’ and (2) ‘personal space’. Relative position refers to the ordinal position of the focal individual relative to all other oryx as the herd moves away from the entry gate, such as in a race (Fig. 3A). A lower numeric score—e.g., a ‘relative position’ of 1 or 2—indicates that the focal individual has moved further from the entry gate than all or most oryx in the herd. A higher numeric score—e.g., a ‘relative position’ of 10—indicates that the focal individual is closer to the entry gate than other oryx moving through the pasture. ‘Relative position’ captures an individual’s tendency to lead or follow other group members. The roles of ‘leaders’ and ‘followers’ are important to collective movement and often correspond to behavioral traits like boldness and shyness, respectively (Harcourt et al. 2009; King et al. 2009). Personal space is the number of conspecifics within one body length of the focal individual (Fig. 3B), and captures the extent to which the space immediately around an individual is occupied by conspecifics. Within-group position affects the time spent performing vigilance behaviors in some ungulates (Burger et al. 2000), and is affected by both individual-level traits (e.g., social tolerance) and group dynamics (e.g., the movements and social tolerance of other group members). An individual’s position within a group may incur either costs or benefits to its fitness (Couzin and Krause 2003), for example through a trade-off between resource acquisition and predation risk.
Variables used to capture the social context of a focal oryx (in black). Relative position (A) scores the focal oryx’s position relative to other herd members as it moves away from the entry gate. The dashed red arrow indicates directionality away from the gate and into the novel pasture. Personal space (B) counts the number of conspecifics within one body length of the focal oryx. The dashed red line indicates a radius of one oryx body length; the solid red line represents the total area considered for the personal space metric
One observer (CAR) annotated head posture and movement from aerial footage for all focal animals. Three observers (CAR, KM, SZG) annotated relative position and personal space from aerial footage. Continuous focal animal sampling (Altmann 1974) was used for all behavior and social context scoring (start and stop time and duration of each head-up/moving state). Agreement among observers was assessed using a two-way mixed-effects, absolute agreement, single-rater Intra-class correlation (ICC; McGraw and Wong 1996). Observers achieved an inter-rater reliability score of 0.85 or higher in annotations of relative position (0.882) and personal space (0.851) for two oryx, indicating that raters had a high degree of agreement and scored social context variables similarly.
We performed a principal components analysis (PCA) to explore the relative effects of individual characteristics, measured behaviors, and social context, consistent with previous analyses of animal personality (Carter et al. 2013; Massen et al. 2013b; Díaz López 2020). Input variables were calculated over the individual’s entire observation and included latency to enter the novel environment, the total proportion of its observation an individual spent in head-up postures, the total proportion of its observation an individual spent moving, age (which explained more variance than dominance or accession date), the coefficient of variation of all durations an individual spent in head-up postures, the coefficient of variation of all durations an individual spent moving, standard deviation of all recorded relative position scores, standard deviation of all recorded personal space counts, and a metric representing vigilance (the total proportion of an individual’s observation spent in head-up postures divided by the total proportion of the observation spent moving). Bartlett's Test of Sphericity was significant (p < 0.001), indicating that our input data met the assumptions for a PCA. We examined the components of the first two PCs, which accounted for ca. 72% of the total variance, and used these components to guide variable selection for generalized linear mixed models (GLMMs).
Exploration of continuous behavior annotations revealed that the median duration of posture and movement states across all individuals was 5.5 s. We used this mean duration of behavioral states, rounded to the nearest whole second, to discretize our continuous behavior annotations. For each 6-s interval, we calculated the proportion of time each focal individual spent in head-up postures, the proportion of time each focal individual spent moving, and summary values (minimum and mean) and variation (variance and standard deviation) of social context variables. We then analyzed these discretized behavior data in a repeated-measures framework.
We constructed two GLMMs to test our predictions that social context (relative position and personal space) and individual-level factors (e.g., age and social ranking) affect oryx behavior, as measured by (1) head-up postures and (2) movement. We selected a Beta distribution with a logit link, appropriate for continuous proportion data. We included a fixed effect for individual identity to directly quantify differences among individuals, and because we repeatedly measured a relatively small number (n = 7) of unequally sampled individuals. Autocorrelation and partial autocorrelation functions indicated that repeated measures of head-up and movement postures were temporally autocorrelated up to one 6-s interval; we thus, also included a first-order autoregressive covariance structure (AR1). Based on PCA results, we included the standard deviation of each social context variable as predictor variables. To exclude collinear pairs of terms (Pearson’s correlation ≥ 0.7), we competed possible models with at most one summary metric (minimum or mean) of each social context variable using the Akaike information criterion corrected for small sample sizes (AICc; Burnham and Anderson 2002). All models were fit using the glmmTMB package (Brooks et al. 2017) in R (R Core Team 2021).
Results
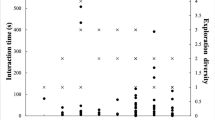
The novel environment test lasted for approximately 11.5 min. Following an initial latency period of 409 s (Table 1), the seven focal oryx entered the experimental pasture over a period of 46 s. We observed most individuals for at least four minutes after entry. However, one oryx (“Rizzo”) was not visible in the video footage after roughly 2.4 min. “Short Horns” entered the pasture first, and remained at the front of the herd for 23% of the observation—the longest time of any single oryx. Two other focal animals were also briefly at the front of the herd, one high-ranking (“Ruby,” for < 5% of the observation) and one low-ranking (“Savannah,” 8.5%). Individuals exhibited variable proportions of head-up postures and movement throughout the observation, and across individuals (Fig. 4).
The first two principal components in our PCA accounted for ca. 73% of the total variance in the data set (Table 2; Fig. 5). PC1 was strongly influenced by variables associated with vigilance and caution, as indicated by substantial positive loadings for a summary metric representing vigilance, the total proportion of time an individual spent in head-up postures, and variation in the durations an individual spent moving. PC2 was negatively influenced by latency, and positively influenced by age and variation in social context (standard deviation of personal space and relative position). Overall, the PCA indicated strong differentiation among identifiable individuals. “Cat” and “Rizzo”—the final three oryx to enter the novel environment—ranked on the high end of PC1, while “Savannah” ranked on the low end. “Ruby”, “Esmerelda”, and “Short Horns” comprised one apparent cluster, whereas “Nancy” (well differentiated along PC2) and “Savannah” (well differentiated along PC1) were relatively isolated from other oryx.
The first two principal components accounted for ca. 73% of the variance in the data set. The first component was characterized by vigilance (ratio between proportion of time in head-up posture/ proportion of time in movement), variance in time spent in movement, and the second component was characterized by social context, latency and age, head-up. Focal oryx were well differentiated along the two axes, consistent with their different social contexts and individual behaviors while exploring the novel environment
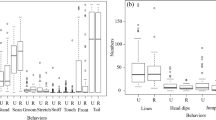
The final model for the proportion of time an oryx spent moving per 6-s interval included five fixed effects: (1) minimum personal space, (2) standard deviation in personal space, (3) minimum relative position, (4) standard deviation in relative position, and (5) individual identity (Table 3), supporting our prediction that social context significantly influences behavioral responses to a novel environment. Based on partial autocorrelation functions of the repeated-measures time series for each focal oryx, the final model also included an autoregressive covariance structure of order 1 (i.e., AR1) to account for temporal autocorrelation within individuals. No individual-level attributes (e.g., age, social dominance, or latency to enter the enclosure) were significantly related to the prevalence of movement by focal oryx (Fig. 6). A candidate model that included mean relative position instead of minimum relative position performed nearly as well (delta AICc < 2) as the final model, indicating relative equivalence among these summary metrics of relative position. Standardized residuals from the final model appeared normally distributed, and did not exhibit any dependence structures with either predictor variables or the fitted values. Nonparametric dispersion tests comparing the variance of raw residuals to that of simulated residuals (Hartig 2021) revealed no significant under- or overdispersion. The final model achieved a root mean squared error (RMSE) equivalent to a change of 0.09 in the proportion of time spent performing movement behaviors, a 20% improvement over the null model. Predictions from the final model achieved a correlation of 0.59 with observed movement proportions, compared to 0.18 in the null model.
Response curves and parameter estimates for the final model for oryx movement. The variables minimum personal space, standard deviation in personal space, minimum relative position, standard deviation in relative position, and individual identity most strongly influenced the proportions of time focal oryx spent moving. Because individual identity has relatively few levels (7), it was included as a fixed effect. Estimated coefficients for individual identity are offsets from “Savannah”, the oryx that exhibited the mean proportion moving closest to the group-level mean
In general, focal oryx at the front of the herd moved more than those toward the back of the herd. Focal oryx with few conspecifics within one body length also moved more than oryx with many neighbors nearby. Focal oryx whose personal space was in flux (i.e., the number of nearby conspecifics varied a great deal across 6-s intervals) also moved more than those with less variation in the number of nearby animals. When these social context cues were accounted for, most focal oryx still exhibited individual-specific variation in movement rates—particularly “Esmeralda” and “Short Horns”, which exhibited significantly more movement than expected, given their social environment.
The final model for the proportions of time focal oryx spent in head-up postures included three fixed effects: (1) standard deviation in relative position, (2) standard deviation in personal space, and (3) individual identity, further supporting our prediction that social context significantly influences behavioral responses to a novel environment (Table 4). During exploratory data analysis, it appeared that younger animals (< 5 years old) spent significantly less time in head-up postures than older (≥ 5 years old) animals (Welch’s t-test, p = 0.001). However, this disparity faded when social context was accounted for: no individual-level attributes exhibited significant relationships with the prevalence of head-up postures in GLMMs. Instead, focal oryx behavior again exhibited strong relationships with variation in social context (Fig. 7). As variation in the number and spatial distribution of nearby conspecifics increased, oryx spent a greater proportion of time with their head raised, potentially observing their social environment. The final model achieved a RMSE equivalent to a change of 0.03 in the proportion of head-up behaviors observed in a 6-s interval, a 17% improvement over the null model. Predicted values from the final model achieved a correlation of 0.68 with observed head-up proportions, compared to 0.41 in the null model. When social context was accounted for, several oryx exhibited individual-specific variation in vigilance behaviors—especially “Rizzo”, which exhibited strikingly greater head-up posture than expected, based on her social environment, and “Savannah”, which exhibited the opposite trend.
Response curves and parameter estimates for the final model for oryx head-up postures. Standard deviation in personal space, standard deviation in relative position, and individual identity most strongly influenced the proportions of time focal oryx spent in head-up postures. Estimated coefficients for individual identity reflect offsets from “Cat”, the oryx that exhibited the mean proportion of head-up postures closest to the group-level mean
Overall, both the PCA and GLMMs indicated strong relationships between changing social context and individual behavior. Variation in relative position and/or personal space significantly influenced both movement and head-up postures, with larger standard deviations associated with more movement and head-up postures. When social context was accounted for, oryx exhibited differentiable responses when traversing the novel environment, as indicated by the significance of individual identity in both models, and the spread and clustering of focal oryx in the PCA.
Discussion
Animal personality traits affect an individual’s ability to compete for resources, evade predators, and assert social dominance (Réale and Festa-Bianchet 2003; Dingemanse et al. 2004; Dingemanse and Réale 2005). Growing evidence indicates that animal personality may also have direct implications for the success of conservation translocations. For example, bolder captive-born Tasmanian devils were the only individuals that survived release (Sinn et al. 2014; Blake et al. 2018), and more exploratory juvenile desert tortoises exhibited higher survival rates after translocation (Germano et al. 2017). Linkages between personality traits and translocation outcomes may also be nuanced: post-release survival in translocated European mink was positively related to boldness in all years, but positively related to exploration in only some years (Haage et al. 2017). Animal personality assessments may thus, be a valuable tool for improving translocation outcomes, by identifying the most suitable individuals to release in a given environment. However, personality assessment methods typically evaluate one isolated individual at a time—even for social species that are rarely isolated from conspecifics in nature, and for which social isolation may cause stress that could confound test results (Sibbald et al. 2009; Perez et al. 2012). In this study, we proposed a method to measure spatial neophobia (i.e., latency to enter) and exploration (i.e., movement and posture after entering) for a group of social ungulates, using aerial footage to minimize invasiveness and disruption to animal management operations, and using social context variables to account for the likely influence of dynamic social cues on focal animal responses.
Our exploratory PCA indicated multiple variables of relevance and verified that the method presented here may be a promising approach to differentiate individuals during a novel environment test under shifting social contexts. The first principal component reflected caution or vigilance, with strong positive loadings for a summary metric for vigilance, time spent in head-up postures, and variation in movement (Fig. 5). The last three focal oryx to enter the enclosure, “Cat”, “Rizzo”, and “Nancy”, were high on this component: all were also described as “flighty” or reactive by animal care personnel, and spent comparatively more time in head-up postures, as indicated by both the overall proportion of time spent with head up, and a summary metric of vigilance. “Savannah”, “Esmeralda”, and “Short Horns” were the first focal oryx to enter the enclosure, and both “Savannah” and “Esmeralda” (though not “Short Horns”) were described as “calm” by animal care personnel.
The second principal component reflected variation in the animal’s social context, latency, and age: it contained positive loadings for standard deviation in relative position, standard deviation in personal space, and age, and a strong negative loading for latency. At one extreme of this component were two of the oldest individuals that maintained positions at the front of groups, “Short Horns” and “Rizzo” (Fig. 4). Notably, “Short Horns” was the first to enter the novel environment and was later joined by others, while “Rizzo” spent a large proportion of time standing still with her head up (Fig. 4) as other oryx moved around her. Both of these oryx experienced high variation in their social environment, either as a result of their own movement, or the movements of nearby conspecifics. At the other extreme of PC2, “Savannah” and “Nancy” were more constant in relative position and personal space throughout the trial—and were also the two youngest oryx analyzed.
Our final generalized linear mixed models (GLMMs) confirmed these interpretations of PCA dimensions, as well as our prediction that social context affects individual behavior: larger variation in relative position and/or personal space was generally associated with increased movement and head-up postures. Variation in an oryx’s social environment reflects the level of social turnover an individual experiences while making behavioral decisions—which are especially critical in a novel environment. For example, frequent exiting and entering of conspecifics in an oryx’s personal space, or many changes in an oryx’s relative position due to fluctuating spatial distribution of the herd, may prompt an oryx to observe its social environment in greater detail (i.e., maintain head-up posture) or react (i.e., move). Explicitly including predictor variables that capture aspects of an animal’s social environment accounts for such behavioral influences beyond the novel environment itself, helping to isolate other behavioral responses that may be of greater interest.
The fixed effects for individual identity included in our final GLMMs also confirmed the patterns of intraspecific variation apparent in the PCA. “Savannah” scored low on both PC1 (caution / vigilance) and PC2 (social context variability / latency / age), and received low coefficient estimates in both final models, indicating generally low prevalence of movement and head-up postures. In some cases, combining PCA and GLMMs enabled nuanced interpretations of focal animal tendencies. For example, “Short Horns” and “Esmeralda” both experienced high social variation, reflected in their position on PC2. However, their high individual coefficient estimates in the model for movement identify a tendency for high movement distinct from high social variability—especially for “Esmeralda”, a hand-raised individual noted as particularly calm by animal care personnel.
This case study presents a promising first application of a streamlined novel environment test to assess behavioral responses in a social setting; however, it has several limitations. First, it represents a single observation of a single captive population. Future applications should aim for multiple replications (Dall and Griffith 2014), particularly if the objective is to characterize personality, which requires multiple trials to establish consistency (Greggor et al. 2015). Second, we considered relatively coarse response and explanatory variables. Finer-resolution variables, such as more specific behavioral states (e.g., stress-related behaviors such as head-tossing or elevated ear- or tail-twitching, or social behaviors such as displacement or threat displays) and other social context variables that are distinguishable in aerial footage (e.g., distance or directionality to dominant individuals), may enable more detailed evaluations of behavioral responses to a novel environment. Third, we used a Helikite and a camera with an advanced gyroscope to collect aerial imagery. While aerostats provide a non-invasive, noiseless platform with unlimited flight time, other readily accessible, maneuverable, and cost-effective options are available. Video footage with a similar perspective and wide field of view may be obtained with an unmanned aerial vehicle (i.e., a drone), or by an external observer in an elevated location (e.g., an observation tower). Finally, though no focal animals had accessed the experimental pasture in three months, the pasture used in this study was not strictly novel. We present these methods to develop and refine novel environment tests under conditions common to many captive institutions, and emphasize that the behavioral findings in this case study should be interpreted cautiously.
Despite these limitations, the methods presented here highlight emerging considerations for advancing conservation translocation protocols. First, response variables in spatial neophobia and exploration assessments should directly translate to fitness-related behaviors (i.e., obtaining food) and potential trade-offs with other essential behaviors (i.e., finding refuge and caring for offspring). Such decisions may be critical during the period immediately after release, when translocated animals must explore a novel environment and identify areas with sufficient resources to ensure their survival. Second, while personality traits like neophobia and exploration are, by definition, relatively consistent for each individual, some plasticity and variation across social contexts are expected (Webster and Ward 2011). Repeated assessments in the presence of conspecifics, using variables that explicitly characterize social context, would quantify this range of variation.
Third, previous studies on animal collective behavior indicate that particularly dominant, active, or exploratory individuals may influence the behavior of an entire group (King et al. 2018). Measuring animal personality traits may, thus, be particularly valuable when assembling a reintroduction cohort. Bolder or more exploratory individuals may be more likely to leave an existing group (Kurvers et al. 2012; Aplin et al. 2013), and groups with bold individuals may be more likely to split into sub-groups (Michelena et al. 2009). In contrast, groups with a majority of shy individuals may stay closer together, and explore or disperse over smaller distances (Sibbald et al. 2009). Existing relationships within focal groups (e.g., how central an individual is to their social network, or where they sit on a dominance hierarchy) may also explain individual behavior in novel environments and may, thus, enrich such assessments. Managers could utilize information gained from replicate personality assessments of candidate animals, combined with knowledge of animal social relationships, to construct reintroduction cohorts with a mix of traits designed to maximize post-release survival and long-term success in a particular reintroduction environment (Watters and Meehan 2007; Delgado et al. 2018).
In addition to the methods described here, other technologies are expanding the capacity to efficiently and precisely analyze the movement and behavior of animal groups. For example, open-source tools for machine learning can automatically extract fine-scale information on multiple animal trajectories, yielding insights into the social and environmental forces that drive the movement of animal groups (Hughey et al. 2018; Torney et al. 2018). One computer vision toolkit can reliably measure the orientation and posture of multiple interacting individuals from a wide range of taxa (Graving et al. 2019). High-resolution GPS tracking and advances in quantitative analysis of animal movement data have revealed high-resolution social networks, and quantified the dynamics of collective decision-making under natural conditions (Jacoby and Freeman 2016; Snijders et al. 2017; Westley et al. 2018). Together, these developments may streamline pre-release assessments, post-release monitoring, and targeted surveillance, to fully integrate the study of animal behavior and personality with conservation outcomes.
To advance these goals, we demonstrated a minimally invasive, replicable approach to assessing spatial neophobia and exploration in a group setting, while accounting for the effects of social context. Overall, social context variables had strong, positive effects on the prevalence of head-up postures and movement, upholding our initial expectation while demonstrating that individual-level behavioral tendencies may be extracted from novel environment tests in a group setting. Ultimately, post-release monitoring efforts that track the relationships among survival, productivity, and different behavioral traits will be necessary to assess the application and effectiveness of these methods under natural conditions. GPS tracking will continue to be an important tool for measuring individual- and group-level home range sizes, dispersal distances, and survival. Similarly, observations collected by in situ monitoring teams, drones, or camera traps will be needed to reveal how individual behavioral traits affect body condition, social status, and integration with resident populations. We encourage further developments along this path, to enable conservation managers and decision-makers to leverage insights from animal behavior to improve outcomes for reintroductions and other translocation programs.
References
Altmann J (1974) Observational study of behavior: sampling methods. Behaviour 49(3–4):227–266. https://doi.org/10.1163/156853974x00534
Aplin, L.M., Farine, D.R., Mann, R.P., Sheldon, B.C., 2014. Individual-level personality influences social foraging and collective behaviour in wild birds. Proc. R. Soc. B Biol. Sci. 281. https://doi.org/10.1098/rspb.2014.1016
Aplin LM, Farine DR, Morand-Ferron J, Cole EF, Cockburn A, Sheldon BC (2013) Individual personalities predict social behaviour in wild networks of great tits (Parus major). Ecol Lett 16:1365–1372. https://doi.org/10.1111/ele.12181
Archard, G.A., Braithwaite, V.A., 2010. The importance of wild populations in studies of animal temperament. J. Zool. 281, no-no. https://doi.org/10.1111/j.1469-7998.2010.00714.x
Benson-Amram S, Holekamp KE (2012) Innovative problem solving by wild spotted hyenas. Proc r Soc B Biol Sci 279:4087–4095. https://doi.org/10.1098/rspb.2012.1450
Blake RR, Shaw DJ, Culshaw GJ, Martinez-Pereira Y (2018) Poincaré plots as a measure of heart rate variability in healthy dogs. J Vet Cardiol 20:20–32. https://doi.org/10.1016/j.jvc.2017.10.006
Both C, Dingemanse NJ, Drent PJ, Tinbergen JM (2005) Pairs of extreme avian personalities have highest reproductive success. J Anim Ecol 74:667–674. https://doi.org/10.1111/j.1365-2656.2005.00962.x
Bourg NA, McShea WJ, Thompson JR, McGarvey JC, Shen X (2013) Initial census, woody seedling, seed rain, and stand structure data for the SCBI SIGEO Large Forest Dynamics Plot. Ecology 94:2111–2112. https://doi.org/10.1890/13-0010.1
Boyer N, Réale D, Marmet J, Pisanu B, Chapuis J-L (2010) Personality, space use and tick load in an introduced population of Siberian chipmunks Tamias sibiricus. J Anim Ecol 79:538–547. https://doi.org/10.1111/j.1365-2656.2010.01659.x
Bremner-Harrison S, Prodohl PA, Elwood RW (2004) Behavioural trait assessment as a release criterion: boldness predicts early death in a reintroduction programme of captive-bred swift fox (Vulpes velox). Anim Conserv 7:313–320. https://doi.org/10.1017/S1367943004001490
Brooks M, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Maechler M, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R Journal 9(2):378–400 https://doi.org/10.32614/RJ-2017-066
Burger J, Sallna C, Gochfeld M (2000) Factors affecting vigilance in springbok: Importance of vegetative cover, location in herd, and herd size. Acta Ethol 2:97–104. https://doi.org/10.1007/s102119900013
Burnham, K.P., Anderson, D.R., 2002. Model selection and inference: a practical information-theoretic approach, 2nd ed. Springer-Verlag, New york. https://doi.org/10.1007/b97636
Carter AJ, Feeney WE, Marshall HH, Cowlishaw G, Heinsohn R (2013) Animal personality: What are behavioural ecologists measuring? Biol Rev 88:465–475. https://doi.org/10.1111/brv.12007
Cheyne SM (2009) Challenges and opportunities of primate rehabilitation—Gibbons as a case study. Endanger Species Res 9:159–165. https://doi.org/10.3354/esr00216
Christensen JW, Keeling LJ, Nielsen BL (2005) Responses of horses to novel visual, olfactory and auditory stimuli. Appl Anim Behav Sci 93:53–65. https://doi.org/10.1016/j.applanim.2005.06.017
Couzin ID, Krause J (2003) Self-Organization and Collective Behavior in Vertebrates.
Creel S, Schuette P, Christianson D (2014) Effects of predation risk on group size, vigilance, and foraging behavior in an African ungulate community. Behav Ecol 25:773–784. https://doi.org/10.1093/beheco/aru050
Dall SRX, Griffith SC (2014) An empiricist guide to animal personality variation in ecology and evolution. Front Ecol Evol 2:3. https://doi.org/10.3389/fevo.2014.00003
De Vries H, Stevens JMG, Vervaecke H (2006) Measuring and testing the steepness of dominance hierarchies. Anim Behav 71:585–592. https://doi.org/10.1016/j.anbehav.2005.05.015
Delgado M, del M., Miranda M, Alvarez SJ, Gurarie E, Fagan WF, Penteriani V, di Virgilio A, Morales JM (2018) The importance of individual variation in the dynamics of animal collective movements. Philos. Trans. R. Soc. B Biol. Sci. https://doi.org/10.1098/rstb.2017.0008
Díaz López B (2020) When personality matters: personality and social structure in wild bottlenose dolphins, Tursiops truncatus. Anim Behav 163:73–84. https://doi.org/10.1016/j.anbehav.2020.03.001
Dingemanse NJ, Both C, Drent PJ, Tinbergen JM (2004) Fitness consequences of avian personalities in a fluctuating environment. Proc r Soc B Biol Sci 271:847–852. https://doi.org/10.1098/rspb.2004.2680
Dingemanse NJ, Réale D (2005) Natural selection and animal personality. Behaviour 142:1159–1184. https://doi.org/10.1163/156853905774539445
Fogarty S, Cote J, Sih A (2011) Social personality polymorphism and the spread of invasive species: a model. Am Nat 177:273–287. https://doi.org/10.1086/658174
Foris, B., Zebunke, M., Langbein Id, J., Melzer Id, N., Loor, J.J., 2018. Evaluating the temporal and situational consistency of personality traits in adult dairy cattle. https://doi.org/10.1371/journal.pone.0204619
Friard O, Gamba M (2016) BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol Evol 7:1325–1330. https://doi.org/10.1111/2041-210X.12584
Germano JM, Nafus MG, Perry JA, Hall DB, Swaisgood RR (2017) Predicting translocation outcomes with personality for desert tortoises. Behav Ecol 28:1075–1084. https://doi.org/10.1093/beheco/arx064
Goldenberg SZ, Owen MA, Brown JL, Wittemyer G, Oo ZM, Leimgruber P (2019) Increasing conservation translocation success by building social functionality in released populations. Glob Ecol Cons 18:e00604. https://doi.org/10.1016/j.gecco.2019.e00604
Graving, J.M., Chae, D., Naik, H., Li, L., Koger, B., Costelloe, B.R., Couzin, I.D., 2019. Deepposekit, a software toolkit for fast and robust animal pose estimation using deep learning. Elife 8. https://doi.org/10.7554/eLife.47994
Greenberg, R., Mettke-Hofmann, C., 2001. Ecological aspects of neophobia and neophilia in birds. In Current Ornithology. Edited by Nolan Jr V. Thompson CF, 119–178.
Greggor, A.L., Thornton, A., Clayton, N.S., 2015. Neophobia is not only avoidance: Improving neophobia tests by combining cognition and ecology. Curr. Opin. Behav. Sci. https://doi.org/10.1016/j.cobeha.2015.10.007
Haage M, Bergvall UA, Maran T, Kiik K, Angerbjörn A (2013) Situation and context impacts the expression of personality: the influence of breeding season and test context. Behav Processes 100:103–109. https://doi.org/10.1016/j.beproc.2013.08.009
Haage M, Maran T, Bergvall UA, Elmhagen B, Angerbjörn A (2017) The influence of spatiotemporal conditions and personality on survival in reintroductions–evolutionary implications. Oecologia 183:45–56. https://doi.org/10.1007/s00442-016-3740-0
Harcourt JL, Ang TZ, Sweetman G, Johnstone RA, Manica A (2009) Social feedback and the emergence of leaders and followers. Curr Biol 19:248–252. https://doi.org/10.1016/j.cub.2008.12.051
Hartig, F. 2021. “DHARMa: Residual Diagnostics for Hierarchical (Multi-Level / Mixed) Regression Models. R package version 0.4.3. https://CRAN.R-project.org/package=DHARMa
Hughey, L.F., Hein, A.M., Strandburg-Peshkin, A., Jensen, F.H., 2018. Challenges and solutions for studying collective animal behaviour in the wild. Philos. Trans. R. Soc. B Biol. Sci. https://doi.org/10.1098/rstb.2017.0005
Hunter LTB, Skinner JD (1998) Vigilance behaviour in African ungulates: the role of predation pressure. Behaviour 1998:195–211. https://doi.org/10.1163/156853998793066320
Jacoby DMP, Freeman R (2016) Emerging network-based tools in movement ecology. Trends Ecol Evol. https://doi.org/10.1016/j.tree.2016.01.011
King AJ, Fehlmann G, Biro D, Ward AJ, Fürtbauer I (2018) Re-wilding collective behaviour: an ecological perspective. Trends Ecol Evol. https://doi.org/10.1016/j.tree.2018.03.004
King AJ, Johnson DDP, Van Vugt M (2009) The origins and evolution of leadership. Curr Biol. https://doi.org/10.1016/j.cub.2009.07.027
Kurvers RHJM, Nolet BA, Prins HHT, Ydenberg RC, Van Oers K (2012) Boldness affects foraging decisions in barnacle geese: an experimental approach. Behav Ecol 23:1155–1161. https://doi.org/10.1093/beheco/ars091
Langbein J, Puppe B (2004) Analysing dominance relationships by sociometric methods—a plea for a more standardised and precise approach in farm animals. Appl Anim Behav Sci 87:293–315. https://doi.org/10.1016/j.applanim.2004.01.007
Mainwaring MC, Beal JL, Hartley IR (2011) Zebra finches are bolder in an asocial, rather than social, context. Behav Processes 87:171–175. https://doi.org/10.1016/j.beproc.2011.03.005
Martin-Wintle MS, Shepherdson D, Zhang G, Huang Y, Luo B, Swaisgood RR (2017) Do opposites attract? Effects of personality matching in breeding pairs of captive giant pandas on reproductive success. Biol Conserv 207:27–37. https://doi.org/10.1016/j.biocon.2017.01.010
Maskrey DK, White SJ, Wilson AJ, Houslay TM (2018) Who dares does not always win: risk-averse rockpool prawns are better at controlling a limited food resource. Anim Behav 140:187–197. https://doi.org/10.1016/j.anbehav.2018.04.023
Massen JJM, Antonides A, Arnold A-MK, Bionda T, Koski SE (2013a) A behavioral view on chimpanzee personality: Exploration tendency, persistence, boldness, and tool-orientation measured with group experiments. Am J Primatol 75:947–958. https://doi.org/10.1002/ajp.22159
May TM, Page MJ, Fleming PA (2016) Predicting survivors: animal temperament and translocation. Behav Ecol 27:969–977. https://doi.org/10.1093/beheco/arv242
Mendl M (1999) Performing under pressure: Stress and cognitive function. Appl Anim Behav Sci 65:221–244. https://doi.org/10.1016/S0168-1591(99)00088-X
Merrick MJ, Koprowski JL (2017) Should we consider individual behavior differences in applied wildlife conservation studies? Biol Conserv 209:34–44. https://doi.org/10.1016/j.biocon.2017.01.021
Mertes K, Stabach JA, Songer M, Wacher T, Newby J, Chuven J, Al Dhaheri S, Leimgruber P, Monfort S (2019) Management background and release conditions structure post-release movements in reintroduced ungulates. Front Ecol Evol 7:470. https://doi.org/10.3389/fevo.2019.00470
Michelena P, Sibbald AM, Erhard HW, McLeod JE (2009) Effects of group size and personality on social foraging: the distribution of sheep across patches. Behav Ecol 20:145–152. https://doi.org/10.1093/beheco/arn126
Olsen HF, Klemetsdal G (2019) Validation of a temperament test in the Norwegian horse breeds. Appl Anim Behav Sci 219:104836. https://doi.org/10.1016/j.applanim.2019.104836
Perals D, Griffin AS, Bartomeus I, Sol D (2017) Revisiting the open-field test: what does it really tell us about animal personality? Anim Behav 123:69–79. https://doi.org/10.1016/j.anbehav.2016.10.006
Perez EC, Elie JE, Soulage CO, Soula HA, Mathevon N, Vignal C (2012) The acoustic expression of stress in a songbird: Does corticosterone drive isolation-induced modifications of zebra finch calls? Horm Behav 61:573–581. https://doi.org/10.1016/j.yhbeh.2012.02.004
Peterson LM, Weckerly FW (2018) Social behavior and changes in foraging behavior in a gregarious ungulate. J Mammal 99:1422–1429. https://doi.org/10.1093/jmammal/gyy129
Quinn JL, Cresswell W (2005) Personality, anti-predation behaviour and behavioural plasticity in the chaffinch Fringilla coelebs. Behaviour 142:1377–1402. https://doi.org/10.1163/156853905774539391
R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Réale D, Festa-Bianchet M (2003) Predator-induced natural selection on temperament in bighorn ewes. Anim Behav 65:463–470. https://doi.org/10.1006/anbe.2003.2100
Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ (2007) Integrating animal temperament within ecology and evolution. Biol Rev 82:291–318. https://doi.org/10.1111/j.1469-185X.2007.00010.x
Schuett W, Dall SRX (2009) Sex differences, social context and personality in zebra finches, Taeniopygia guttata. Anim Behav 77:1041–1050. https://doi.org/10.1016/j.anbehav.2008.12.024
Sibbald AM, Erhard HW, McLeod JE, Hooper RJ (2009) Individual personality and the spatial distribution of groups of grazing animals: an example with sheep. Behav Processes 82:319–326. https://doi.org/10.1016/j.beproc.2009.07.011
Sih A, Bell A, Johnson JC (2004) Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378. https://doi.org/10.1016/J.TREE.2004.04.009
Sih A, Watters JV (2005) The mix matters: Behavioural types and group dynamics in water striders. Behaviour 142:1417–1431. https://doi.org/10.1163/156853905774539454
Sinn DL, Cawthen L, Jones SM, Pukk C, Jones ME (2014) Boldness towards novelty and translocation success in captive-raised, orphaned Tasmanian devils. Zoo Biol 33:36–48. https://doi.org/10.1002/zoo.21108
Snijders L, Blumstein DT, Stanley CR, Franks DW (2017) Animal social network theory can help wildlife conservation. Trends Ecol Evol. https://doi.org/10.1016/j.tree.2017.05.005
Spiegel O, Leu ST, Bull CM, Sih A (2017) What’s your move? Movement as a link between personality and spatial dynamics in animal populations. Ecol Lett 20:3–18. https://doi.org/10.1111/ele.12708
Stamps JA, Swaisgood RR (2007) Someplace like home: Experience, habitat selection and conservation biology. Appl Anim Behav Sci 102:392–409. https://doi.org/10.1016/j.applanim.2006.05.038
Teixeira CP, de Azevedo CS, Mendl M, Cipreste CF, Young RJ (2007) Revisiting translocation and reintroduction programmes: the importance of considering stress. Anim Behav. https://doi.org/10.1016/j.anbehav.2006.06.002
Torney CJ, Lamont M, Debell L, Angohiatok RJ, Leclerc LM, Berdahl AM (2018) Inferring the rules of social interaction in migrating caribou. Philos. Trans. R. Soc. B Biol. Sci. 373. https://doi.org/10.1098/rstb.2017.0385
Traisnel G, Pichegru L (2019) Boldness at the nest predicts foraging and diving behaviour of female but not male African penguins. Anim Behav 150:113–125. https://doi.org/10.1016/j.anbehav.2019.02.003
van Oers K, Klunder M, Drent PJ (2005) Context dependence of personalities: risk-taking behavior in a social and a nonsocial situation. Behav Ecol 16:716–723. https://doi.org/10.1093/beheco/ari045
Veissier I, le Neindre P (1992) Reactivity of Aubrac heifers exposed to a novel environment alone or in groups of four. Appl Anim Behav Sci 33:11–15. https://doi.org/10.1016/S0168-1591(05)80079-6
Verhoeven GJJ, Loenders J, Vermeulen F, Docter R (2009) Helikite aerial photography—a versatile means of unmanned, radio controlled, low-altitude aerial archaeology. Archaeol Prospect 16:125–138. https://doi.org/10.1002/arp.353
Watters JV, Meehan CL (2007) Different strokes: Can managing behavioral types increase post-release success? Appl Anim Behav Sci 102:364–379. https://doi.org/10.1016/j.applanim.2006.05.036
Webster MM, Ward AJW (2011) Personality and social context. Biol Rev 86:759–773. https://doi.org/10.1111/j.1469-185X.2010.00169.x
Westley PAH, Berdahl AM, Torney CJ, Biro D (2018) Collective movement in ecology: From emerging technologies to conservation and management. Philos. Trans. R. Soc. B Biol. Sci. 373. https://doi.org/10.1098/rstb.2017.0004
Acknowledgements
We are grateful to John McEvoy and Sarah Macey for assistance with Helikite and camera setup. We thank Melissa Songer and Peter Leimgruber for feedback on an early draft of this manuscript and for institutional support, and we thank SCBI Police and security officers for facilitating transport and site access. We are grateful for a grant through the Smithsonian Women’s Committee that was used to purchase the Helikite used in this study. We also thank the editor Scott Y.S. Chui and three reviewers for their careful consideration and recommendations regarding this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling editors: Scott Y.S. Chui and Elissa Z. Cameron.
This article is a contribution to the special issue on “Individual Identification and Photographic Techniques in Mammalian Ecological and Behavioural Research – Part 2: Field Studies and Applications” — Editors: Leszek Karczmarski, Stephen C.Y. Chan, Scott Y.S. Chui and Elissa Z. Cameron
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
See Appendix Fig. A1 and Table A1.
Scimitar-horned oryx (Oryx dammah) are large African antelope adapted to the semi-arid seasonal grasslands of North and Central Africa. Wild oryx once ranged across the Sahel, but declined due to overhunting and habitat degradation. The last report of wild oryx occurred in the mid-1980s, and the species was declared Extinct in the Wild in 2000
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mertes, K., Ressijac, C.A., Moraes, R.N. et al. Assessing neophobia and exploration while accounting for social context: an example application in scimitar-horned oryx. Mamm Biol 102, 1357–1371 (2022). https://doi.org/10.1007/s42991-022-00271-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42991-022-00271-1