Abstract
Purpose
Post-exercise hypotension (PEH) following prolonged dynamic exercise is induced by an increase in systemic vascular conductance via skeletal muscle vasodilation, which may occur not only in the arteries but also in the veins, and the vasodilated regions may contribute to greater venous pooling in the exercised limbs. However, the contribution of venous distention to PEH is unclear. Therefore, we aimed to evaluate venous compliance in previously exercised muscles in a preliminary study.
Methods
Seven participants performed a single 60-min session of upright cycle ergometry at 60% of heart rate reserve. Calf venous compliance was measured using venous congestion plethysmography before and 45 min following exercise in Trial 1. Stroke volume and femoral arterial blood flow were determined using ultrasonography at the same time points in Trial 2. Heart rate and blood pressure were monitored during both trials.
Results
Mean arterial pressure significantly decreased between the start and ~ 30 min following the end of exercise (82 ± 6 mmHg vs. 76 ± 6 mmHg, respectively, in Trial 1; 79 ± 6 mmHg vs. 74 ± 5 mmHg, respectively, in Trial 2; P < 0.05). Pre- and post-exercise stroke volume did not significantly differ. Blood flow and vascular conductance in the femoral artery significantly increased from 641 ± 84 mL/min and 8.13 ± 0.79 mL/min/mmHg pre-exercise to 773 ± 121 mL/min and 10.55 ± 1.00 mL/min/mmHg post-exercise, respectively (P < 0.05). Pre- and post-exercise calf venous compliance did not significantly differ.
Conclusion
Our findings demonstrate that vasodilation in the active limb increases during exercise, but the resulting PEH does not affect venous compliance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Physical activity is well known to reduce the risk of cardiovascular disease; for example, regular aerobic and dynamic resistance training have anti-hypertensive effects [10, 15, 25, 28]. In addition, an acute period of dynamic exercise reduces arterial blood pressure in both healthy individuals and patients with hypertension [5, 9, 21]. Post-exercise hypotension (PEH) has a positive effect to prevent hypertension, but may also be associated with severe orthostatic hypotension following exercise that predisposes toward syncope [8, 20].
In most individuals, PEH is induced by an increase in the systemic vascular conductance that is incompletely offset by the ongoing greater cardiac output [5, 9, 21]. PEH is closely related to systemic vascular conductance, which is the sum of the vascular conductances of the parallel arteries supplying several organs and tissues. Previous studies have shown that PEH arises from the greater vascular conductance mainly resulted from vasodilation in the skeletal muscles of the exercised and non-exercised limbs, but not in the splanchnic and renal circulations [2, 29].
Previous studies of PEH have focused primarily on the arterial response, whereas little attention has been given to the post-exercise venous response. These studies have consistently shown that cardiac output is maintained by an increase in heart rate, even though stroke volume is lower following exercise [14, 31]. Stroke volume is affected by venous return, which is reduced by venous pooling in regions such as the lower limbs and viscera. After dynamic aerobic exercise, there is an increase venous pooling in the legs due to sudden cessation of muscle pumping [5, 16] and sympathoinhibition [6]. Venous walls contain sparsely innervated smooth muscle [33], which may result in increased venous compliance due to reduced venoconstriction, attributed to sympathoinhibition following exercise. Thus, the more substantial venous pooling caused by greater venous compliance in the previously exercised limb during recovery may play a role in determining the degree of PEH. In addition, the sustained increase of arterial inflow in the exercised limbs during PEH may contribute to venous retention. However, the venous vascular response during PEH has been poorly characterized. Therefore, we current study aimed to better characterize the change in venous compliance associated with hypotension following dynamic exercise. The results of this study provide essential insights into the impact of acute exercise on venous hemodynamic response.
Methods
Participants
The required sample size was calculated using PS software (version 3.1.6). We aimed to identify a 5-mmHg difference in mean arterial blood pressure before and after exercise (i.e., post-exercise hypotension). The sample size, calculated using an α of 0.05 and a power of 0.80, was seven. Therefore, seven healthy, normotensive, and non-smoking volunteers (five men and two women) aged 20–32 years, with a mean ± SD height, body mass, and body mass index (BMI) of 172 ± 10 cm, 60 ± 8 kg, and 20.3 ± 1.8 cm/m2, respectively, participated in this preliminary study. Written informed consent was obtained prior to participation. The study protocol was approved by the Institutional Ethics Committee of the Prefectural University of Hiroshima and it was conducted in accordance with the guidelines of the Declaration of Helsinki.
The participants were sedentary in terms of their normal day-to-day activity, performing no regular endurance training and participating in < 2 h of aerobic exercise per week. Each participant underwent an examination before performing the main protocol. The participants arrived at the laboratory after abstaining from caffeine and exercise for at least 1 day, and from food and drink for at least 3 h prior to performing the main protocol. Each participant underwent an incremental cycling exercise test using a bicycle ergometer (232CXL; Combi, Tokyo, Japan) to determine their appropriate work rate for the main protocol. They performed three 5-min sessions of continuous graded cycle ergometer exercise at 30, 60, and 90 W each, while maintaining a cadence at 60 r/min, followed by a 3-min rest in an upright position. Their heart rates were monitored using three-lead electrocardiography (ECG) (BP-88 S; Colin, Tokyo, Japan) during the exercise. To determine their work rates during the main protocol, a linear graph of the relationship between their steady-state heart rates at the end of each stage and the work rates was created, and the work rate corresponding to 60% of the resting heart rate (RHR) reserve (calculated as 0.6 × [(220 − age) − RHR] + RHR) was estimated. The room temperature was maintained at 24 ± 1 °C throughout the trial with a thermal feedback device.
Protocol
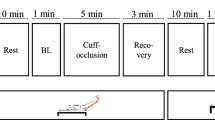
The main protocol, which consisted of a 60-min period of upright cycling at 60 rpm and individually determined work rate, was performed twice on separate days. Female participants performed during the follicular phase of their menstrual cycles. The participants arrived in the laboratory at 10:00, whereupon each individual emptied their bladder and changed into shorts and a t-shirt. Following this, they laid down in a supine position for the application of instuments and remained in this position for at least 30 min. Calf venous compliance (Trial 1) and central and femoral hemodynamics (Trial 2) were assessed in a random order. In both trials, participants remained in a supine position for 30 min before commencing the exercise and continued in the same position for an additional 60 min after concluding the exercise session. Calf venous compliance and central and femoral hemodynamic parameters were measured 15 min before the exercise and 45 min after the exercise (Fig. 1).
Scheme of the protocol. Calf venous compliance (Trial 1) and central and femoral hemodynamics (Trial 2) were assessed in a random order. In both trials, the participants remained in a supine position for 30 min before exercise and for an additional 60 min after the exercise period. The exercise consisted of a 60-min upright cycling at 60% of heart rate reserve. Calf venous compliance and central and femoral hemodynamic parameters were measured 15 min before the exercise (pre) and 45 min after the exercise (post)
Measurements
Heart rate was continuously monitored using a three-lead ECG throughout both trials. Blood pressure was monitored using an autonomic manometer (BP-306; Colin), and with a cuff placed on the left arm, every 5 min starting from 30 min before the start of exercise and continuing until 60 min after the end of exercise during both Trials 1 and 2. Blood pressure during exercise was measured during both trials using a mercury manometer at 20 and 50 min after the start of exercise, conducted on the left arm, aligned with the level of the heart.
Central and Femoral Hemodynamics
Stroke volume was determined using an echocardiograph (EUB6500; Hitachi Medical, Tokyo, Japan). To obtain a parasternal long-axis view, a 2.5-MHz annular phased-array sector probe was placed at the point of maximal cardiac impulse on the chest wall, in the third intercostal space. Beat-by-beat measurements of left ventricular volumes were conducted by the same investigator during all the trials, and each measurement was made for at least 10 s (6–9 beats). The transducer was placed at the same anatomic position during each measurement period, with care taken to keep the same images on the screen throughout the experiments. Simultaneous recording of M-mode and two-dimensional B-mode images were acquired and stored on the hard disk of the machine, later archived onto a digital video disc for subsequent offline analysis. Data were subsequently digitally converted, and the R–R intervals, end-diastolic diameters (EDDs), and end-systolic diameters (ESDs) were analyzed using image analysis software (Image J; NIH, Bethesda, Maryland) on a PC. The left ventricular ejection fraction was calculated using Pombo’s formula, in which end-diastolic volume (EDV) = π/3*EDD3 and end-systolic volume (ESV) = π/3*ESD3. Stroke volume was calculated as EDV − ESV. Cardiac output was calculated as stroke volume multiplied by heart rate (determined by measuring the R–R interval).
The blood flow in the right femoral artery was determined using simultaneous pulse, and echo-Doppler ultrasonography was used to measure the mean blood velocity (MBV) and arterial blood vessel diameter. The MBV was obtained on a beat-by-beat basis using a pulsed Doppler ultrasonography apparatus (LOGIQS6; GE Medical System, Tokyo, Japan) and a linear 5.0-MHz probe at an insonation angle of 55–60°. The diameter was measured simultaneously using an imaging frequency of 12.0 MHz. The femoral artery was assessed at a site approximately 2–3 cm distal to the inguinal ligament of the right thigh. The sampling volume was positioned in the center of the vessel and adjusted to cover its width. Doppler trace data for each cardiac cycle were analyzed using LOGIQS6 software, and the MBV for each target artery was calculated. Blood flow was calculated using the MBV and cross-sectional area of each vessel, and normalized to a time constant of 1 s. Blood flows were measured over a 15-min period 15 min before and 45 min after the end of the exercise.
Calf Venous Compliance
Calf venous compliance was measured using venous congestion plethysmography according to models described by Halliwill et al. [7, 35]. Changes in limb volume were measured using mercury-in-silastic strain gauges (model EC6; D. E. Hokanson, Indianapolis, IN, USA). The strain gauges were electronically calibrated in situ using circuitry developed by Hokanson et al. [12] that produces a 1% rise in gauge resistance. This change in resistance is equivalent to a 0.5% increase in gauge length or limb circumference, and is, therefore, equivalent to a 1% rise in limb volume. Changes in limb volume are expressed as mL per deciliter of tissue. A venous collecting cuff (18 cm in width) was placed around the right thigh proximal to the knee, and a strain gauge (30–36 cm in length) was placed around the calf at the point of maximum girth. The pressure in the venous collecting cuff was controlled with an electromagnetic valve connected to the cuff and a compressed air cylinder. The electromagnetic valve was controlled by an electric signal converted via D/A from the computer. The collecting cuff on the right thigh was inflated to 60 mmHg for 8 min after 2 min of baseline recording (no cuff inflation), and then deflated at 1 mmHg/s over 1 min to 0 mmHg. A rapid increase in limb volume was evoked within 3–4 min of increasing the cuff pressure to 60 mmHg, representing the maximum blood volume stored in the veins at the given pressure, followed by a slower, fairly linear volume increase caused by net filtration into the extravascular space.
The filtration-dependent increase was calculated using a model developed by Skoog et al. [35], in which limb volume is corrected during cuff pressure inflation and deflation. We assessed the venous vascular properties using the corrected limb volume curve. Pressure-volume curves were generated from the pressure-volume relationship as the pressure was reduced from 60 mmHg to 10 mmHg at 1 mmHg/s. Data points below 10 mmHg were excluded, owing to the difficulty of accurately measuring the true intravenous pressure at low cuff pressures [7]. Pressure-volume curves were created using the quadratic regression model: (Δlimb volume) = β0 + β1 × (cuff pressure) + β2 × (cuff pressure)2, where Δ is the change. The regression parameters β1 and β2 were used together as an estimate of compliance, such that compliance = β1 + β2 × 2 × (cuff pressure); i.e., compliance was defined as a derivative of the pressure-volume curve. Calf venous capacitance was evaluated as the change in limb volume between the start of cuff inflation and 8 min following cuff inflation at 60 mmHg. Calf venous outflow was calculated using the rate of change in limb volume over the 1 min taken for cuff deflation from 60 to 0 mmHg.
The coefficient of variation for plethysmography measurements taken 1 week apart from seven healthy participants was calculated to be 9.7% (1.9%–18.9%).
Statistical Analysis
Values are expressed as mean ± SD. The effects of Trials 1 and 2 on the temporal changes in heart rate and blood pressure were evaluated using a two-way analysis of variance (ANOVA). To test the effect of time on each variable, repeated-measures ANOVA was used. When a significant difference was identified, this was compared with the pre-exercise value using Dunnett’s post-hoc test. To identify differences in hemodynamic parameters before and after exercise (45 min; during the same phase as the venous compliance measurement), the paired t-test was used. The effects of condition (pre- or post-exercise) and pressure on venous compliance and limb volume were evaluated using two-way ANOVA, and when a significant difference was identified, this was further interrogated using a paired t-test. Statistical significance was accepted when P < 0.05. Data were analyzed using SPSS v.12.0 for Windows (IBM, Chicago, IL, USA).
Results
Effects of Exercise
The heart rate and blood pressure of the participants during exercise did not significantly differ between Trials 1 and 2. In both trials, participants exhibited a significant increase in systolic blood pressure (SBP) and mean arterial blood pressure (MAP) during exercise (Table 1; before: SBP 122 ± 10 mmHg and MAP 82 ± 6 mmHg; after 50 min of exercise: SBP 136 ± 11 mmHg and MAP 86 ± 6 mmHg in Trial 1; before: SBP 115 ± 12 mmHg and MAP 79 ± 6 mmHg; after 50 min of exercise: SBP 148 ± 17 mmHg and MAP 88 ± 4 mmHg in Trial 2; P < 0.05). Conversely, diastolic blood pressure (DBP) did not change significantly (before: DBP 63 ± 6 mmHg; after 50 min of exercise: DBP 60 ± 8 mmHg in Trial 1; before: DBP 61 ± 7 mmHg; after 50 min of exercise: DBP 58 ± 10 mmHg in Trial 2; P > 0.05). The heart rate of the participants significantly increased during exercise (before: 60 ± 10 beats/min; after 50 min of exercise: 132 ± 5 beats/min in Trial 1; before: 54 ± 10 beats/min; after 50 min of exercise: 134 ± 8 beat/min in Trial 2; P < 0.05).
Post-exercise Changes
The time-courses of the post-exercise changes in heart rate and blood pressure did not significantly differ between Trials 1 and 2 (P > 0.05). The heart rate of the participants had largely recovered by 25 min post-exercise (before: 57 ± 10 beats/min; 25 min post-exercise: 64 ± 13 beats/min in Trial 1; before: 54 ± 10 beats/min; 25 min post-exercise: 61 ± 11 beats/min in Trial 2). MAP consistently began to decrease in the participants approximately 30 min after the conclusion of exercise (before: 82 ± 6 mmHg; 30 min post-exercise: 78 ± 6 mmHg in Trial 1; before: 79 ± 6 mmHg; 30 min post-exercise: 74 ± 5 mmHg in Trial 2).
Central and Femoral Hemodynamics
The central and femoral hemodynamic data before and 45 min after the end of exercise in Trial 2 are shown in Fig. 2. SBP and DBP significantly decreased during recovery (before: 115 ± 12 and 61 ± 7 mmHg; after: 107 ± 11 and 56 ± 6 mmHg, P < 0.05). Heart rate (Fig. 2D), stroke volume (Fig. 2E), and cardiac output had not changed significantly 45 min after the end of exercise when compared to their pre-exercise levels (heart rate, before: 54 ± 10, after: 57 ± 12 beats/min; stroke volume, before: 91 ± 24, after: 87 ± 13 mL; cardiac output, before: 4.66 ± 0.98, after: 5.01 ± 1.32 L/min; P > 0.05). As a result, systemic vascular conductance (Fig. 2F) tended to increase during the post-exercise period, but the magnitude of this change did not reach significance (before: 59.1 ± 11.8, post: 67.9 ± 13.5 mL/min/mmHg; P = 0.107). Arterial blood flow (Fig. 2G) and vascular conductance (Fig. 2H) in the leg were significantly higher after exercise (blood flow, before: 641 ± 84, after: 773 ± 121 mL/min; vascular conductance, before: 8.13 ± 0.79, after: 10.55 ± 1.00 mL/min/mmHg; P < 0.05).
Responses of blood pressure, heart rate, stroke volume, systemic vascular conductance, femoral arterial blood flow, leg vascular conductance, calf venous capacitance, and venous outflow at baseline (pre-exercise [pre]) and 45 min following exercise (post). Values are means ± SD. P values were calculated using the paired t-test. # P < 0.05 vs. baseline
Calf Venous Compliance
The calf volume and venous compliance, corrected for net fluid filtration, before and after exercise (45 min after cessation) in Trial 1, are shown in Fig. 3. The pre- and post-exercise volume changes in response to venous occlusion did not differ significantly (P = 0.090). β0 after exercise was lower than that pre-exercise (P < 0.05); however, β1 and β2 were not significantly changed by exercise (before: y = 0.3691 ± 0.2261 + 0.1064 ± 0.0199 × (cuff pressure) − 0.00099 ± 0.00019 × (cuff pressure)2; after: y = − 0.4502 ± 0.3790 + 0.1162 ± 0.0173 × (cuff pressure) − 0.00105 ± 0.00016 × (cuff pressure)2, β1; P = 0.256, β2; P = 0.528). Thus, venous compliance in the calf did not increase during the PEH following a 60-min cycling exercise. In addition, the calf venous capacitance (Fig. 2I) and outflow (Fig. 2J) were similar before and after exercise. Therefore, the venous vascular properties of the calf did not change during PEH.
Discussion
The objective of the present preliminary study was to determine whether calf venous compliance increases during PEH. The main findings are that the post-exercise calf venous vascular properties do not differ from the pre-exercise properties, and that arterial inflow and vasodilation increase following exercise in the previously active limb, but this does not result in greater venous pooling.
The arterial responses underlying PEH have been well studied. However, venous responses to exercise, including vessel properties, may also be important determinants of the extent of PEH. The veins usually contain approximately two-thirds of the total blood volume at rest; therefore, small changes in the venous bed can substantially affect blood pressure control [17, 32, 33]. For example, Hagberg et al. [4] found that PEH is induced by the relatively lower cardiac output of older participants with hypertension, even though their systemic vascular resistance is high. This decrease in cardiac output has been attributed to a reduction in stroke volume, secondary to insufficient venous return following exercise [1, 17, 33]. This phenomenon has also been observed in endurance-trained men [34]. In addition, chronic exercise training has been shown to be associated with higher venous compliance in young and older participants [11, 24]. Finally, some studies have demonstrated that venous compliance increases after endurance training for 6–8 weeks [13, 26]. Thus, venous compliance might be increased by an acute bout of exercise because of greater venous pooling [5], less venoconstriction [3, 6], and greater venous distention [5]. However, in the present study, we found no differences in the stroke volume or venous compliance (β1 and β2), or in the capacitance or outflow before and after exercise, even though the arterial blood flow and vascular conductance in the leg significantly increased after exercise.
Stewart et al. [36] compared the calf blood flow, venous capacitance, compliance in participants with postural tachycardia syndrome (POTS) and a control group, both in a supine position and during head-up tilt testing. The study revealed that there were no significant differences in calf venous capacitance and compliance between the two groups or between postures. However, the calf vascular resistance was significantly lower in participants with POTS than in the controls. This result suggests that the pooling observed in patients with POTS is a result of blunted arterialvasoconstriction in the limbs, rather than any significant venous changes. These data are consistent with the results of the present study, in which we found that the venous vascular properties of previously exercised limbs are not directly linked to PEH and are unaffected by greater arterial vasodilation and flow. Although we have not determined the mechanism underlying the vasodilation in the active limb during the hypotension that follows aerobic exercise, this vasodilation is likely to be mediated through changes in the local release of vasodilator substances. Increases in the release of nitric oxide, histamine, and prostaglandins may contribute to the change in TVC following exercise [8, 19, 23]. Nevertheless, the mechanism of PEH remains to be determined.
Calf venous compliance is often evaluated using venous occlusion plethysmography, according to the method described by Halliwill et al. [7]. This method evaluates venous compliance based on the overall volume increase during venous stasis, without separating the net fluid filtration and the venous capacitance responses. The pressure-volume curve and β0 would have been influenced by the unstressed volume, resting venous pressure, fluid leak, and fluid accumulation, as a result of the congestion associated with cuff inflation. In addition, this method may influence post-capillary venous pressure, owing to changes in blood flow [7, 32, 33]. Therefore, calf venous compliance was estimated using a model developed by Skoog et al. [35] in the present study. In this model, limb volume is corrected for net fluid filtration, to provide an estimate of the change in limb volume owing to blood pooling. Therefore, the venous compliance measured by the model employed in the present study might have been affected by venous responses. Most previous studies of venous compliance have been performed during orthostatic stress [35, 36] or exercise training [11, 13, 24, 26]. To our knowledge, this is the first study to compare the pre- and post-exercise calf venous compliances and evaluate venous vascular properties during PEH.
The present study had several limitations. We calculated the required sample size using the mean arterial blood pressure as the output. However, had we determined the required sample size using the difference in venous compliance (β1) between the arm and leg, as per a previous study [7], it would have been 149 (PS software, version 3.1.6). Therefore, the results of this preliminary investigation should be interpreted with caution. In addition, the study was performed with participants in a supine position. Previous studies have shown that a supine position promotes an increase in TVC, whereas a seated position is associated with a decrease in cardiac output during PEH [30]. Therefore, in the present study, the seated position may have reduced venous return, secondary to a reduction in venous compliance during PEH, subsequently leading to reductions in stroke volume and cardiac output. Therefore, further studies are needed to better understand venous compliance during PEH.
Clinical Applications
Physical activity has been shown to be an important means of preventing the development of hypertension in both normotensive and prehypertensive individuals. Indeed, aerobic and dynamic resistance exercise training, whether individually or in combination, were found to be equally effective in lowering blood pressure in normotensive, prehypertensive, and hypertensive individuals [28]. The extent of PEH correlates with the magnitude of the blood pressure reduction achieved with exercise training. This result suggests that the long-term reduction in blood pressure is largely the result of PEH [10]. Therefore, PEH may represent an easy-to-use predictor for the efficacy of exercise training as an antihypertensive lifestyle measure [37].
Although the present study was a preliminary investigation with a small sample size, it suggested that, unlike arterial properties, the venous vascular properties of the exercised limbs remained unchanged during the hypotension that follows acute exercise in young normotensive participants. Previous studies have shown that venous compliance is reduced by the high venomotor tone observed at rest in hypertensive rats [22, 27]. There is also evidence to suggest that sympathetic nerve activity decreases following exercise in hypertensive humans [36] and animals [18]. In addition, PEH is induced by the lower stroke volume in older individuals with hypertension and endurance-trained men [4, 34]. Venous function may thus be a determinant of PEH, especially in hypertension patients and endurance-trained men; therefore, further investigations are warranted.
Availability of data and materials
The datasets analyzed in this study are not publicly available due to a privacy policy but are available from the corresponding author on reasonable request.
Abbreviations
- ANOVA:
-
Analysis of variance
- DBP:
-
Diastolic blood pressure
- ECG:
-
Electrocardiogram
- EDD:
-
End-diastolic diameter
- EDV:
-
End-diastolic volume
- ESD:
-
End-systolic diameter
- ESV:
-
End-systolic volume
- MAP:
-
Mean arterial blood pressure
- MBV:
-
Mean blood velocity
- PEH:
-
Post-exercise hypotension
- POTS:
-
Postural tachycardia syndrome
- RHR:
-
Resting heart rate
- SBP:
-
Systolic blood pressure
References
Brito LC, Queiroz ACC, Forjaz CLM. Influence of population and exercise protocol characteristics on hemodynamic determinants of post-aerobic exercise hypotension. Braz J Med Biol Res. 2014;47(8):626–36.
Endo MY, Shimada K, Miura A, Fukuba Y. Peripheral and central vascular conductance influence on post-exercise hypotension. J Physiol Anthropol. 2012;31(1):32. https://doi.org/10.1186/1880-6805-31-32.
Floras JS, Sinkey CA, Aylward PE, Seals DR, Thoren PN, Mark AL. Postexercise hypotension and sympathoinhibition in borderline hypertensive men. Hypertension. 1989;14(1):28–35.
Hagberg JM, Montain SJ, Martin WH. Blood pressure and hemodynamic responses after exercise in older hypertensives. J Appl Physiol. 1987;63(1):270–6.
Halliwill JR. Mechanisms and clinical implications of post-exercise hypotension in humans. Exerc Sport Sci Rev. 2001;29(2):65–70.
Halliwill JR, Taylor JA, Eckberg DL. Impaired sympathetic vascular regulation in humans after acute dynamic exercise. J Physiol. 1996;495(Pt1):279–88.
Halliwill JR, Minson CT, Joyner MJ. Measurement of limb venous compliance in humans: technical considerations and physiological findings. J Appl Physiol. 1999;87(4):1555–63.
Halliwill JR, Sieck DC, Romero SA, Buck TM, Ely MR. Blood pressure regulation X: what happens when the muscle pump is lost? Post–exercise hypotension and syncope. Eur J Appl Physiol. 2013;114(3):561–78.
Hamer M. The anti-hypertensive effects of exercise: integrating acute and chronic mechanisms. Sports Med Rev. 2006;36(2):109–16.
Hecksteden A, Grütters T, Meyer T. Association between postexercise hypotension and long-term training-induced blood pressure reduction: a pilot study. Clin J Sport Med. 2013;23(1):58–63.
Hernandez JP, Franke WD. Effects of a 6-mo endurance-training program on venous compliance and maximal lower body negative pressure in older men and women. J Appl Physiol. 2005;99(3):1070–7.
Hokanson DE, Sumner DS, Strandness DE Jr, Hokanson. An electrically calibrated plethysmograph for direct measurement of limb blood flow. IEEE Trans Biomed Eng. 1975;22(1):2.
Iida H, Nakajima T, Kurano M, Yasuda T, Sakamaki M, Sato Y, Yamasoba T, Abe T. Effects of walking with blood flow restriction on limb venous compliance in elderly subjects. Clin Physiol Funct Imaging. 2011;31(6):472–6.
Jones H, George K, Edwards B, Atkinson G. Is the magnitude of acute post-exercise hypotension mediated by exercise intensity or total work done? Eur J Appl Physiol. 2007;102:33–40.
Joyner MJ, Green DJ. Exercise protects the cardiovascular system: effects beyond traditional risk factors. J Physiol. 2009;587(Pt 23):5551–8.
Kenney MJ, Morgan DA. Sciatic nerve stimulation induces hypotension but not renal or lumbar sympathoinhibition in hypertensive Dahl rats. Clin Auton Res. 1993;3(3):163–8.
Kenney MJ, Morgan DA, Mark AL. Sympathetic nerve responses to sustained stimulation of somatic afferents in Dahl rats. J Hypertens. 1991;9(10):963–8.
Kulics JM, Collins HL, DiCarlo SE. Postexercise hypotension is mediated by reductions in sympathetic nerve activity. Am J Physiol. 1999;276(1):H27–32.
Lockwood JM, Wilkins BW, Halliwill JR. H1 receptor-mediated vasodilatation contributes to postexercise hypotension. J Physiol. 2005;563(Pt 2):633–42.
Lucas SJ, Cotter JD, Murrell C, Wilson L, Anson JG, Gaze D, George KP, Ainslie PN. Mechanisms of orthostatic intolerance following very prolonged exercise. J Appl Physiol. 2008;105(1):213–25.
MacDonald JR. Potential causes, mechanisms, and implications of post exercise hypotension. J Hum Hypertens. 2002;16(4):225–36.
Martin DS, Rodrigo MC, Appelt CW. Venous tone in the developmental stages of spontaneous hypertension. Hypertension. 1998;31(1):139–44.
McCord JL, Beasley JM, Halliwill JR. H2-receptor-mediated vasodilation contributes to postexercise hypotension. J Appl Physiol. 2006;100(1):67–75.
Monahan KD, Dinenno FA, Seals DR, Halliwill JR. Smaller age-associate reductions in leg venous compliance in endurance exercise-trained men. Am J Physiol. 2001;281(3):H1267–73.
Mora S, Cook N, Buring JE, Ridker PM, Lee IM. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116(19):2110–8.
Oue A, Saito M, Iimura Y. Effect of short-term endurance training on venous compliance in the calf and forearm differs between continuous and interval exercise in humans. Physiol Rep. 2019;7(17):e14211. https://doi.org/10.14814/phy2.14211.
Overbeck HW. Hemodynamics of early experimental renal hypertension in dogs. Normal limb blood flow, elevated limb vascular resistance, and decreased venous compliance. Circ Res. 1972;31(5):653–63.
Pescatello LS, Buchner DM, Jakicic JM, Powell KE, Kraus WE, Bloodgood B, Campbell WW, Dietz S, Dipietro L, George SM, Macko RF, McTiernan A, Pate RR, Piercy KL. Physical activity to prevent and treat hypertension: a systematic review. Med Sci Sports Exerc. 2019;51(6):1314–23.
Pricher MP, Holowatz LA, Williams JT, Lockwood JM, Halliwill JR. Regional hemodynamics during postexercise hypotension. I. Splanchnic and renal circulations. J Appl Physiol. 2004;97(6):2065–70.
Raine NM, Cable NT, George KP, Campbell IG. The influence of recovery posture on post-exercise hypotension in normotensive men. Med Sci Sports Exerc. 2001;33(3):404–12.
Rezk CC, Marrache RC, Tinucci T, Mion D Jr, Forjaz CL. Post-resistance exercise hypotension, hemodynamics, and heart rate variability: influence of exercise intensity. Eur J Appl Physiol. 2006;98(1):105–12.
Rothe CF. Reflex control of veins and vascular capacitance. Physiol Rev. 1983;63(4):1281–342.
Rowell LB. Human cardiovascular control. New York: Oxford University Press; 1993.
Senitko AN, Charkoudian N, Halliwill JR. Influence of endurance exercise training status and gender on postexercise hypotension. J Appl Physiol. 2002;92(6):2368–74.
Skoog J, Lindenberger M, Ekman M, Holmberg B, Zachrisson H, Länne T. Reduced venous compliance: an important determinant for orthostatic intolerance in women with vasovagal syncope. Am J Physiol. 2016;310(3):R253–61.
Stewart JM. Pooling in chronic orthostatic intolerance: arterial vasoconstrictive but not venous compliance defects. Circulation. 2002;105(19):2274–81.
Wegmann M, Hecksteden A, Poppendieck W, Steffen A, Kraushaar J, Morsch A, Meyer T. Postexercise hypotension as a predictor for long-term training-induced blood pressure reduction: a large-scale randomized controlled trial. Clin J Sport Med. 2018;28(6):509–15.
Funding
This study was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Sports, Science, and Technology of Japan (no.16K01728 to MYE).
Author information
Authors and Affiliations
Contributions
MYE conceived and designed research; MK and HK performed experiments; MYE and HK analyzed data; YF, AO and AM interpreted results of experiments; MYE prepared figures; MYE and AO drafted manuscript; MYE and AO edited and revised manuscript; All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Consent to participate
Written informed consent was obtained from all participants.
Consent to publication
The authors affirm that all participants provided informed consent for the publication of all the data and Figures.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. The study was approved by the Ethics Committee of Prefectural University of Hiroshima (Approval No. HH005).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Endo, M.Y., Kanda, M., Fukuba, Y. et al. Calf Venous Compliance During Hypotension After an Acute bout of Aerobic Exercise in Humans. J. of SCI. IN SPORT AND EXERCISE (2023). https://doi.org/10.1007/s42978-023-00252-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42978-023-00252-x