Abstract
Purpose
Anthracyclines have been one of the standard therapies for breast cancer (BC), and dose-related cardiotoxicity is one serious side effect. Exercise is an effective strategy for the prevention and management of BC, endorsed by experts in both exercise and oncology. However, there is a great deal of confusion about the effectiveness of exercise on anthracycline-induced cardiotoxicity and the exercise prescription (i.e., timing, type, and intensity) for cardiotoxicity, which limits its application in clinical settings. The aim of this article is to review the safety of exercise in BC patients receiving anthracyclines and its effectiveness in preventing cardiotoxicity.
Methods
Six electronic databases were searched using terms related to exercise, BC, anthracyclines, and cardiotoxicity for retrieving clinical randomized controlled trials in either Chinese or English. A summary of the included literature was also provided.
Results
Of 202 records screened, 10 were eligible. A total of 434 BC patients (stage I–IIIC, mean age ranged from 43.5 to 52.4 years) were included. The main findings were that: (1) Acute (a single bout) moderate-to-vigorous aerobic exercise could prevent NT-proBNP elevation beyond the threshold of acute myocardial injury; (2) Long-term (> 8 weeks) moderate-to-high intensity aerobic exercise (continuous or interval) could improve or maintain left ventricular ejection fraction and cardiorespiratory fitness in BC patients. However, the optimal timing, type, and intensity of exercise for people with BC to prevent cardiotoxicity remain unclear.
Conclusion
Moderate-to-vigorous intensity exercise may be an effective non-pharmacological approach to mitigate cardiotoxicities induced by anthracyclines in women with BC. However, the optimal exercise prescription for preventing cardiotoxicity remains unclear.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2020, breast cancer (BC) has become the most prevalent cancer in the world [51]. It is estimated that there are about 2.26 million new cases of BC worldwide, of which approximately 420,000 are in China [8, 51]. Advances in modern detection strategies and treatments have led to improved survival rates for BC [32]. Anthracyclines, such as doxorubicin and epirubicin, are one of the basic chemotherapeutic agents for BC, and anthracycline-containing regimens have been shown to reduce mortality in women with breast cancer by 38% [13]. However, patients may experience adverse cardiovascular events related to cancer treatment or exacerbation of underlying cardiovascular diseases [7]. Anthracycline-induced cardiotoxicity could significantly impact the cardiovascular system, causing damage to both the heart and vascular endothelial cells [34]. It has been reported that cardiotoxicity may cause treatment interruptions, and cardiovascular disease has become the leading cause of non-cancer-related mortality in BC survivors [3]. Therefore, it would be prudent to consider strategies to mitigate the effects of cardiotoxicity during treatment.
Cardiotoxicity begins with myocardial damage and gradually progresses to cardiac dysfunction [9, 34]. If a decline in cardiac function is detected, myocardial damage has already occurred [9]. Therefore, the detection of myocardial damage through biochemical markers, such as N-terminal brain natriuretic peptide precursor (NT-proBNP), cardiac troponin, and creatine kinase isoenzymes (CK-MB), can be an earlier sign of cardiotoxicity compared to cardiac function [29, 35, 56]. Echocardiographic indexes, such as LVEF and GLS, are also commonly used to detect cardiotoxicity [22, 55]. Furthermore, peak oxygen uptake (VO2peak), as an indicator of the cardiorespiratory fitness and cardiac reserve function, can be estimated through a graded exercise test, which could more precisely detect early cardiac injury and predict future cardiac events [26, 44]. It has been widely reported that the VO2peak can be improved by physical activity, including structured exercise, in apparently healthy populations and patients with various chronic diseases [6, 50].
Numerous strategies, including continuous infusion, dosage reduction, liposomal formulations, and cardioprotective drugs (e.g., dexrazoxane) have been utilized clinically to reduce the risk of cardiotoxicity induced by anthracyclines [12]. However, the risk of anthracyclines-induced cardiotoxicity is still high [27]. In an analysis of nearly 23,000 cancer survivors receiving anthracycline chemotherapy with a median follow-up of nine years, the rates of clinical and subclinical cardiotoxicity were 6.3% and 17.9%, respectively [33]. Anthracyclines induce cardiotoxicity through a number of pathways, with the more commonly reported mechanisms including topoisomerase II inhibition, oxidative stress, and cardiomyocyte apoptosis [53]. A growing body of evidence indicates that mitochondrial dysfunction is strongly associated with cardiotoxicity [45]. There is clear evidence to support the benefits of exercise on alleviating numerous chemotherapy side effects. Preclinical studies have shown that exercise mitigates and reverses cardiotoxicity, without compromising anthracycline’s antitumor effects [4, 41, 42, 54]. It is well known that cancer survivors should ‘avoid inactivity’ [1]. The American College of Sports Medicine (ACSM) and American Heart Association (AHA) recommend cancer survivors engage in at least 150 min of moderate-intensity exercise or 75 min of vigorous-intensity exercise, or an equal combination of both, per week [7, 18, 47].
There still needs to be more clarity among clinicians and BC patients about the effectiveness of exercise in reducing cardiotoxicity and the implemention of exercise prescriptions in clinical settings. This includes defining the type, intensity, duration, and frequency of exercise. Moreover, most BC patients cannot tolerate the recommended exercise amounts due to weakness, a common side effect of chemotherapy [24]. Several systematic reviews and two narrative reviews concluded that exercise might be an effective non-pharmacological intervention for improving aerobic capacity and reducing cardiotoxicity among BC patients. However, the conclusion was based on a limited number of randomized controlled trials (RCTs) and observational studies [27, 36, 39, 40, 52]. It is crucial to recognize the limitations of existing reviews. First, the RCTs included in these reviews contained a small number of subjects, and most of these RCTs were published before 2023, with only three relevant RCTs published since then. Second, the effects of exercise on cardiotoxicity-related indicators were inconsistent. Third, these reviews focused more on the beneficial effects of aerobic exercise than cardiotoxicity. The efficacy of different types of exercise in anthracycline-induced cardiotoxicity is still uncertain. One recent systematic review by Ma et al. [36] unveiled that while aerobic exercise increased VO2peak, it had no significant effect on biomarkers of cardiac injury. Conversely, resistance exercise can improve the function of the cardiovascular muscles [17, 27], thereby improving the aerobic system and reducing cardiotoxicity. In summary, the objectives of this review were to summarize RCTs published between 2010 and 2023 and to: (1) describe the efficacy of exercise (aerobic and/or resistance) on anthracycline-induced cardiotoxicity in clinical settings; (2) provide recommendations for clinicians and BC patients to prevent anthracycline-induced cardiotoxicity, including the timing, type (aerobic, resistance and flexibility exercise), duration, frequency, intensity and precautions of exercise interventions.
Methods
Registration and Protocol
This review protocol was registered in PROSPERO on October 2, 2022 (CRD42022364256), and the guidelines in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement were followed [38].
Search Strategy
The literature search was conducted using the following six electronic databases: China National Knowledge Infrastructure, Wan Fang Data Knowledge Service Platform, VIP Chinese Journal Service Platform, Web of Science, PubMed, and the Cochrane Library. The retrieval strategy was Mesh combined Entry Terms and was determined by two researchers (LHM and LHY). The search targeted candidate studies published from January 1, 2010, to March 29, 2023. The search terms, a combination of four filters, were used to select relevant articles: (“Exercise” OR “Physical Activity” OR “Training”) AND (“Breast Neoplasms” OR “Breast Cancer” OR “Breast Tumor”) AND (“Anthracyclines” OR “Doxorubicin” OR “Epirubicin” OR “Pirarubicin”) AND (“Cardiotoxicity” OR “Cardiac Toxicity” OR “Cardiomyopathy” OR “Heart Failure” OR “Myocarditis” OR “Arrhythmia” OR “Cardiac Function”). In addition, the references of included articles were screened to identify potentially eligible studies.
Inclusion and Exclusion Criteria
Inclusion criteria based on PICOS principles involved: (1) participants: BC patients scheduled to receive anthracycline chemotherapy; (2) intervention: exercise intervention encompassing either acute (a single bout) or long-term exercise interventions (aerobic, resistance or combined); (3) comparison: usual cancer care; (4) Outcomes related to the cardiotoxicity: biochemical markers,echocardiographic index, and cardiorespiratory fitness, etc.; (5) study design: clinical RCTs.
Exclusion criteria were: (1) repeatability study; (2) Subjects were included in addition to BC patients, but also patients with other types of cancer, such as melanoma. (3) animal models or cell model research; (4) studies published as conference proceedings or reviews; (5) observation groups receiving interventions other than exercise, such as nutrition or cardioprotective drugs (e.g., dexrazoxane).
Study Selection and Data Extraction
Study selection and data extraction were independently carried out by two researchers (LHM and LHY). The following information was extracted: title, author, publication year, subjects, drug dosage, exercise intervention program details (type, intensity, session duration, time, and frequency), the timing of exercise intervention (before, during, and after chemotherapy), adherence, and outcome indicators. When two researchers (LHM and LHY) disagreed, a third researcher was consulted (SD).
Risk of Bias in Individual Studies
The risk of bias in human studies was independently assessed during data extraction using the Cochrane risk-of-bias tool by two researchers (LHM and LHY) [20]. The tool included seven items: (1) random sequence generator, (2) allocation concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) incomplete outcome data, (6) selective reporting, and (7) other bias. Each item was assigned a score of ‘high’ (bias with the potential to seriously alter the results), ‘low’ (bias unlikely to seriously alter the results), or ‘unclear’ (indicating lack of information, or uncertainty over potential bias) [20].
Results
Search Results
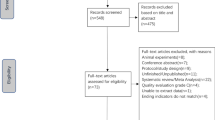
Figure 1 provides details of the study exclusion and inclusion process. The initial search yielded 200 records from six databases, and two additional studies were identified through a reference search of eligible studies. After removing duplicates and screening the titles and abstracts, 13 full articles were retrieved and assessed for eligibility. The final number of included studies was ten (one was in Chinese and nine were in English) [2, 10, 16, 21, 25, 28, 29, 31, 35, 56].
Studies description
The quality assessment is shown in Table 1. ‘Random sequence generator’ and ‘allocation concealment’ were graded as low risk in eight studies, while the risk was unclear in two studies [35, 56]. ‘Blinding of participants and personnel’ were assessed as high risk in 10 studies due to the need for supervision during exercise interventions. Nine studies were graded as low risk for ‘blinding outcome assessment’, and one study was graded as unclear. Ten studies were rated as being at low risk of ‘incomplete outcome data’ and ‘selective reporting’, and as unclear for ‘other bias’. Across the seven domains of bias, the proportion (range) of studies rated as ‘high’ was 14.3%; ‘low’, 64.3% (28.6% to 71.4%); and ‘unclear’, 21.4% (14.3% to 57.1%).
The experimental design of included studies is shown in Table 2. A total of 434 participants were involved in this review, consisting of stage I–IIIC BC patients scheduled to receive anthracyclines for (neo) adjuvant chemotherapy. The mean age of participants ranged from 43.5 to 52.4 years, and sample sizes ranged from 20 to 102. Anthracycline doses were reported in 7/10 studies [2, 10, 16, 21, 25, 28, 29]. One study conducted acute high-intensity aerobic exercise before chemotherapy, and the exercise adherence was 100% [29]. The remaining nine studies conducted long-term (8 weeks–12 months) continuous moderate-to-high training or high-intensity interval training (HIIT) during chemotherapy, with exercise adherence ranging from 63.2% to 98% [10, 21, 25, 28, 31, 35, 56]. Three out of then articles reported aerobic combined with resistance exercise [2, 10, 16], and the remaining seven articles reported aerobic exercise.
Discussion
This systematic review described the role of exercise in the prevention and treatment of anthracycline-induced cardiotoxicity in women with BC (I–IIIC stage). The main findings of this review were that (1) acute moderate-to-vigorous aerobic exercise could avoid elevated NT-proBNP levels; (2) long-term moderate-to-high intensity aerobic exercise (continuous or interval) or a combination of aerobic and resistance exercises could increase or maintain echocardiographic indexes and cardiorespiratory fitness in BC patients. However, the effectiveness of aerobic exercise on biochemical markers of myocardial injury was inconsistent. Owing to the lack of high-quality RCTs and the differences in training variables such as timing, intensity, duration, and adherence of aerobic exercise performed in each study, some caution is warranted in interpreting these results.
NT-proBNP is synthesized and secreted by the myocardium in response to increased hemodynamic stress, including increased left ventricular end-diastolic volume and pressure [49]. Kirkham et al. [29] found an transient increase in NT-proBNP levels between 24 and 48 hours after the scheduled starting time of participants’ first doxorubicin treatment. Aerobic exercise at 70% heart rate reserve (HRR) in the exercise intervention group prevented NT-proBNP from surpassing the threshold for acute myocardial injury (> 300 pg/mL) ([23, 29], whereas NT-proBNP elevation in the control group exceeded this acute myocardial injury threshold. Another facet of the same trial by Kirkham et al. [28] revealed a significant increase in NT-proBNP levels at 7–14 days after the final doxorubicin treatment, despite acute aerobic exercise at 70% HRR (once per cycle) was conducted prior to four cycles of chemotherapy. Studies by Zhang et al. [56] and Ma et al. [35] reported that a 16-week HIIT regimen (three times a week) at 90%–95% HRmax also prevented NT-proBNP elevations above the threshold for acute myocardial injury. Notably, the study by Kirkham et al. [28] did not continuously monitor changes in NT-proBNP, and both studies by Zhang et al. [56] and Ma et al. [35] did not report the time of blood collection and lacked follow-up, so it is unclear how long the effects of acute and long-term exercise would last. Above mentioned findings may suggest that acute exercise can mitigate myocardial injury induced by a single injection of anthracyclines, which is a stress response with a temporary beneficial effect. In contrast, acute exercise (once per cycle) prior to four chemotherapy cycles cannot offset the myocardial injury caused by prolonged accumulation of anthracyclines. Since cardiotoxicity is a chronic condition, regular long-term exercise is required to effectively mitigate anthracycline-induced myocardial damage to achieve the lifelong protective effect of exercise.
Adherence rates across the studies in this review ranged from 63.2% to 98% for sessions lasting 8 weeks ~ 12 months, and decreased with a longer duration of the intervention. It is significant to find strategies to improve adherence to long-term exercise intervention. Notably, exercise adherence, an element related to the effectiveness of exercise, was not described in two studies within this review [35, 56]. Exercise is generally deemed safe for cancer survivors during and after cancer treatment, and no adverse events were reported in the included studies of this review. However, cancer survivors often experience a variety of acute, chronic, and late side effects of cancer and its treatment, which can create barriers (i.e., side effects, co-morbid conditions and emotional changes) to exercise adherence [19]. Optimal exercise benefits are more likely to be achieved in younger patients [35, 56], because clinical experience suggests that younger patients are more willing to adopt exercise intervention during chemotherapy and have better compliance [5]. To comprehensively understand the effects of exercise on cardiotoxicity, future studies should describe more details (i.e., adherence, barriers, and adverse events) during implementation. Clinicians might play a critical role in assisting BC patients in identifying and overcoming challenges in long-term exercise adhere, thus they could have lifelong benefits. According to the clinical experience of our research team, patients should be provided with education on the benefits of exercise, regular feedback, timely progression or regression, and enhanced supervision.
Although exercise prescriptions used in the included studies are consistent with the current ACSM guideline for cancer survivors [1, 7]. The optimal timing, modality, and intensity of exercise for individuals with BC are still unclear. Regarding the timing of exercise interventions, one study implemented exercise before chemotherapy and nine studies conducted interventions during chemotherapy, which was related to the convenience of availability and adherence monitoring of patients during chemotherapy. Future studies are required to investigate how the timing of exercise (before, during, and after anthracycline exposure) might influence cardiotoxicity outcomes. All studies included in this review focused on aerobic exercise interventions, which demonstrated positive changes in LVEF and cardiorespiratory fitness. These positive changes were also found in interventions combining aerobic and resistance exercise [10]. Resistance exercise, commonly prescribed for improving neuromuscular functions such as muscle mass, strength, and endurance, has also been shown to be safe and beneficial in attenuating various treatment-related adverse effects, such as cancer-related fatigue and diminished physical functioning, and improving health-related quality of life in cancer patients [11, 14]. Traditionally, resistance exercise is prescribed to improve skeletal muscle strength and muscle mass [27, 37], which leads to the optimization of cardiac function and improvement of their daily life (e.g., stair climbing, carrying objects) for BC patients. Resistance exercise can also promote the production of factors that resist inflammation [15], which is considered to be one of the mechanisms of cardiotoxicity [57]. Furthermore, resistance exercise-induced arterial compliance may reduce arterial stiffness by effectively enhancing nitric oxide bioavailability and reducing endothelial senescence [30]. Future studies could explore the effects of resistance exercise or combined exercise in reducing cardiotoxicity.
All studies included in this review used moderate-to-vigorous intensity or HIIT exercise. However, there are certain obstacles in adhering to higher intensity exercise for vulnerable groups such as those who do not often exercise, have significant side effects of chemotherapy, or have underlying diseases. Those people should start exercise at lower intensity and gradually increase the intensity as their body adapt. Future research is needed to explore and directly compare the effects of (1) low-intensity exercise with the same volume as moderate-to-vigorous intensity exercise, and (2) different exercise intensities, in mitigating cardiotoxicity.
The variations in results among the studies in this review are related to differences in exercise prescription elements and differences between subjects across the studies. The reviewed studies encompassed BC patients of different ages (mean age 43.5–52.4 years) or stages (stage I–III). Grouping patients of diverse ages and disease stages raises concerns because potential differences in age and disease severity could influence exercise response. In addition, some studies in this review did not report the dose of anthracyclines received by BC patients, and others reported different doses of anthracyclines, which were known to be associated with cardiotoxicity [48].
For clinicians providing exercise guidance to BC patients receiving anthracycline-based chemotherapy and expressing willingness to engage in exercise training, some special considerations are as follows: (1) patients should perform a supervised moderate-to-vigorous exercise with heart rate and RPE monitored during exercise [52], (2) initiating exercise with a small volume to prevent adverse cardiovascular events at the beginning, then the frequency, intensity, and time of exercise can be incrementally increased based on tolerance. In addition, lymphedema and bone metastases require special attention. Lymph node dissection: patients have a risk of developing lymphedema in the upper limbs, shoulders, and back, and should wear appropriate compression cuffs or garments during resistance exercise [46]. Bone metastases: modalities for exercise should be chosen to avoid direct musculoskeletal loading on metastatic lesions or muscles proximal to these metastatic lesions. Careful attention on balance and safety should be given to reduce the risk of falls and injuries [43]. Patients who are not suitable candidates for resistance or endurance exercise and those with bone pain should be monitored during and after exercise [11].
To better interpret the present results, the following limitations should be acknowledged. First, the systematic review included only ten studies with small sample sizes and inherent risk of bias. Second, some studies lacked the necessary details for quality assessment. Third, the terms ‘anthracyclines’ only focused on doxorubicin, epirubicin, and pirirubicin, commonly used clinically in China, potentially excluding relevant studies. Finally, the large variability among studies (e.g., the timing of exercise interventions, duration of sessions, the dose of anthracyclines, and outcome measures) made result comparison difficult and pooling unsuitable. Therefore, a meta-analysis was not conducted (Table 3).
Conclusions
Overall, a limited number of quality-controlled studies suggest that both acute moderate-to-vigorous aerobic exercise and long-term moderate-to-high intensity aerobic exercise (continuous or interval) or a combination of aerobic and resistance exercises could protect against anthracycline-induced cardiotoxicity in BC patients. Despite these findings, the optimal timing, type, and intensity of exercise for preventing cardiotoxicity in individuals with BC remain unclear. Recruiting a large sample of BC patients is challenging in a clinical setting, so studies with well-described procedures and harmonized outcome measures may improve the quality and comparability of evidence. Future clinical studies should prioritize quality control, provide detailed intervention descriptions, coordinate outcome indicators comprehensively, and incorporate long-term follow-up to further investigate the effects of exercise on cardiotoxicity. This provides evidence for guiding clinical staff and patients in implementing exercise to prevent cardiotoxicity.
Data Availability
The datasets used during the current study are available from the corresponding author on reasonable request.
References
American College of Sports Medicine, Liguori G, Feito Y, Roy Brad A. ACSM’s guidelines for exercise testing and prescription, 11th ed. Philadelphia: Wolters Kluwer; 2022.
Antunes P, Joaquim A, Sampaio F, Nunes C, Ascensão A, Vilela E, Teixiera M, Capela A, Amarelo A, Marques C, Viamonte S, Alves A, Esteves D. Effects of exercise training on cardiac toxicity markers in women with breast cancer undergoing chemotherapy with anthracycline: a randomized controlled trial. Eur J Prev Cardiol. 2023;30(9):844–55. https://doi.org/10.1093/eurjpc/zwad063.
Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, Dent S, Douglas PS, Durand JB, Ewer M, Fabian C, Hudson M, Jessup M, Jones LW, Ky B, Mayer EL, Moslehi J, Oeffinger K, Ray K, Ruddy K, Lenihan D. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: american society of clinical oncology clinical practice guideline. J Clin Oncol. 2017;35(8):893–911. https://doi.org/10.1200/JCO.2016.70.5400.
Ascensão A, Magalhães J, Soares J, Ferreira R, Neuparth M, Marques F, Oliveira J, Duarte J. Endurance training attenuates doxorubicin-induced cardiac oxidative damage in mice. Int J Cardiol. 2005;100(3):451–60. https://doi.org/10.1016/j.ijcard.2004.11.004.
Bélanger LJ, Plotnikoff RC, Clark A, Courneya KS. A survey of physical activity programming and counseling preferences in young-adult cancer survivors. Cancer Nurs. 2012;35(1):48–54. https://doi.org/10.1097/NCC.0b013e318210220a.
Benda NM, Seeger JP, Stevens GG, Hijmans-Kersten BT, van Dijk AP, Bellersen L, Lamfers EJ, Hopman MT, Thijssen DH. Effects of high-intensity interval training versus continuous training on physical fitness, cardiovascular function and quality of life in heart failure patients. PLoS ONE. 2015;10(10): e0141256. https://doi.org/10.1371/journal.pone.0141256.
Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, Zucker DS, Matthews CE, Ligibel JA, Gerber LH, Morris GS, Patel AV, Hue TF, Perna FM, Schmitz KH. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51(11):2375–90. https://doi.org/10.1249/MSS.0000000000002116.
Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). 2021;134(7):783–91. https://doi.org/10.1097/CM9.0000000000001474.
Cardinale D, Iacopo F, Cipolla CM. Cardiotoxicity of Anthracyclines. Front Cardiovasc Med. 2020;7:26. https://doi.org/10.3389/fcvm.2020.00026.
Chung WP, Yang HL, Hsu YT, Hung CH, Liu PY, Liu YW, Chan SH, Tsai KL. Real-time exercise reduces impaired cardiac function in breast cancer patients undergoing chemotherapy: a randomized controlled trial. Ann Phys Rehabil Med. 2022;65(2):101485. https://doi.org/10.1016/j.rehab.2021.101485.
Cong MH, Shi HP. Consensus of Chinese experts on exercise therapy for cancer patients. Sci Sin Vitae. 2022;52(1):1–16. https://doi.org/10.1360/ssv-2022-0028[Inchinese].
Corremans R, Adão R, De Keulenaer GW, Leite-Moreira AF, Brás-Silva C. Update on pathophysiology and preventive strategies of anthracycline-induced cardiotoxicity. Clin Exp Pharmacol Physiol. 2019;46(3):204–15. https://doi.org/10.1111/1440-1681.13036.
Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–717. https://doi.org/10.1016/S0140-6736(05)66544-0.
Fairman CM, Zourdos MC, Helms ER, Focht BC. A Scientific rationale to improve resistance training prescription in exercise oncology. Sports Med. 2017;47(8):1457–65. https://doi.org/10.1007/s40279-017-0673-7.
Fernández-Rodríguez R, Monedero-Carrasco S, Bizzozero-Peroni B, Garrido-Miguel M, Mesas AE, Martínez-Vizcaíno V. Effectiveness of resistance exercise on inflammatory biomarkers in patients with type 2 diabetes mellitus: a systematic review with meta-analysis. Diabetes Metab J. 2023;47(1):118–34. https://doi.org/10.4093/dmj.2022.0007.
Foulkes SJ, Howden EJ, Haykowsky MJ, Antill Y, Salim A, Nightingale SS, Loi S, Claus P, Janssens K, Mitchell AM, Wright L, Costello BT, Lindqvist A, Burnham L, Wallace I, Daly RM, Fraser SF, La Gerche A. Exercise for the prevention of anthracycline-induced functional disability and cardiac dysfunction: the BREXIT study. Circulation. 2023;147(7):532–45. https://doi.org/10.1161/CIRCULATIONAHA.122.062814.
Gentil P, de Lira CAB, Coswig V, Barroso WKS, Vitorino PVO, Ramirez-Campillo R, Martins W, Souza D. Practical recommendations relevant to the use of resistance training for COVID-19 survivors. Front Physiol. 2021;12:637590. https://doi.org/10.3389/fphys.2021.637590.
Gilchrist SC, Barac A, Ades PA, Alfano CM, Franklin BA, Jones LW, La Gerche A, Ligibel JA, Lopez G, Madan K, Oeffinger KC, Salamone J, Scott JM, Squires RW, Thomas RJ, Treat-Jacobson DJ, Wright JS; American Heart Association Exercise, Cardiac Rehabilitation, and Secondary Prevention Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; and Council on Peripheral Vascular Disease. Cardio-oncology rehabilitation to manage cardiovascular outcomes in cancer patients and survivors: a scientific statement from the American heart association. Circulation. 2019;139(21):e997–1012. https://doi.org/10.1161/CIR.0000000000000679.
Henriksson A, Arving C, Johansson B, Igelström H, Nordin K. Perceived barriers to and facilitators of being physically active during adjuvant cancer treatment. Patient Educ Couns. 2016;99(7):1220–6. https://doi.org/10.1016/j.pec.2016.01.019.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods Group, Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. https://doi.org/10.1136/bmj.d5928.
Hornsby WE, Douglas PS, West MJ, Kenjale AA, Lane AR, Schwitzer ER, Ray KA, Herndon JE 2nd, Coan A, Gutierrez A, Hornsby KP, Hamilton E, Wilke LG, Kimmick GG, Peppercorn JM, Jones LW. Safety and efficacy of aerobic training in operable breast cancer patients receiving neoadjuvant chemotherapy: a phase II randomized trial. Acta Oncol. 2014;53(1):65–74. https://doi.org/10.3109/0284186X.2013.781673.
Howden EJ, Bigaran A, Beaudry R, Fraser S, Selig S, Foulkes S, Antill Y, Nightingale S, Loi S, Haykowsky MJ, La Gerche A. Exercise as a diagnostic and therapeutic tool for the prevention of cardiovascular dysfunction in breast cancer patients. Eur J Prev Cardiol. 2019;26(3):305–15. https://doi.org/10.1177/2047487318811181.
Januzzi JL, van Kimmenade R, Lainchbury J, Bayes-Genis A, Ordonez-Llanos J, Santalo-Bel M, Pinto YM, Richards M. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J. 2006;27(3):330–7. https://doi.org/10.1093/eurheartj/ehi631.
Jones LW, Courneya KS. Exercise counseling and programming preferences of cancer survivors. Cancer Pract. 2002;10(4):208–15. https://doi.org/10.1046/j.1523-5394.2002.104003.x.
Jones LW, Fels DR, West M, Allen JD, Broadwater G, Barry WT, Wilke LG, Masko E, Douglas PS, Dash RC, Povsic TJ, Peppercorn J, Marcom PK, Blackwell KL, Kimmick G, Turkington TG, Dewhirst MW. Modulation of circulating angiogenic factors and tumor biology by aerobic training in breast cancer patients receiving neoadjuvant chemotherapy. Cancer Prev Res (Phila). 2013;6(9):925–37. https://doi.org/10.1158/1940-6207.CAPR-12-0416.
Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007;50(15):1435–41. https://doi.org/10.1016/j.jacc.2007.06.037.
Kang DW, Wilson RL, Christopher CN, Normann AJ, Barnes O, Lesansee JD, Choi G, Dieli-Conwright CM. Exercise cardio-oncology: exercise as a potential therapeutic modality in the management of anthracycline-induced cardiotoxicity. Front Cardiovasc Med. 2022;8:805735. https://doi.org/10.3389/fcvm.2021.805735.
Kirkham AA, Eves ND, Shave RE, Bland KA, Bovard J, Gelmon KA, Virani SA, McKenzie DC, Stöhr EJ, Waburton DER, Campbell KL. The effect of an aerobic exercise bout 24 h prior to each doxorubicin treatment for breast cancer on markers of cardiotoxicity and treatment symptoms: a RCT. Breast Cancer Res Treat. 2018;167(3):719–29. https://doi.org/10.1007/s10549-017-4554-4.
Kirkham AA, Shave RE, Bland KA, Bovard JM, Eves ND, Gelmon KA, McKenzie DC, Virani SA, Stöhr EJ, Warburton DER, Campbell KL. Protective effects of acute exercise prior to doxorubicin on cardiac function of breast cancer patients: a proof-of-concept RCT. Int J Cardiol. 2017;245:263–70. https://doi.org/10.1016/j.ijcard.2017.07.037.
Kresnajati S, Lin YY, Mündel T, Bernard JR, Lin HF, Liao YH. Changes in arterial stiffness in response to various types of exercise modalities: a narrative review on physiological and endothelial senescence perspectives. Cells. 2022;11(22):3544. https://doi.org/10.3390/cells11223544.
Lee K, Kang I, Mack WJ, Mortimer J, Sattler F, Salem G, Dieli-Conwright CM. Feasibility of high intensity interval training in patients with breast Cancer undergoing anthracycline chemotherapy: a randomized pilot trial. BMC Cancer. 2019;19(1):653. https://doi.org/10.1186/s12885-019-5887-7.
Li H, Liu Z, Yuan L, Fan K, Zhang Y, Cai W, Lan X. Radionuclide-based imaging of breast cancer: state of the art. Cancers (Basel). 2021;13(21):5259. https://doi.org/10.3390/cancers13215459.
Lotrionte M, Biondi-Zoccai G, Abbate A, Lanzetta G, D’Ascenzo F, Malavasi V, Peruzzi M, Frati G, Palazzoni G. Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. Am J Cardiol. 2013;112(12):1980–4. https://doi.org/10.1016/j.amjcard.2013.08.026.
Lv H, Tan R, Liao J, Hao Z, Yang X, Liu Y, Xia Y. Doxorubicin contributes to thrombus formation and vascular injury by interfering with platelet function. Am J Physiol Heart Circ Physiol. 2020;319(1):H133–43. https://doi.org/10.1152/ajpheart.00456.2019.
Ma ZJ. Effect of anthracycline combined with aerobic exercise on the treatment of breast cancer. Pak J Pharm Sci. 2018;31(3):1125–9.
Ma ZY, Yao SS, Shi YY, Lu NN, Cheng F. Effect of aerobic exercise on cardiotoxic outcomes in women with breast cancer undergoing anthracycline or trastuzumab treatment: a systematic review and meta-analysis. Support Care Cancer. 2022;30(12):10323–34. https://doi.org/10.1007/s00520-022-07368-w.
Mavropalias G, Sim M, Taaffe DR, Galvão DA, Spry N, Kraemer WJ, Häkkinen K, Newton RU. Exercise medicine for cancer cachexia: targeted exercise to counteract mechanisms and treatment side effects. J Cancer Res Clin Oncol. 2022;148(6):1389–406. https://doi.org/10.1007/s00432-022-03927-0.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9, W64. https://doi.org/10.7326/0003-4819-151-4-200908180-00135.
Murray J, Bennett H, Bezak E, Perry R. The role of exercise in the prevention of cancer therapy-related cardiac dysfunction in breast cancer patients undergoing chemotherapy: systematic review. Eur J Prev Cardiol. 2022;29(3):463–72. https://doi.org/10.1093/eurjpc/zwab006.
Naaktgeboren WR, Binyam D, Stuiver MM, Aaronson NK, Teske AJ, van Harten WH, Groen WG, May AM. Efficacy of physical exercise to offset anthracycline-induced cardiotoxicity: a systematic review and meta-analysis of clinical and preclinical studies. J Am Heart Assoc. 2021;10(17): e021580. https://doi.org/10.1161/JAHA.121.021580.
Parry TL, Hayward R. Exercise training does not affect anthracycline antitumor efficacy while attenuating cardiac dysfunction. Am J Physiol Regul Integr Comp Physiol. 2015;309(6):R675–83. https://doi.org/10.1152/ajpregu.00185.2015.
Pfannenstiel K, Hayward R. Effects of resistance exercise training on doxorubicin-induced cardiotoxicity. J Cardiovasc Pharmacol. 2018;71(6):332–9. https://doi.org/10.1097/FJC.0000000000000574.
Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, Bandera EV, Hamilton KK, Grant B, McCullough M, Byers T, Gansler T. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–74. https://doi.org/10.3322/caac.21142.
Ross R, Blair SN, Arena R, Church TS, Després JP, Franklin BA, Haskell WL, Kaminsky LA, Levine BD, Lavie CJ, Myers J, Niebauer J, Sallis R, Sawada SS, Sui X, Wisløff U; American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Cardiovascular and Stroke Nursing; Council on Functional Genomics and Translational Biology; Stroke Council. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the american heart association. Circulation. 2016;134(24):e653–99. https://doi.org/10.1161/CIR.0000000000000461.
Russo M, Della Sala A, Tocchetti CG, Porporato PE, Ghigo A. Metabolic aspects of anthracycline cardiotoxicity. Curr Treat Options Oncol. 2021;22(2):18. https://doi.org/10.1007/s11864-020-00812-1.
Schmitz KH, Ahmed RL, Troxel A, Cheville A, Smith R, Lewis-Grant L, Bryan CJ, Williams-Smith CT, Greene QP. Weight lifting in women with breast-cancer-related lymphedema. N Engl J Med. 2009;361(7):664–73. https://doi.org/10.1056/NEJMoa0810118.
Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvão DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, Schneider CM, von Gruenigen VE, Schwartz AL; American College of Sports Medicine. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42 (7):1409–26. https://doi.org/10.1249/MSS.0b013e3181e0c112.
Shan K, Lincoff AM, Young JB. Anthracycline-induced cardiotoxicity. Ann Intern Med. 1996;125(1):47–58. https://doi.org/10.7326/0003-4819-125-1-199607010-00008.
Skovgaard D, Hasbak P, Kjaer A. BNP predicts chemotherapy-related cardiotoxicity and death: comparison with gated equilibrium radionuclide ventriculography. PLoS ONE. 2014;9(5):e96736. https://doi.org/10.1371/journal.pone.0096736.
Stenner HT, Eigendorf J, Kerling A, Kueck M, Hanke AA, Boyen J, Nelius AK, Melk A, Boethig D, Bara C, Hilfiker A, Berliner D, Bauersachs J, Hilfiker-Kleiner D, Eberhard J, Stiesch M, Schippert C, Haverich A, Tegtbur U, Haufe S. Effects of six month personalized endurance training on work ability in middle-aged sedentary women: a secondary analysis of a randomized controlled trial. J Occup Med Toxicol. 2020;15:8. https://doi.org/10.1186/s12995-020-00261-4.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Tranchita E, Murri A, Grazioli E, Cerulli C, Emerenziani GP, Ceci R, Caporossi D, Dimauro I, Parisi A. The beneficial role of physical exercise on anthracyclines induced cardiotoxicity in breast cancer patients. Cancers. 2022;14(9):2288. https://doi.org/10.3390/cancers14092288.
Varghese SS, Eekhoudt CR, Jassal DS. Mechanisms of anthracycline-mediated cardiotoxicity and preventative strategies in women with breast cancer. Mol Cell Biochem. 2021;476(8):3099–109. https://doi.org/10.1007/s11010-021-04152-y.
Yang HL, Hsieh PL, Hung CH, Cheng HC, Chou WC, Chu PM, Chang YC, Tsai KL. Early moderate intensity aerobic exercise intervention prevents doxorubicin-caused cardiac dysfunction through inhibition of cardiac fibrosis and inflammation. Cancers. 2020;12(5):1102. https://doi.org/10.3390/cancers12051102.
Yang H, Wright L, Negishi T, Negishi K, Liu J, Marwick TH. Research to practice: assessment of left ventricular global longitudinal strain for surveillance of cancer chemotherapeutic-related cardiac dysfunction. JACC Cardiovasc Imaging. 2018;11(8):1196–201. https://doi.org/10.1016/j.jcmg.2018.07.005.
Zhang N, Kong Y, Li H, Sun FY, Liu Y. Impact of aerobic exercise on cardiac function of breast cancer patients receiving anthracyclines. J Pract Med. 2016;32(19):3183–6. https://doi.org/10.3969/j.issn.1006-5725.2016.19.019.[Inchinese].
Zhang QL, Yang JJ, Zhang HS. Carvedilol (CAR) combined with carnosic acid (CAA) attenuates doxorubicin-induced cardiotoxicity by suppressing excessive oxidative stress, inflammation, apoptosis and autophagy. Biomed Pharmacother. 2019;109:71–83. https://doi.org/10.1016/j.biopha.2018.07.037.
Acknowledgements
We would like to express my deepest gratitude to our team members, Prof. Yong Wang (Cancer Hospital, Chinese Medical College of Science) and Prof. Lijing Gong (Beijing Sport University), for their kindness and patience. Their have valuable guidance has been a constant support at every stage of our research. Furthermore, we would like to thank the laboratory of the Ministry of Education, Beijing Sport University, for providing an environment to complete this review.
Funding
This work was supported by the National Key Research and Development Program Project (2020YFC2006705).
Author information
Authors and Affiliations
Contributions
HL: data curation, writing—original draft. HL: writing—review and editing. BW: writing—review and editing. XJ: writing—review and editing. JY: writing—review and editing. YZ: writing—review and editing. DS: writing—review and editing. YZ: conceptualization, methodology, writing—review and editing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
All authors have read and approved the publication of this manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, H., Liu, H., Wang, B. et al. Exercise Interventions for the Prevention and Treatment of Anthracycline-Induced Cardiotoxicity in Women with Breast Cancer: A Systematic Review. J. of SCI. IN SPORT AND EXERCISE (2024). https://doi.org/10.1007/s42978-023-00256-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42978-023-00256-7