Abstract
In Namibia, leopards (Panthera pardus) are widely distributed, used commercially as trophy animals and are often persecuted for perceived or real predation on livestock and valuable game species outside protected areas. Therefore, leopard populations living in protected areas might be important source populations and for maintaining connectivity. Little data on their population sizes and densities are available from the northern part of the country, particularly from protected areas. Here, we estimated leopard densities using a spatial capture–recapture approach in northern Namibia: (i) the Khaudum National Park (KNP) in north-east Namibia with an annual average rainfall of 450 mm and (ii) the Lower Hoanib River (LHR) in north-west Namibia with an annual average rainfall of 25 mm. With an effort of 2430 and 2074 camera trap nights in the KNP and LHR, respectively, 11 adult female and six adult male leopards were identified in the KNP, whilst only one adult female leopard was detected once in the LHR. For the KNP, a maximum likelihood approach (using the package SECR) revealed a density estimate of 2.74 leopards/100 km2, whereas a Bayesian approach (using the package SPACECAP) revealed a density estimate of 1.83 leopards/100 km2. For the LHR, no density estimate could be determined and it is suggested that the leopard density in such an arid environment is low. These are the first leopard density estimates based on camera trap surveys provided for these protected areas and thus of importance for further monitoring programs to understand leopard population dynamics. We discuss our findings with current habitat changes and conservation measures in both study areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leopards (Panthera pardus) are large carnivores with elusive habits and secretive behaviour, and are therefore difficult to count (Bailey 1993; Stander 1998; Balme et al. 2009a). Leopard distribution has shrunk across its range (Jacobson et al. 2016), due to the depletion of natural prey (Datta et al. 2008; Athreya et al. 2014), lethal removal due to real or perceived human-leopard conflict (Ray et al. 2005; Thorn et al. 2013; Swanepoel et al. 2015), unsustainable hunting practices (Lindsey et al. 2006; Packer et al. 2009; Gray and Prum 2012; Braczkowski et al. 2015), habitat loss and landscape fragmentation (Henschel et al. 2008; Jacobson et al. 2016). As a consequence, the leopard status in the Red List of the International Union for Conservation of Nature (IUCN) has recently been up-listed from “Near Threatened” to “Vulnerable” (Stein et al. 2016).
Leopards in Namibia are widely distributed across the country (Hanssen and Stander 2004; Stein et al. 2011a). They live in a matrix of protected areas, freehold farms and communal lands. Outside of protected areas, they can be trophy hunted and/or suffer persecution due to perceived or real conflict with humans (Stein et al. 2011a; NAPHA 2019). Previous interviews in north-central Namibia (n = 23) revealed that 15% of farmers would shoot leopards on sight and 60% would remove leopards after livestock depredation (Stein et al. 2010). Santangeli et al. (2016) estimated that 67% of freehold farmers interviewed (n = 276) did not report such persecution to the authorities.
The areas where leopards are protected are patchy, making them valuable source populations (Allen et al. 2020). The presence of competitors such as spotted hyenas (Crocuta crocuta) and lions (Panthera leo) in protected areas might suppress leopard density (Packer et al. 2011), but it was shown that leopards are well adapted to coexist with larger competitors (Stein et al. 2015; Balme et al. 2017a,b). For developing conservation strategies and a metapopulation management plan for the species, it is critical to obtain leopard numbers and population trends. It is also crucial to identify hotspots of densities across a country with a range of different human perceptions towards this predator and various land management schemes (Farhadinia et al. 2019; Gubbi et al. 2020).
So far, five studies provided leopard density estimates for eight study sites across Namibia using camera traps and a capture–recapture approach. In two study sites located in freehold farmlands in the Namib Desert in south-western Namibia, leopard density was estimated at 0.6 and 0.9 leopards/100 km2, respectively (Edwards et al. 2015); similar to the Mudumu North Complex (MNC), a network of conservancies, community forests and protected areas in the Zambezi strip in north-east Namibia with an estimate of 0.6 leopards/100 km2 (Hanssen et al. 2015). In the freehold farmlands in east-central and north-central Namibia, leopard density was estimated at 3.1 leopards/100 km2 and 2.8 leopards/100 km2, respectively (NAPHA 2019). Stein et al. (2011a) estimated in the protected Waterberg Plateau Park in north-central Namibia, 1.0 leopard/100 km2 and in the neighboring freehold farmlands 3.6 leopards/100 km2. The fifth study was conducted in a fully fenced private reserve in north-central Namibia which excluded any movements in and out of the reserve and estimated 14.5 leopards/100 km2, the highest leopard density reported so far for any area in Namibia (Noack et al. 2019).
Here, we present the first leopard camera trap data for two protected areas located in the north of Namibia. We conducted camera trap surveys in the Khaudum National Park (KNP) in north-east Namibia and in the Lower Hoanib River (LHR) in the Kunene region in north-west Namibia. The KNP is characterized by high vegetation cover and average rainfall of 450 mm, while the LHR is one of the most arid areas of southern Africa with an average rainfall of 25 mm (Fig. A1 and A2). Both study sites are surrounded by communal conservancies established between 1998 and 2003 to give local communities rights to wildlife management and tourism. Such initiative is also known as community-based nature resources management (CBNRM) and aims to promote the sustainable use of natural resources to maintain numbers of wildlife species and positively impact the attitude of people towards wildlife (Boudreaux and Nelson 2011; Stormer et al. 2019). Due to this, in both study areas, there was, and is, a notable increase in tourism and of the local population who settled in the area. Approximately 36–65% of this local population lives below the poverty line (NPC 2015). The successful implementation of the CBNRM brought also some challenges, including an overall increase in human-wildlife conflict cases (MET 2019, https://communityconservationnamibia.com/ accessed February 2021). Regular aerial surveys, annual game counts and intensive monitoring are being conducted in these conservancies to implement adaptive management, ensure sustainable use of biodiversity in communal lands and reach the goals set by the conservancies (Stuart-Hill et al. 2005; Gibson and Craig 2015; Craig and Gibson 2016).
In the communal conservancies south of the KNP, there is substantial conflict between leopards and humans. Stander et al. (1997) reported that within 4 years, 11 of 15 collared leopards were killed after attacking domestic animals at settlements. In this area, the leopard hunting quota remained stable during the last 20 years and is set at three male leopards annually (Piet Beytell pers. comm.). In the west of the KNP, small-scale farms have been developed (Gibson and Craig 2015) and due to this, an increase of conflict between humans and carnivores has been reported (Piet Beytell pers. comm.). Little information is available on human-carnivore conflict in the north of the KNP and along the border with Botswana.
Two decades ago, Stander (1998) estimated a density of 1.5 leopards/100 km2 in the Khaudum Game Reserve (KGR) and the adjacent Nyae Nyae Conservancy in the south using spoor counts. This was before the KGR was declared as the Khaudum National Park in 2007 (GGRN 2008) and no study has provided a density estimate since then. The KNP hosts all large carnivore species occurring in the country, i.e. brown hyena (Parahyaena brunnea), cheetah (Acinonyx jubatus), leopard, lion, spotted hyena and wild dog (Lycaon pictus). There were little changes in the landscape in the KNP during the last three decades (Piet Beytell pers. comm.), but during the past 5 years, new management plans were implemented within the KNP. Restoration and opening of water points led to an increased use of the area by elephants (Loxodonta africana), that previously used to occur in the KNP seasonally (MET 2019). Elephants have now settled and increased in numbers, with yet unknown impact on the vegetation and other species. A fire management control plan has also recently been established aiming to reduce the extensive uncontrolled wildfires that used to annually affect large areas of the park (MET 2016). Eland (Tragelaphus oryx), blue wildebeest (Connochaetes taurinus) and impala (Aepyceros melampus) were recently reintroduced and aerial surveys are being conducted to monitor the population of wildlife species (Beytell 2017). The outcomes of these management plans may lead to changes in the vegetation and the prey abundance over the next years with possible effects on the leopard population.
In the LHR, there are no previous leopard density estimates and no study on leopards had been carried out. The study area hosts all large carnivore species present in Namibia except wild dogs and prey availability and density is low due to the scarce and localized rainfall. In the LHR, where the camera trap survey was conducted, there is no livestock grazing. Recently, tourism concessions were granted and water sources opened to attract wildlife. The latter led to an intensified use of the area by elephants with a negative impact on the surrounding vegetation (Curtis 2017). The south of the study area borders with the Palmwag concession where tourism is the main activity. In the towns and settlements located north and east of the LHR, the human population and the livestock have increased in the last two decades, leading to a rise of human-carnivore conflicts (MET/NACSO 2018). Another consequence was a noticeable degradation of the vegetation condition and thus grazing and browsing capacities (Verlinden and Kruger 2017; Inman et al. 2020a). This degradation was intensified by continuous droughts (Masih et al. 2014; Inman et al. 2020b) and opening of water sources (Klintenberg and Verlinden 2008). Currently, in north-west Namibia, livestock numbers outweigh the estimated numbers of all the wildlife species combined and the areas where wildlife numbers dominate have low overall biomass due to their marginal arid character (Craig and Gibson 2016).
Precise and accurate population estimates repeated over time are required to understand how changes in the landscape, prey population, human presence and other factors may affect leopard density (Gese 2001; Balme et al. 2009b). Over the past three decades, considerable advances in the application of photographic techniques in capture–recapture studies of individually recognisable animals have facilitated increasingly robust bases for obtaining such data across a variety of species and habitats, both terrestrial and marine (e.g. Hammond et al. 1990; Schneider et al. 2019; Karczmarski et al. 2022a, b). Such datasets provide crucial information on mortality, birth, survival and recruitment rates and can be used to evaluate the effect of conservation measures and management strategies on population dynamics and performance (Boitani and Fuller 2000; Karanth et al. 2006; Boyd et al. 2009; Balme et al. 2009b, 2012). Regular camera trap surveys of large carnivores are a powerful tool when carried out with a continuous monitoring program of wildlife population numbers and landscape changes. Such surveys allow to understand the impact of different threats such as anthropogenic persecution, prey depletion and habitat loss on threatened carnivore populations (Karanth et al. 2006). Here, we determine leopard density estimates in the KNP and LHR with two of the currently most popular spatial capture–recapture packages, SECR and SPACECAP, to increase comparability of future density estimates.
Methods
Study areas
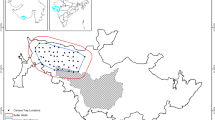
The KNP is located in north-east Namibia and covers 3842 km2 (Fig. 1). It is at the western edge of the Kavango-Zambezi Transfrontier Conservation Area (KAZA TFCA), which promotes the free movement of wildlife across the borders of Angola, Botswana, Namibia, Zambia and Zimbabwe. The area therefore plays an important role as a population source for various species, and has a high value for wildlife connectivity and dispersal of animals (KAZA TFCA 2014; MET 2019). The KNP is bordered by communal conservancies to the north and south, small-scale farmlands to the west, and the Botswana border to the east. The reserve is unfenced except for the eastern boundary which forms the international border with Botswana. The KNP is covered by northern Kalahari sandveld forest and it belongs to the north-eastern Kalahari woodland biome. The camera trap study was conducted in the southern section of the KNP in an area of 609 km2 (Fig. 2). Here, quartzite soils with calcrete predominate, favoring Acacia broadleaf vegetation with patches of Baikiaea plurijuga and Terminalia sericea on deeper sands. Within the KNP, the average annual rainfall is 450 mm and ranges from 400 to 500 mm, starting in November and lasting up to April. The minimum average temperature ranges from 4 °C to 6 °C and the maximum average temperature ranges from 32 °C to 34 °C (Mendelson et al. 2002).
Map of Namibia with the locations of the two study areas (filled red polygons), i.e. the southern section of Khaudum National Park (KNP) and the Lower Hoanib River (LHR). The presence of towns and settlements and the different land-uses are shown as indicated by the legend. The insert shows in black the location of Namibia on the African continent
The second study area was located in the Kunene region and has a strong geological and landscape heterogeneity encompassing the gravel and sandy plains north and south of the LHR in north-west Namibia (Fig. 1). The study area was located east of the Skeleton Coast National Park, with the towns of Puros and Sesfontein located to the north and east respectively, and the Palmwag concession to the south, covering an area of 567 km2 (Fig. 3). The study area includes both Nama Karoo and desert biomes. In the plains, the vegetation is scarce and dominated by sparse Acacia tortilis, some Acacia erioloba and Boscia foetida, thriving typically along the drainage lines. The dominant shrubs in the area are Calicorema capitata, Petalidium variabile, Salvadora persica, Adenolobus pechuelii and Euphorbia damarana, whilst Stipagrostis uniplumis and Stipagrostis giessii are the dominant grasses during good rainy seasons. The average annual precipitation for the area is 25 mm and ranges from 0 to 50 mm. The minimum average temperature ranges from 8 °C to 10 °C and the maximum average temperature ranges from 26 °C to 28 °C (Mendelsohn et al. 2002). Coastal fog, which occurs predominantly during the cold-dry (approximately May to August) and hot-dry (approximately September to January) seasons, reaches up to 60 km inland. This fog is ecologically important as it is the main source of water in the area (Seely et al. 1998).
Camera trap surveys
Capture-recapture models assume that every individual within a survey area has more than a zero probability of being detected by the camera traps (Karanth et al. 2002). Therefore, the distance between camera trap stations must be small enough that every individual has the chance to encounter at least one camera trap station during the survey and no home range can fit between stations. To ensure adequate data collection, the trap spacing and trap array were chosen according to available home range data from the nearest leopard population to the study area as suggested by Sollmann et al. (2012). As leopard females have smaller home ranges than males (Bailey 1993; Stander et al. 1997), spatial movement data from 12 Global Positioning System (GPS) collared females in east-central Namibia were used. These animals were studied during a mean period of 484 days (± SD 104 days) between 2012 and 2016 and their home range sizes (95% kernel density estimator (KDE)) were at least 25 km2 (IZW Namibia on Movebank www.movebank.com). We considered the use of this figure as conservative because data collected in the KNP and adjacent communal land by Stander et al. (1997) estimated the smallest female home range to be 183 km2 using very high frequency (VHF) collars. With the aim of setting one to two camera trap stations per female home range, we chose a grid with a four kilometer cell width which equals to a density of 6.25 camera traps/100 km2.
In the KNP, the location of each camera trap station was selected by overlaying the grid on a satellite photo of the study area. The GPS coordinates of every centre of the cell were selected as a reference and visited on the ground. Within a 500 m radius of every reference location, particular landscape features known to be used by leopards (Bailey 1993) were identified to deploy the camera traps. Such features included animal trails surrounding dense vegetation patches or bordering riverine habitat, and roads and tracks leading to main roads or water points. Although survey designs with a high perimeter-to-area ratio should be avoided (Karanth and Nichols 2011), the camera traps in the KNP could not always be placed optimally because long distance walks away from tracks maintained by the park authority had to be avoided to reduce the risk of encountering elephants in the dense bush. The irregular shape of the camera trap setup (Fig. 2) is therefore the result of the trade-off between the optimal distribution of the cameras, the safe access to the camera trap stations and the landscape features known to be used by leopards.
In the LHR, due to the arid environment and scarcity of sites to potentially detect leopards, we followed a more flexible setting of the cameras within the grid cell to increase capture and recapture probabilities, even when camera spacing became more uneven (Sun et al. 2014). Thus, we considered the whole grid cell for the placement of each camera trap station. Features such as dry riverbeds, animal trails in the mountain valleys and tracks bordering mountains, drainage lines and vegetated areas were chosen for camera trap placement. The scarce vegetation and the distribution of geographic features such as gravel and sandy plains between granite mountains determined the distance between camera traps (Fig. 3).
From the 25th of October 2016 until the 12th of January 2017, 76 camera traps were deployed in 38 stations during 79 days to sample an area of 609 km2 in KNP (Fig. 2). From the 30th of January 2017 until the 1st of April 2017, 68 camera traps were deployed in 34 stations during 61 days to sample an area of 567 km2 in the LHR (Fig. 3). In each study area, the grid size selected and the number of camera traps available allowed to cover an area equivalent to at least two home ranges of male leopards from the nearest leopard population where data was available (Royle et al. 2014). This was based on data from east-central Namibia where the mean home range size (95% KDE) of eight GPS collared leopard males was 286.6 km2 (SD = 160.5 km2) (IZW Namibia unpublished data on Movebank www.movebank.com). At each camera trap station, two Reconyx HC600 HyperFire (Reconyx Inc, Holeman, Wisconsin, USA) were deployed on each side of the animal trail or road and set at an approximate distance of 2 to 3 m from the path. Every unit was housed in a steel protective box and was powered with 12 lithium batteries or rechargeable nickel–metal hybrid (NiMH) batteries. To avoid flash interference, the camera traps were set with a lateral offset of several meters (2–7 m) determined by the availability of bushes or trees to camouflage them. The cameras were programmed to high sensitivity, no interval between pictures (RapidFire) and three pictures per trigger.
Data analysis
Each leopard was identified by its unique pelage composed of spots and rosette patterns (Fig. A3) (Bailey 1993). All pictures were classified by two of the authors (RP and SE) without discrepancies. Leopard sex was assigned using sex-specific morphological features such as the presence of testes and dewlap of males. Females can look similar to young males (approximately 2 years old) if the area below the tail is not visible in the picture (Balme et al. 2012; Braczkowski et al. 2016). In this study, the presence or absence of testes and/or dewlap was seen in at least one picture in all photographed individuals that were identifiable to the individual level (86%), thus we were able to sex all individuals.
Individual detection histories were produced using 24 h sampling occasions following the recommendation in Goldberg et al. (2015). Given that leopards are mainly active between dusk and dawn (Balme et al. 2009a; Martins and Harris 2013), a sampling occasion was defined as starting at 12:00 midday and ending at 11:59 the following day. Such an approach avoids the “midnight problem” whereby an individual visiting a camera trap either side of midnight in a single night would be recorded as present during two consecutive nights (Jordan et al. 2011). This way, leopard photo records were classified into independent events when consecutive photos of the same individual were recorded in different 24-h sampling occasion, thus representing the active period of the diel cycle of leopards (du Preez et al. 2014).
To calculate leopard density, we used spatial capture–recapture (SCR) models. Such models allow for individual movement outside of the surveyed grid, thereby overcoming the problems associated with defining the survey area in traditional, non-spatial capture–recapture models (Royle et al. 2014). SCR models assume that every individual i has a permanent, unobserved activity centre si, and that the probability of encountering an individual is a monotonically decreasing function of the distance from the activity centre to a camera trap (j) yij (Sollmann et al. 2011). The models combine a state model that represents the geographic distribution of individual home ranges, which is treated as a homogeneous Poisson point process model, with an observation model, which estimates the probability of encountering an individual at a given detector, e.g. camera trap, to the distance of the detector from the activity centre of the individual (Borchers and Efford, 2008).
Data analyses were conducted with the package SECR (Efford 2012) using a maximum likelihood framework and with the package SPACECAP (Gopalaswamy et al. 2012) using a Bayesian modelling framework; both implemented in R (R Development Core Team 2014). Both SECR and SPACECAP packages account for camera trap failures by indicating the effort (active vs. non-active camera traps) in number of occasions (i.e. trapping nights) and calculate the detection probability based on the effort specified by the user.
Using the package SECR (Efford 2012), models were run to select the most appropriate detection (observational) process, either half-normal or negative exponential, using model Akaike's Information Criterion (AIC) values, adjusted for small sample size (AICc). The hazard rate detection process was not considered, as this is only recommended in situations in which the survey area is fully surrounded by a natural or artificial boundary, given that density estimates from it do not reach a plateau fairly promptly with an increasing buffer width (Efford 2017). The quantile–quantile plot from the hazard rate detection process on a null model confirmed this for our data, with no plateau being met even with many (n = 100,000) Markov Chain Monte Carlo (MCMC) iterations. Six density models were run, using the most appropriate detection process, in which g0 (λ0), the probability of capture at the centre of an individual's home range and sigma (б), a function of the scale of animal movement were affected by various factors: (1) the null model in which both g0 and sigma were constant (λ0 ~ 1, б ~ 1), (2) the behaviour b1 model in which g0 was affected by a reaction of individuals to camera traps (λ0 ~ b, б ~ 1), (3) a second behaviour model named as the learned response b2 in which both g0 and sigma were affected by a reaction of individuals to camera traps (λ0 ~ b, б ~ b). Three sex models were run in which leopard sex was coded as a factor affecting estimates of g0 and sigma; (4) the full sex model (λ0 ~ sex, б ~ sex), (5) the g0 sex model (λ0 ~ sex, б ~ 1), and (6) the sigma sex model (λ0 ~ 1, б ~ sex). All models were ranked using AICc values. Population closure was tested for by performing the closure test (Otis et al. 1978) within the SECR package.
SPACECAP requires three input files: (1) a trap deployment file which contains the names and Universal Transverse Mercator (UTM) coordinates of all camera trap stations, (2) an animal capture file, which contains the capture history details of each individual, including the animal identity, camera trap identity, and sampling occasion for each leopard event, and (3) a state space file, which is a maximum polygon convex (MCP) of the area containing the camera traps plus a buffer around it that intends to encompass the home ranges of leopards detected during the survey but potentially having their home range centre outside the MCP. This area is then covered with a fine scale grid of equally spaced points, each representing a potential activity centre for all individuals in the surveyed population. To create the state space file, a buffer of 20 km was placed around the MCP using QGIS (2015). The fine scale grid was generated with equally spaced points (n = 9119), each 0.821 km apart, giving a total area of 4233 km2 of habitat over which the hypothetical home range centres were uniformly distributed.
The SPACECAP base model considering equal capture probabilities between individuals was used to estimate leopard density using a half normal detection function, with a Bernoulli encounter model and 50,000 MCMC iterations, of which the first 2000 were discarded as a burn-in period, with a thinning rate of 50. An augmentation value of 170 individuals was used, representing 10 times the number of identified individual leopards detected by camera traps in the KNP (Table 1). Along with the density estimate, SPACECAP produces an estimate of sigma (Σ), the spatial scale over which detection declines, Lam0 (λ), the probability of capture at the centre of an individual's home range, Psi (Ψ), the data augmentation value and Nsuper (†), the population size of individuals having their activity centres within the state space area. Model adequacy was assessed using Bayesian P values deduced by SPACECAP from individual encounter histories, with values close to 0 and 1 indicating an inadequate model. The Geweke’s diagnostics, also known as Z scores (Geweke 1992), produced by SPACECAP were used to deduce whether the MCMC chains have converged around a solution, with values lying between − 1.6 and 1.6 when convergence is achieved (Gopalaswamy et al. 2012).
Results
Descriptive camera trap statistics
During the 79 day survey period in the KNP, a total of 2,430 camera trap nights (mean nights per station 65.7 ± SD 19.4) were achieved. At one of the 38 stations, both camera traps failed to record any pictures, thus it was removed from the analysis. A total of 29 independent leopard events were recorded of which 25 events (86%) were identifiable to the individual level, comprising six adult males and 11 adult females (Table 1). Using the closure test in SECR, the data set did not show evidence for population closure (z = − 2.15, p = 0.02).
During the 61 days of survey in the LHR, a total of 2074 camera trapping nights (mean nights per station 61.0 ± SD 0.0) were conducted, with no camera trap failures. Only one adult female leopard was detected once during the entire survey period (Table 1). This individual was photographed already twice by camera traps prior to the survey start. With this single leopard event, no density estimate could be calculated for the LHR study area.
Density estimate for Khaudum National Park (KNP)
Using the R package SECR, the half-normal detection function was identified as best fitting, and therefore was used for the six candidate models selected for estimating leopard density. Examination of AICc values identified the null model, which had a constant g0 and sigma, to be the best fit. No other models had ΔAICc values of < 2.0 (Table 2), therefore sex specific estimates of sigma and g0 are not presented. Leopard density using the null model was estimated at 2.74 leopard/100 km2 (± SD 1.07, 95% confidence interval (CI) 1.31–5.72) and g0 estimated at 0.005 (± SD 0.003, 95% CI 0.002–0.010).
Using the base model that assumes equal capture probabilities across individuals, the R package SPACECAP provided a density estimate of 1.83 leopards/100 km2 (range 1.11–2.50, posterior SD = 0.40). The Bayesian P value was estimated at 0.51, showing model adequacy, and all Z scores from the Geweke's diagnostic were between − 1.6 and 1.6, suggesting chain convergence around a solution for all parameters. Sigma (Σ) was estimated at 4080 m (range 2300–6370 m, posterior SD = 127 m) and Lam0 (λ) estimated at 0.005 (range 0.001–0.010, posterior SD = 0.003) (Table 3).
Discussion
Our study provides valuable first information on leopard densities in two areas in the north of Namibia, the KNP characterized by woodland vegetation and the LHR which is one of the most arid areas of southern Africa. In both areas, leopards are not fenced in and are surrounded by human population with whom they might come into contact. The majority of leopards in Namibia are found on freehold farmland, where they are considered to be the apex predator and interactions occur mainly with brown hyenas, cheetahs and humans (NAPHA 2019). We provide the first leopard density estimate for the southern part of the KNP, using a spatial capture–recapture (SCR) framework which revealed a density of 2.74 leopards/100 km2 with a maximum likelihood approach (SECR) and a density of 1.83 leopards/100 km2 using a Bayesian approach (SPACECAP). This population might be an important source population in northern Namibia.
A closure test did not reveal evidence of population closure, which we assume is due to low numbers of recaptures since ten of the 17 animals were only captured once. When the number of captures and recaptures does not reach an asymptote over time, the population closure assumption is not met (Otis et al. 1978). This limits the power of the tests as previously noted by others (Kawanishi and Sunquist 2004; Soisalo and Cavalcanti 2006; Weingarth et al. 2015). The length of our surveys was within the recommended closed period of < 90 days for large cats (Karanth and Nichols 1998, 2011; Hedges et al. 2015). In the current absence of a specific population closure test for SCR models, this time period is the most appropriate way to meet the closure assumption (Royle et al. 2014). The use of SCR, however, relaxes the closure requirements, and for long living species with low densities and large home ranges, such as large carnivores, the survey period can run for a longer duration to collect sufficient data on leopard captures and recaptures (Royle et al. 2014). On the other hand, high detection rates might be difficult to achieve even with longer time periods when there is a wide number of tracks potentially used by leopards.
Our camera trap study in the KNP followed the study design previously used by Stein et al. (2011b) aiming to standardize methodology across sites in Namibia to obtain comparable datasets. We selected camera trap locations within 500 m of the centre of each 4 × 4 km grid cell (Stein et al. 2011b) and placed the camera traps at landscape features known to be used by leopards (Bailey 1993; Karanth and Nichols 2011). Such a study design might have led to the omission of some suitable camera trapping sites and thus affected the detectability of leopards during the study (Sun et al. 2014). The placement of camera traps of the survey carried out in the LHR had to be more flexible because of the scarcity of features used by leopards in the Namib Desert. This flexibility is advisable because it is likely to increase capture probability which will provide a more robust population density estimate, even if the spacing of camera traps becomes uneven (Sun et al. 2014). In areas of expected low density, we suggest maximizing leopard capture rather than prioritizing spatial distribution of camera trap locations (Sun et al. 2014).
Camera traps in the KNP were set along roads and sometimes in the vicinity of water sources where leopards benefitted from the permanent water availability. Thus, the estimated density might be higher than in the rest of the KNP where water availability is ephemeral and prey density potentially lower. We therefore call for caution when extrapolating our density estimates outside the study area.
This study gives an insight into the leopard population in the north-east of Namibia, where the species interacts with other large carnivores such as lions, spotted hyenas, wild dogs and cheetahs which were also photographed by our camera traps. Their presence might also impact leopard density and explain the low number of captures and recaptures of leopards. The low number of captures and recaptures in the KNP might explain why only the null model was supported during the data analyses. Such scenario shows that calculations of sex-specific estimates of sigma and other parameters such as g0 would be unsuitable (Royle et al. 2014).
In the KNP, wildlife is legally protected from human persecution. Stander et al. (1997) reported a relatively high mortality of leopards outside the KNP due to human-wildlife conflict. Hanssen et al. (2015) estimated with camera traps and SCR models a density of 0.6 leopard/100 km2 for the MNC in the Zambezi strip, the nearest location from our study area (approximately 300 km away). They suggested that interactions between humans and leopards, high trophy hunting quotas in the past and the small size of protected areas in the Zambezi strip are keeping leopards at low density. Balme et al. (2009b) have shown that in southern Africa, the leopard density is strongly influenced by the degree of persecution, thus we suggest to repeat this study in the neighboring communal land and freehold farmlands to assess the effect of human pressure on leopard density.
The KNP is one of the most remote locations of Namibia which has experienced few changes in the last three decades. However, the infrastructure in the KNP has been upgraded within the last five years to promote tourism development, whilst long-term park management plans increased water availability throughout the year, game numbers, fire control, number of rangers and regular monitoring schemes. Such changes are likely to affect wildlife numbers also in the future. In addition, during our camera trap survey, we detected livestock inside the western side of the KNP where it borders with recently developed small-scale farms. This has led to an increase of human-carnivore conflict reported in the area (Piet Beytell pers. comm.). The presence of livestock within the park indicates that herding management measures and damage prevention strategies are required. Thus, regular surveys are required to understand how park management, leopard interactions with their main competitors (i.e. lions and spotted hyenas) and human-leopard conflicts with livestock farmers shapes leopard demography.
In the LHR in north-west Namibia, density estimates could not be calculated because only one leopard was detected once. In addition, the camera traps captured all GPS collared lions (n = 5) of the Desert Lion Project (DLP), all brown hyenas (n = 3) tagged by the Skeleton Coast Brown Hyena Project and all GPS collared cheetahs (n = 3) tagged by our own Cheetah Research Project in the area. Additionally, during a previous survey focusing on cheetahs in this area in 2016, only one leopard was detected by the camera traps, which was the same individual as recorded in this study. Thus, leopards seem to occur at low densities in the LHR and they were not thought to be resident in the area during the last two decades (Stander, pers. comm.). Previous to this study, the only known male leopard in the area was GPS collared by the DLP and found dead after an encounter with a lioness in January 2016; no other male was detected afterwards (Stander, pers. comm.).
With only 25 mm of average rainfall in the LHR, our study provides information from the driest area where a leopard survey was carried out (Ghoddousi et al. 2010; Edwards et al. 2015; Farhadinia et al. 2021). Stein et al. (2011a) determined the entire costal Namib Desert as area of high leopard densities by extrapolating densities across the country. Our results therefore call for caution from extrapolating densities over large areas. Similar to our findings, low leopard densities were described in two other arid Namibian environments by Edwards et al. (2015). They estimated 0.6 and 0.9 leopards/100 km2 in two study sites in freehold farmlands in the southern Namib Desert where rainfall average varies between 80 and 120 mm per year. For the LHR, leopard sightings are regularly reported in areas adjacent to this study area such as in the Palmwag Concession Area, in the Hoanib floodplains and in the Sesfontein Conservancy (Ruben Portas pers. comm.). The heterogeneity of the landscape, the irregular distribution of the prey and vegetation due to the unpredictable and sparse rainfall is likely to result also in an irregular distribution of the leopards in this arid environment. A study based on modelling extrapolation of density estimates in other areas and inclusion of environmental variables such as altitude, land-cover and rainfall revealed a wide range of leopard densities (0.5 to > 3 leopards/100 km2) in the Kunene region which includes the LHR (NAPHA 2019). The wide range of leopard densities suggests a need to intensify camera trap studies in this area, preferably also in study areas of different human pressure on leopards. The determination of the home range sizes and movement patterns of leopards in this area is also likely to be of importance for conservation management of the species, and as such is suggested as a future research priority.
Long-term systematic camera trap studies estimating density across species range are crucial to determine the population trends and should be regularly repeated to establish science based management plans and data driven conservation strategies (Gittleman et al. 2001; Karanth et al. 2006; Balme et al 2009b; NAPHA 2019). In species that are both persecuted and commercially used for trophy hunting, accurate and precise estimates as well as good data on survival and recruitment are key to ensure the long-term conservation of the species through management and responsible quota setting (Gittleman et al. 2001; Balme et al. 2009b; Stein et al. 2011b). This is important because management measures and off-take per year are often not considering population trends (IUCN SSC CAT SG and CITES 2018).Footnote 1 We therefore suggest carrying out additional and regular surveys on leopards across Namibia and establishing a stratified monitoring system to provide robust data and population trends across different habitats that addresses the current knowledge gaps to ensure the long-term conservation of the species.
Availability of data and material
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Code availability
The code supporting the current study is available from the corresponding author on request.
Notes
IUCN SSC CAT SG: International Union for Conservation of Nature Species Survival Commission CAT Specialist Group, CITES: Convention on International Trade in Endangered Species.
References
Allen M, Wang S, Olson LO, Li Q, Krofel M (2020) Counting cats for conservation: seasonal estimates of leopard density and drivers of distribution in the Serengeti. Biodivers Conserv 29:3591–3608. https://doi.org/10.1007/s10531-020-02039-w
Athreya V, Odden M, Linnell JDC, Krishnaswamy J, Karanth KU (2014) A cat among the dogs: leopard Panthera pardus diet in a human-dominated landscape in western Maharashtra, India. Oryx 50:156–162. https://doi.org/10.1017/S0030605314000106
Bailey TN (1993) The African Leopard: ecology and behaviour of a solitary felid. Columbia University Press, New York
Balme GA, Hunter LTB, Slotow R (2009a) Evaluating methods for counting cryptic carnivores. J Wildl Manag 73:433–441. https://doi.org/10.2193/2007-368
Balme GA, Slotow R, Hunter LTB (2009b) Impact of conservation interventions on the dynamics and persistence of a persecuted leopard (Panthera pardus) population. Biol Conserv 142:2681–2690. https://doi.org/10.1016/j.biocon.2009.06.020
Balme GA, Hunter L, Braczkowski AR (2012) Applicability of age-based hunting regulations for African leopards. PLoS ONE 7(4):e35209. https://doi.org/10.1371/journal.pone.0035209
Balme GA, Miller JRB, Pitman RT, Hunter LTB (2017a) Caching reduces kleptoparasitism in a solitary, large felid. J Anim Ecol 86:634–644. https://doi.org/10.1111/1365-2656.12654
Balme GA, Pitman RT, Robinson HS et al (2017b) Leopard distribution and abundance is unaffected by interference competition with lions. Behav Ecol 28:1348–1358. https://doi.org/10.1093/beheco/arx098
Beytell P (2017) Aerial count of roan antelope and other wildlife species in Khaudum National Park. Ministry of Environment and Tourism, Windhoek
Boitani L, Fuller T (2000) Research techniques in animal ecology: controversies and consequences. Columbia University Press, New York
Borchers DL, Efford MG (2008) Spatially explicit maximum likelihood methods for capture-recapture studies. Biometrics 64:377–385. https://doi.org/10.1111/j.1541-0420.2007.00927.x
Boudreaux K, Nelson F (2011) Community conservation in Namibia: empowering the poor with property rights. Econ Aff 31:17–24. https://doi.org/10.1111/j.1468-0270.2011.02096.x
Boyd IL, Bowen WD, Iverson SJ (2009) Marine mammal ecology and conservation: a handbook of techniques. Oxford University Press, Oxford
Braczkowski AR, Balme GA, Dickman A, Macdonald DW, Johnson PJ, Lindsey PA, Hunter LTB (2015) Rosettes, remingtons and reputation: establishing potential determinants of leopard (Panthera pardus) trophy prices across Africa. Afr J Wildl Res 45:158–168. https://doi.org/10.3957/056.045.0158
Braczkowski AR, Balme GA, Dickman A, Fattebert J, Johnson P, Dickerson T, Macdonald DW, Hunter LTB (2016) Scent lure effect on camera-trap based leopard density estimates. PLoS ONE 11(4):e0151033. https://doi.org/10.1371/journal.pone.0151033
Craig GC, Gibson DSTC (2016) Aerial survey of elephants and other animals in north western Namibia. Ministry of Environment and Tourism, Windhoek
Curtis BA (2017) The status of Faidherbia albida trees in the Hoanib River, Namibia. Nami J Envir 1:77–91
Datta A, Osuri A, Naniwadekar R (2008) Empty forests: large carnivore and prey abundance in Namdapha National Park, north-east India. Biol Conserv 141:1429–1435. https://doi.org/10.1016/j.biocon.2008.02.022
Du Preez BD, Loveridge AJ, Macdonald DW (2014) To bait or not to bait: a comparison of camera-trapping methods for estimating leopard Panthera pardus density. Biol Conserv 176:153–161. https://doi.org/10.1016/j.biocon.2014.05.021
Edwards S, Aschenborn O, Gange AC, Wiesel I (2015) Leopard density estimates from semi-desert commercial farmlands, south-west Namibia. Afr J Ecol 54:103–106. https://doi.org/10.1111/aje.12235
Efford M (2012) SECR: Spatially explicit capture-recapture models. R package; version 2.10.4. http://cran.r-project.org/package=secr. Accessed Jan 2017
Efford M (2017) A tutorial on fitting spatially explicit capture-recapture models in secr. http://www.otago.ac.nz/density/pdfs/secr-tutorial.pdf. Accessed May 2017
Farhadinia MS, McClintock BT, Johnson PJ, Behnoud P, Hobeali K, Moghadas P, Hunter LTB, Macdonald DW (2019) A paradox of local abundance amidst regional rarity: the value of montane refugia for Persian leopard conservation. Sci Rep 9:14622. https://doi.org/10.1038/s41598-019-50605-2
Farhadinia MS, Behnoud P, Hobeali K, Mousavi SJ, Hosseini-Zavarei F, Gholikhani N, Akbari N, Braczkowski A, Eslami M, Moghadas P, Macdonald DW (2021) Estimating the density of a small population of leopards (Panthera pardus) in central Iran using multi-session photographic-sampling data. Mamm Biol 101:363–371. https://doi.org/10.1007/s42991-020-00096-w
Gese EM (2001) Monitoring of terrestrial carnivore populations. In: Gittleman JL, Funk SM, Macdonald DW, Wayne RK (eds) Carnivore conservation. Cambridge University Press, New York, pp 372–396
Geweke J (1992) Evaluating the accuracy of sampling-based approaches to the calculation of posterior moment. In: Ch M (ed) Proceedings of the 4th Valencia international meeting on bayesian statistics. Oxford University Press, Oxford
Government Gazette of the Republic of Namibia, No. 3991 (2008) Government notices, No. 35: declaration of an area as a National Park: Khaudum National Park. Available from: http://www.lac.org.na/laws/2008/3991.pdf. Accessed 18 Jan 2018
Ghoddousi A, Hamidi AK, Ghadirian T, Ashayeri D, Khorozyan I (2010) The status of the endangered Persian leopard Panthera pardus saxicolor in Bamu National Park. Iran Oryx 44:551–557. https://doi.org/10.1017/S0030605310000827
Gibson DSTC, Craig GC (2015) Aerial survey of elephants and other wildlife in Khaudum National Park and neighbouring conservancies. Ministry of Environment and Tourism and World Wildlife Fund, Windhoek
Gittleman JL, Funk SM, Macdonald DW (2001) Carnivore conservation. Conservation biology series, 5th edn. Cambridge University Press, New York
Goldberg JF, Tempa T, Norbu N, Hebblewhite M, Mills LS, Wangchuk TR, Lukacs P (2015) Examining temporal sample scale and model choice with spatial capture-recapture models in the common leopard Panthera pardus. PLoS ONE 10(11):e0140757. https://doi.org/10.1371/journal.pone.0140757
Gopalaswamy AM, Royle JA, Hines JE, Singh P, Jathanna D, Kumar NS, Karanth U (2012) Program SPACECAP: software to estimate animal density using spatially explicit capture-recapture models. Methods Ecol Evol 3:1067–1072. https://doi.org/10.1111/j.2041-210X.2012.00241.x
Gray TNE, Prum S (2012) Leopard density in post-conflict landscape, Cambodia: evidence from spatially explicit capture-recapture. J Wildl Manag 76:163–169. https://doi.org/10.1002/jwmg.230
Gubbi S, Sharma K, Kumara V (2020) Every hill has its leopard: patterns of space use by leopards (Panthera pardus) in a mixed use landscape in India. Peer J 8:e10072. https://doi.org/10.7717/peerj.10072
Hammond PS, Mizroch SA, Donovan GP (1990) Individual recognition of cetaceans: Use of photo-identification and other techniques to estimate population parameters. International Whaling Commission (Special Issue 12).
Hanssen L, Stander PE (2004) Namibia large carnivore atlas. Predator Conservation Trust, Windhoek, Namibia. www.predatorconservation.com
Hanssen L, Singwangwa M, Kukuwa V (2015) Camera trap survey to determine the status of leopards in the Mudumu-North Complex, Zambezi Region, Namibia. Report July 2015. Kwando Carnivore Project, Kongola, Zambezi region, Namibia. Available on request from carnivores@iway.na
Hedges L, Lam WY, Campos-Arceiz A, Rayan DM, Laurance WF, Latham CJ, Saaban S, Clements GR (2015) Melanistic leopards reveal their spots: infrared camera traps provide a population density estimate of leopards in Malaysia. J Wildl Manag 79:846–853. https://doi.org/10.1002/jwmg.901
Henschel P, Hunter L, Breitenmoser U, Purchase N, Packer C, Khorozyan I, Bauer H, Marker L, Sogbohossou E, Breitenmoser-Wursten C (2008) Panthera pardus. The IUCN red list of threatened species: e.T15954A5329380. https://downloads.regulations.gov/FWS-HQ-ES-2016-0131-0709/attachment_31.pdf
Inman EN, Hobbs RJ, Tsvuura Z, Valentine L (2020a) Current vegetation structure and composition of woody species in community-derived categories of land degradation in a semiarid rangeland in Kunene region, Namibia. Land Degrad Dev 31:2996–3013. https://doi.org/10.1002/ldr.3688
Inman EN, Hobbs RJ, Tsvuura Z (2020b) No safety net in the face of climate change: the case of pastoralists in Kunene region, Namibia. PLoS ONE 15(9):e0238982. https://doi.org/10.1371/journal.pone.0238982
IUCN SSC CAT SG & CITES (2018) IUCN SSC CAT specialist group position statement on leopard quotas and non-detriment findings. In: Seventieth meeting of the standing committee Rosa Khutor, Sochi (Russian Federation), SC70 Inf. 35. 1–5 October 2018
Jacobson AP, Gerngross J, Lemeris R, Schoonover R, Anco C, Breitenmoser C, Durant SD, Farhadinia MS, Henschel P, Kamler JF, Laguardia A, Rostro-Garcia S, Stein AB, Dollar L (2016) Leopard (Panthera pardus) status, distribution and the research efforts across its range. PeerJ 4:e1974. https://doi.org/10.7717/peerj.1974
Jordan MJ, Barrett RH, Purcell KL (2011) Camera trapping estimates of density and survival of fishers Martes pennanti. Wildl Biol 17:266–276. https://doi.org/10.2981/09-091
Karanth KU, Nichols JD (1998) Estimation of tiger densities in India using photographic captures and recaptures. Ecology 79:2852–2862. https://doi.org/10.1890/0012-9658(1998)079[2852:EOTDII]2.0.CO;2
Karanth KU, Nichols JD (2011) Estimating tiger abundance from camera trap data: field surveys and analytical issues. In: O’Connell AF, Nichols JD, Karanth KU (eds) Camera traps in animal ecology: Methods and analyses. Springer, Tokyo, pp 97–117
Karanth KU, Kumar NS, Nichols JD (2002) Field surveys: estimating absolute densities of tigers using capture–recapture sampling. In: Karanth KU, Nichols JD (eds) Monitoring tigers and their prey: a manual for researchers, managers and conservationists in tropical Asia. Centre for Wildlife Studies, Bangalore, pp 139–152
Karanth KU, Nichols JD, Samba Kumar S, Hines JE (2006) Assesing tiger population dynamics using photographic capture-recapture sampling. Ecology 87:2925–2937. https://doi.org/10.1890/0012-9658(2006)87[2925:atpdup]2.0.co;2
Karczmarski L, Chan SCY, Rubenstein DI, Chui SYS, Cameron EZ (2022a) Individual identification and photographic techniques in mammalian ecological and behavioural research – Part 1: Methods and concepts. Mamm Biol (Special Issue) 102(3). https://link.springer.com/journal/42991/volumes-and-issues/102-3
Karczmarski L, Chan SCY, Chui SYS, Cameron EZ (2022b) Individual identification and photographic techniques in mammalian ecological and behavioural research – Part 2: Field studies and applications. Mamm Biol (Special Issue) 102(4). https://link.springer.com/journal/42991/volumes-and-issues/102-4
Kawanishi K, Sunquist ME (2004) Conservation status of tigers in a primary rainforest of peninsular Malaysia. Biol Conserv 120:329–344. https://doi.org/10.1016/j.biocon.2004.03.005
KAZA TFCA (2014) Kavango Zambezi transfrontier conservation area. Master integrated development plan 2015–2020. http://www.kavangozambezi.org. Accessed 18 Jan 2018
Klintenberg P, Verlinden A (2008) Water points and their influence on grazing resources in central northern Namibia. Land Degrad Dev 19:1–20. https://doi.org/10.1002/ldr.809
Lindsey PA, Alexander R, Frank LG, Mathieson A (2006) Potential of trophy hunting to create incentives for wildlife conservation in Africa where alternative wildlife-based land uses may not be viable. Anim Conserv 9:283–291. https://doi.org/10.1111/j.1469-1795.2006.00034.x
Martins Q, Harris S (2013) Movement, activity and hunting behaviour of leopards in the Cederberg mountains, South Africa. Afr J Ecol 51:571–579. https://doi.org/10.1111/aje.12068
Masih I, Maskey S, Mussá FEF, Trambauer P (2014) A review of droughts on the African continent: a geospatial and long-term perspective. Hydrol Earth Syst Sci 18:3635–3649. https://doi.org/10.5194/hess-18-3635-2014
Mendelsohn J, Jarvis A, Roberts C, Robertson T (2002) Atlas of Namibia. A portrait of the land and its people. New Africa books (Pty) Ltd. David Philip Publishers, Cape Town
MET (2016) Fire management strategy for Namibia’s protected area. Ministry of Environment and Tourism, Windhoek
MET (2019) Management plan for Khaudum National Park 2020/2021–2029/2030. Ministry of Environment and Tourism, Windhoek
MET/NACSO (2018) The state of community conservation in Namibia: Annual report 2017. MET, Windhoek
NAPHA (2019) The Namibian leopard: National census and sustainable hunting practices. Windhoek, Namibia, pp 177. http://www.napha-namibia.com/uploads/media/National_Leopard_Census_-_October_2019_01.pdf. Accessed 23 Mar 2020
Noack J, Heyns L, Rodenwoldt D, Edwards D (2019) Leopard density estimation within an enclosed reserve, Namibia using spatially explicit capture-recapture models. Animals 9:724. https://doi.org/10.3390/ani9100724
NPC (2015) Namibia poverty mapping report. Macroeconomic planning department. National planning commission. https://www.npc.gov.na/download/NPC_Reports/NPC-Poverty%20Mapping%20Report.pdf. Accessed 11 Feb 2021
Otis DL, Burnham KP, White GC, Anderson DR (1978) Statistical inference from capture data on closed animal populations. Wildl Monogr 62:135
Packer C, Kosmala M, Cooley HS, Brink H, Pintea L, Garshelis D, Purchase G, Strauss M, Swanson A, Balme G, Hunter L, Nowell K (2009) Sport hunting, predator control and conservation of large carnivores. PLoS ONE 4(6):e5941. https://doi.org/10.1371/journal.pone.0005941
Packer C, Brink H, Kissui BM, Maliti H, Kushnir H, Caro T (2011) Effects of trophy hunting on lion and leopard populations in Tanzania. Conserv Biol 25:142–153. https://doi.org/10.1111/j.1523-1739.2010.01576.x
Quantum GIS Development Team (2015) QGIS geographic information system. Open Source Geospatial Foundation Project. https://qgis.org/gl/site/. Accessed 21 Jan 2019
Ray JC, Hunter L, Zigouris J (2005) Setting conservation and research priorities for larger African carnivores. Working Paper No. 24. Wildlife Conservation Society, New York.
R Development Core Team (2014) R: A language and environment for statistical computing. Version 3.1.1. R Foundation for statistical computing, Vienna, Austria
Royle JA, Chandler RB, Sollmann R, Gardner B (2014) Spatial capture-recapture. Elsevier, United Kingdom
Santangeli A, Arkumarev V, Rust N, Girardello M (2016) Understanding, quantifying and mapping the use of poison by commercial farmers in Namibia-Implications for scavengers’ conservation and ecosystem health. Biol Conserv 204:205–211. https://doi.org/10.1016/j.biocon.2016.10.018
Schneider S, Taylor GW, Linquist S, Kremer SC (2019) Past, present and future approaches using computer vision for animal re-identification from camera trap data. Methods Ecol Evol 10:461–470. https://doi.org/10.1111/2041-210X.13133
Seely M, Henschel J, Robertson M (1998) The ecology of fog in Namib Desert dunes. In: Proceedings Conference on Fog and Fog Collection, Vancouver, Canada
Soisalo MK, Cavalcanti SMC (2006) Estimating the density of a jaguar population in the Brazilian Pantanal using camera-traps and capture-recapture sampling in combination with GPS radio-telemetry. Biol Conserv 129:487–496. https://doi.org/10.1016/j.biocon.2005.11.023
Sollmann R, Furtado MM, Gardner B, Hofer H, Jácomo ATA, Tôrres NM, Silveira L (2011) Improving density estimates for elusive carnivores: accounting for sex-specific detection and movements using spatial capture-recapture models for jaguars in central Brazil. Biol Conserv 144:1017–1024. https://doi.org/10.1016/j.biocon.2010.12.011
Sollmann R, Gardner B, Belant JL (2012) How does spatial study design influence density estimates from spatial capture-recapture models? PLoS ONE 7(4):e34575. https://doi.org/10.1371/journal.pone.0034575
Stander PE (1998) Spoor counts as indices of large carnivore populations: the relationship between spoor frequency, sampling effort and true density. J Appl Ecol 35:378–385. https://doi.org/10.1046/j.1365-2664.1998.00313.x
Stander PE, Haden PJ, Kaqece II, Ghau II (1997) The ecology of asociality in Namibian leopards. J Zool 242:343–364. https://doi.org/10.1111/j.1469-7998.1997.tb05806.x
Stein AB, Fuller TK, Damery DT, Sievert L, Marker LL (2010) Farm management and economic analyses of leopard conservation in north-central Namibia. Anim Conserv 13:419–427. https://doi.org/10.1111/j.1469-1795.2010.00364.x
Stein A, Andreas A, Aschenborn O (2011a) Namibian national leopard survey. 2011. Final report. Ministry of Environment and Tourism, Windhoek
Stein AB, Fuller TK, DeStefano S, Marker L (2011b) Leopard population and home range estimates in north-central Namibia. Afr J Ecol 49:383–387. https://doi.org/10.1111/j.1365-2028.2011.01267.x
Stein AB, Bourquin SL, McNutt JW (2015) Avoiding intraguild competition: leopard feeding ecology and prey caching in northern Botswana. Afr J Wildl Res 45:247–257. https://doi.org/10.3957/056.045.0247
Stein A, Athreya V, Balme G, Henschel P, Karanth U, Miquelle D (2016) Panthera pardus. The IUCN red list of threatened species: e.T15954A50659089. Gland (switzerland). https://doi.org/10.2305/IUCN.UK.2016-1.RLTS.T15954A50659089.en
Stormer N, Weaver CL, Stuart-Hill G, Diggle D, Naidoo R (2019) Investigating the effects of community-based conservation on attitudes towards wildlife in Namibia. Biol Conserv 233:193–200. https://doi.org/10.1016/j.biocon.2019.02.033
Stuart-Hill G, Diggle R, Munali B, Tagg J, Ward D (2005) The event book system: a community-based natural resource monitoring system from Namibia. Biodivers Conserv 14:2611–2631. https://doi.org/10.1007/s10531-005-8391-0
Sun CC, Fuller AK, Royle AJ (2014) Trap configuration and spacing influences parameter estimates in spatial capture-recapture models. PLoS ONE 9(2):e88025. https://doi.org/10.1371/journal.pone.0088025
Swanepoel LH, Somers MJ, van Hoven W, Schiess-Meier M, Owen C, Snyman A, Martins Q, Senekal C, Camacho G, Boshoff W, Dalerum F (2015) Survival rates and causes of mortality of leopards Panthera pardus in southern Africa. Oryx 49:595–603. https://doi.org/10.1017/S0030605313001282
Thorn M, Green M, Scott D, Marnewick K (2013) Characteristics and determinants of human-carnivore conflict in South African farmland. Biodiver Conserv 22:1715–1730. https://doi.org/10.1007/s10531-013-0508-2
Verlinden A, Kruger AS (2017) Changing grazing systems in central north Namibia. Land Degrad Dev 18:179–197. https://doi.org/10.1002/ldr.769
Weingarth K, Zeppenfeld T, Heibl C, Heurich M, Bufka L, Daniszová K, Müller J (2015) Hide and seek: extended camera-trap session lengths and autumn provide best parameters for estimating lynx densities in mountainous areas. Biodivers Conserv 24:2935–2952. https://doi.org/10.1007/s10531-015-0986-5
Acknowledgements
We thank the Ministry of Environment, Forestry and Tourism (MEFT) in Namibia for permission and support to conduct this study, Hans Swartbooi for his valuable help in the field during the camera trap survey in the Khaudum National Park and Ortwin Aschenborn, the large carnivore coordinator of MEFT at the time, who motivated this study. We thank Emsie Verwey and Wilderness Safaris for their logistic support during the fieldwork in the Skeleton Coast and Philip Stander for the expert information provided. We also thank Vera Menges for providing the home range sizes of the leopard females and males in east-central Namibia and Ivan Palmegiani for assisting with the home range calculations. We further thank Rahel Sollmann and Miha Krofel for their valuable comments on a previous version of this manuscript. We greatly appreciate the guidance provided by Leszek Karczmarski and his excellent editorial handling of our manuscript. Finally, we thank Alex Braczkowski and an anonymous reviewer for their time and constructive comments that considerably improved the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The publication of this article was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – project number 491292795. This study was funded by the Ministry of Environment, Forestry and Tourism (MEFT) in Namibia, WWF Germany and the Messerli Foundation in Switzerland. Sarah Edwards received a post-doctoral bursary from the University of Pretoria, South Africa.
Author information
Authors and Affiliations
Contributions
Conceived the study: RP, JM, KHU, Designed the experiments: RP, SE, JM. Performed the experiments and collected the data: RP, PB. Analyzed the data: RP, SE. Wrote the paper: RP, SE, BW. Commented on the manuscript: JM, KHU, PB.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
To methods applied and the study design were approved by the MEFT and the National Commission of Research, Science and Technology of Namibia.
Consent to participate
All authors agreed to participate to this manuscript and all have contributed to its content and current version.
Consent for publication
All authors agreed to submit this manuscript to the Special Issue of Mammalian Biology for publication.
Additional information
Handling editors: Leszek Karczmarski and Stephen C.Y. Chan.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is a contribution to the special issue on “Individual Identification and Photographic Techniques in Mammalian Ecological and Behavioural Research – Part 2: Field Studies and Applications” — Editors: Leszek Karczmarski, Stephen C.Y. Chan, Scott Y.S. Chui and Elissa Z. Cameron.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Portas, R., Wachter, B., Beytell, P. et al. Leopard Panthera pardus camera trap surveys in the arid environments of northern Namibia. Mamm Biol 102, 1185–1198 (2022). https://doi.org/10.1007/s42991-022-00237-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42991-022-00237-3