Abstract
Muscular hypertrophy depends on metabolic exhaustion as well as mechanical load on the muscle. Mechanical tension seems to be the crucial factor to stimulate protein synthesis. The present meta-analysis was conducted to determine whether stretching can generate adequate mechanical tension to induce muscle hypertrophy. We used PubMed, Web of Science, and Scopus to search for literature examining the effects of long-term stretching on muscle mass, muscle cross-sectional area, fiber cross-sectional area, and fiber number. Since there was no sufficient number of studies investigating long-lasting stretching in humans, we only included original animal studies in the current meta-analysis. Precisely, we identified 16 studies meeting the inclusion criteria (e. g. stretching of at least 15 min per day). The 16 studies yielded 39 data points for muscle mass, 11 data points for muscle cross-sectional area, 20 data points for fiber cross-sectional area, and 10 data points for fiber number. Across all designs and categories, statistically significant increases were found for muscle mass (d = 8.51; 95% CI 7.11–9.91), muscle cross-sectional area (d = 7.91; 95% CI 5.75–10.08), fiber cross-sectional area (d = 5.81; 95% CI 4.32–7.31), and fiber number (d = 4.62; 95% CI 2.54–6.71). The findings show an (almost) continuous positive effect of long-term stretching on the listed parameters, so that it can be assumed that stretch training with adequate intensity and duration leads to hypertrophy and hyperplasia, at least in animal studies. A general transferability to humans—certainly with limited effectiveness—can be hypothesized but requires further research and training studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To achieve muscular hypertrophy, strength training needs—in addition to metabolic exhaustion—a high mechanical load on the muscle, which leads to micro-traumatization of the muscle fibers [63]. In this regard, the crucial factor is high mechanical tension on the muscle. Resulting hypertrophy effects depend on an increased (myofibrillar) protein synthesis rate, which is stimulated via corresponding signaling pathways. In particular, activation of the Akt/mTOR/p70S6K signaling pathway appears to be of high importance for the stimulation of muscular protein synthesis and is primarily induced by mechanical loading [1, 17, 46]. A corresponding mechanical stimulus can be initiated not only by high loads in strength training, but also through stretching with appropriate intensity. Smith et al. [55] demonstrated that mechanical stress generated by stretching can be sufficient to induce delayed onset muscle soreness (DOMS) [55]. Accordingly, it can be assumed that stretching stimuli can cause adequate micro-traumatization. The resulting repair processes can trigger hypertrophy-stimulating signaling pathways to increase protein synthesis rates [29]. The resulting activation of stretch-activated channels alters the cytoplasmic membrane and initiates signal transduction processes via mTOR [59, 61].
Against this background, the following hypothesis can be derived: stretch training performed with sufficient intensity leads to high mechanical load that can trigger muscular hypertrophy as a long-term training effect. This hypothesis has already been discussed previously: “It is well known that application of chronic stretch is a very potent model for inducing muscle enlargement” [36]. However, to date, studies examining adaptations of stretch training have generally focused either on increasing range of motion (ROM), or on other parameters describing flexibility [38, 40]. Moreover, acute effects of stretching interventions on muscular performance mostly show negative effects regarding maximum strength and explosive power [13, 71].
Initial human studies show that long-term stretching interventions for several weeks can induce hypertrophic effects and/or increase maximum strength. For example, Simpson et al. [53] were able to achieve an average increase of 5.6% in muscle cross-sectional area through a stretching intervention with a duration of three minutes, three days per week, for 6 weeks. Panidi et al. [44] found an increase in muscle cross sectional area (MCSA) of 23% ± 14% after a 12-week stretching intervention with stretching durations up to 15 min per training session. Nelson et al. [43] demonstrated a 29% increase in maximal strength after stretching the calf muscles for 4 × 30 s, 3 days a week for 10 weeks. In addition, Kokkonen et al. [34] achieved significant improvements in various performance tests, such as 1 RM knee extension and knee flexion, standing long jump, and high jump, with static stretching for 40 min per session, 3 days per week for 10 weeks.
Longitudinal studies using animal experiments have been available for some time and have demonstrated significant hypertrophy effects after continuous stretching from 30 min to 24 h per day over an intervention period of several weeks, reflected by an increase in muscle mass (MM), MCSA, fiber cross sectional area (FCSA) and/or hyperplasia effects with an increased fiber number (FN) [8, 10, 15, 23, 25]. Data of muscle weight were collected by removing the connective tissue and weighing the wet muscle weight. MCSA and FN were investigated by placing the muscle in a solution in which the different muscle fibers were stained in different colors (fast twitch fiber stained lightly, slow twitch fibers stained darkly). Subsequently, the muscle cross-section and fiber cross-sectional area were determined from a given number of fibers (for example 500 slow twitch and 200 fast twitch fibers in Antonio et al. [10] using light micrography images and an image analysis computer program). In addition, in vitro condition a significant increase in maximum strength was demonstrated by continuous stretching, so that these hypertrophy effects are functional in animals [3, 4]. The muscle fiber type was determined by ATPase-activity using an ATPase staining method and fiber number was investigated by counting fibers running from origin to insertion [10]
Since animal studies play a vital role in research to investigate human health, and systematic reviews or meta-analyses provide a suitable basis for drawing evidence-based conclusions concerning a research topic, we decided to create a transparent overview of the available information on effects of long-lasting stretching intervention on muscle tissue, especially to check if the applicability of the training method appears worthwhile for human studies [31]. There is one meta-analysis available from Kelley [33] that has addressed this issue before. In Kelley’s meta-analysis, however, the muscular overload was not generated exclusively by stretching but also by other methods (weight training, ablation), so that no conclusion could be drawn about the specific effects of long-term stretching. Moreover, comprehensive analysis on distinct outcomes such as MM, MCSA, FCSA and FN are not available in the study by Kelley [33]. Consequently, a distinct base of empirical evidence needs to be researched to investigate the questions of the present meta-analysis. In particular, the present meta-analysis of animal studies aims to provide a comprehensive and differentiated overview of the effects of (continuous) stretching interventions on MM, MCSA and FCSA, and on hyperplasia effects (FN). Subsequently, the relevance of these results with regard to the potential use of stretching training with the goal of muscle and strength building in athletic and therapeutic training will be discussed.
Methods
The following search terms were defined to search PubMed, Web of Science, and Scopus databases: [(“hypertrophy” OR “hyperplasia”) AND (“stretch-induced growth” OR “stretch-induced hypertrophy” OR “fiber number” OR “fiber length” OR “sarcomere length” OR “sarcomere number”) AND “skeletal muscle”) NOT (“exercise induced” OR “endocrine” OR “nervous system” OR “electrical stimulation” OR “cardiomyocytes”]. The search strategy was limited to English language sources only.
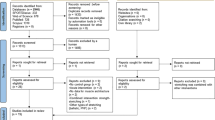
A total of 89 publications were found from this combination of terms. The references found in these publications were examined for further relevant studies. However, this did not yield any additional studies. After reviewing the titles, 47 studies remained, which were then screened to exclude studies that only indirectly investigated structural adaptations and those studies that focused more on hormonal adaptations, muscle fiber distribution, or signal transduction pathways without collecting the target parameters of muscle mass, muscle cross-sectional area, fiber cross-sectional area, fiber length, or fiber number. After this step, 23 studies remained, which were then subjected to full-text analysis using inclusion and exclusion criteria established in advance of the meta-analysis for the final selection.
The following parameters were defined as inclusion criteria:
- Objective measurement of muscle mass and/or muscle cross-sectional area and/or fiber count and/or fiber cross-sectional area and/or fiber length and/or number of muscle fibers.
- Stretching interventions of at least one week.
- Stretching times of at least 15 min per day.
- Specification of mean values and standard deviations.
- Studies on animals.
Accordingly, the following were considered exclusion criteria:
- No measurement of muscle mass and/or muscle cross-section and/or fiber number and/or fiber cross-section and/or fiber length and/or number of muscle fibers.
- Missing or insufficient information on the duration of the intervention and on the stretching times.
- Missing data concerning mean values and standard deviations, absence of absolute values.
- Missing data of number of test animals.
- Missing control group/control condition.
The final sample in the meta-analysis included 16 studies, whereby some studies with multiple effect sizes were included in the analysis because they either included different variables (e.g. muscle mass, fiber cross-section and/or hyperplasia effects) or because they described the effects of different intervention periods (a few days to several months). Figure 1 illustrates the procedure for study selection and Table 1 details the included studies.
Quality Assessment
The quality assessment was based on the Delphi list [62]. The Delphi method was chosen as a reliable and valid tool for the assessment of the quality of the included studies [54]. The assessment items for the current meta-analyses can be found in Table 2. The evaluation was performed by two independent raters. If question 2 received an affirmative answer, it was assumed that the age of the test animals, the species or breed of the animal as well as the initial weight were given. In all studies listed, mean values and standard deviations were given (see inclusion criteria) and the objective of the study was clearly stated. In none of the studies was information provided on blinding of the “care provider” and “outcome provider.” Only Czerwinski et al.[23] provided information on randomization.
Meta-analytic Procedure
Using the meta-analysis software RevMan, version 5.4.1 [22], 5 separate analyses were performed for the following parameters: muscle mass, muscle cross-sectional area, muscle fiber cross-sectional area, muscle fiber length, and number of muscle fibers. The following parameters from each of the studies were included in the analysis: number of experimental animals, and the respective mean values and standard deviations of the experimental and control conditions. Since several studies involved different durations, the studies were listed in alphabetical order with a lowercase letter to allow assignment of the elongation period to the respective representation in the forest plot. We used a random effects model to take into account any heterogeneity resulting from the use of different species in the studies and all other potential between-study differences (study characteristics are summarized in Table 1).Footnote 1
Tables 3–7 report the empirical M, SD and N for the parameters muscle mass, muscle cross sectional area, fiber cross sectional area, muscle fiber number, and fiber length. For all analyses, the standardized mean difference (with inverse variance weighting) and its 95% confidence interval were computed as the effect size of interest in RevMan.Footnote 2 Since for the evaluation of MM, MCSA, FCSA, FN and FL in laboratory studies, animals had to be dissected and flight muscles (ALD, PAT) had to be removed, no pre-post comparison of the same subjects could be performed. Therefore, the SMD was calculated for the comparison of the post-treatment experimental and a respective control group.
Results
Muscle Mass
The included studies show that in animal experiments a significant increase in muscle mass can be achieved by stretching intervention over several weeks. The effect size across all studies was d = 8.51, P < 0.001, 95% CI 7.11–9.91. Stretching was performed with varying durations per day (minimum 2 × 15 min) up to 24 h stretching over up to 6 weeks [25]. There were positive effects found on muscle mass in most studies, expect for one intervention performed by Brown et al. [18], see Table 3. The highest increases in muscle mass in the listed studies were obtained by Antonio and Gonyea [8] with a 37-day stretching intervention and an increase of 318% ± 39.1% and d = 7.01, 95% CI 3.77–10.24. Other high percentage increases were obtained by Antonio and Gonyea [9] with an increase of 294.3% ± 39.1% with d = 11.96, 95% CI 7.27–16.66 in muscle mass, Alway [2] with an increase of 161.5% ± 7.9% with d = 6.64, 95% CI 5.43–7.85, and Carson et al. [20, 21] with 178.7% ± 7.1% d = 20.82, 95% CI 15.44–26.32.
Muscle Cross-Sectional Area
Changes in muscle cross-sectional were all positive. Here, an effect strength of d = 7.91, P < 0.001, 95% CI 5.75–10.08 was recorded. Frankeny et al. [25] measured an increase in muscle cross-section of 111% compared to the control muscle. Alway [3] also recorded muscle cross-sectional increases of 100% (see Table 4).
Fiber Cross-Sectional Area
For the effects on fiber hypertrophy, an increase due to the stretching intervention was also determined (almost) consistently. The effect size here was d = 5.81, P < 0.001, 95% CI 4.32–7.31. The changes in fiber cross-section ranged from − 0.75% to 141.6% (± 32.6%), with these two values being more of an outlier, as all other results ranged from + 27.8% to + 63.8% (see Table 5).
Fiber Number (Hyperplasia)
With regard to the number of fibers, the studies also show significant increases as an adaptation to permanent stretching. Here, the calculated effect size across the studies is d = 4.62, P < 0.001, 2.54–6.71. In two studies, a decrease in the number of fibers − 0.7% ± 3.6% with d = − 0.29, 95% CI − 1.34–0.77 in Antonio and Gonyea [8] and − 6.7% ± 4.6% with d = − 1.3, 95% CI − 2.6–0.0 in Antonio and Gonyea [9] was initially determined after a certain intervention period, which, however, was no longer present at a later test in the same study, so that an increase in the number of fibers was also recorded in this study (see Table 6).
Fiber Length
The fiber length was only taken into account in three studies. The effect size determined was d = 7.86, P < 0.001, 95% CI 4.00–11.72. Here, percentage increases were 26.1% ± 7.3% (d = 3.31, 95% CI 1.52–5.09 [8]. Studies by Alway [3] determined muscle length changes of approx. 25% compared to the control muscle due to the stretching intervention (see Table 7).
Discussion
Based on the studies and the effect sizes determined in this meta-analysis, it can be assumed that (continuous) stretching (from 30 min to 24 h per day in a longitudinal section over several days to weeks) induces muscular tension in animal muscles, which leads to the following morphological adaptations of the stretched muscles: an increase in muscle mass, muscle cross-section, fiber cross-section, fiber length, and/or number of muscle fibers. This is confirmed by the results of other studies whose experimental investigations were similar to the analyzed studies, but which could not be included in the statistical analyses due to exclusion criteria or missing information in the method description [6, 12, 15, 35, 69].
Several studies show that there seems to be a correlation between stretching time and stretching intensity with achieved muscle mass increase [15, 25, 35], assuming an upper limit or optimum of stretching duration. In studies by Frankeny et al. [25] and Bates [15], although further increases due to an increase in stretching duration can be detected, the stretching optimum (effort relative to return) seems to be 30 min: “We conclude that daily stretching for as little as 30 min per day is a powerful inducer of growth in normal and dystrophic muscle” [25]. Antonio et al. [10] achieved maximal muscle mass gains of 318% with a progressively increased stretching load and an intermittent stretching protocol. The increases in muscle mass is consistent in almost all studies listed in this meta-analysis except for one measured parameter by Brown et al. [18] due to stretching the PAT for 16 days in old female chicken (28 month old).
The muscle mass gains are attributed by most authors to muscle fiber hypertrophy and muscle fiber hyperplasia. For muscle hyperplasia, uninterrupted continuous stretching seems to be the initiating stimulus, since the muscle fiber is not given sufficient time to regenerate. This stimulates increased satellite cell activation, which leads to the formation of new muscle fibers [8]. Another explanation is that reaching a critical muscle fiber size by hypertrophy effects leads to the splicing of the muscle fiber into several muscle fibers. This could be responsible for hyperplasia [8, 10].
Hypertrophy
Induced tension or mechanical stress on the individual sarcomeres are thought to be responsible for the hypertrophy effects achieved by stretching, such that the mechanical stimulus on the muscle is the adaptation-inducing stressor and thus the crucial stimulus for muscle mass gains [49, 67]. The muscle responds to this stimulus by increasing its serial sarcomere number [66] and the accumulation of myofibrils triggers an increase in cross-sectional area [4, 8, 20, 25]. The increase in muscle mass due to long duration stretching interventions has been clearly demonstrated in animal studies. Various studies with animals have also demonstrated an increased rate of protein synthesis by stretching [16, 28, 29]. Whether and to what extent the results of this study are transferable to humans have not yet been adequately investigated. Several of the studies integrated in this meta-analysis specifically request this step [15, 29]. Critically, protein synthesis differs between humans and animals. Garibotto et al. [27] and Tessari et al. [58] list protein synthesis rates of 2% and 1.5%, respectively, for leg muscles. Early experiments made by Williams and Goldspink indicate 2–3 days for length adaptation of muscle in mice, but 2–3 weeks in cats and humans [67]. For the species primarily studied in this meta-analysis (chickens/quail), Sayegh and Lajtha [50] indicate a lower protein synthesis rate compared to mice. However, the protein synthesis rate is dependent on the species, but also on other factors such as gender or hormones (e.g., testosterone) [60], age, and muscle fiber distribution or the expression of myosin heavy chains [42, 52]. The highest increases in muscle length reported in the literature were found to be up to 60% depending on the duration of stretching by Antonio and Gonyea [8] or up to 77% by Antonio et al. [9].
With regard to fiber hypertrophy in animal experiments, no uniform statement can be made. Antonio et al. [10] found an increase in the cross-section of FT as well as ST fibers, whereas Alway, [3] and Roman and Alway [47], for example, do not highlight any increase in the muscle cross-section of FT fibers. The hypertrophy of ST fibers seems to be regulated by the calcineurin/NFAT signal transduction pathway [48]. This is significant as the studies listed in this meta-analysis are primarily concerned with prolonged exercise leading to ST fiber adaptations [10, 20, 21, 25, 29, 30].
Hyperplasia
Referring to the finding of Antonio et al. [10] that the amount of increase in fiber count is related to the duration as well as the amount of the stretching stimulus, it can be hypothesized that traditional strength training methods do not achieve adequate stretching of the muscles. A stretching intervention lasting several hours to several days, as performed in animal experiments, has of course not been carried out. The proliferation and activation of satellite cells is held responsible for the hyperplasia effects [8, 10, 29]. This seems to occur—at least in animal experiments—when a muscle is seriously damaged by mechanical stress [36, 57].
Maximum Strength and Speed Strength
Studies by Alway [3, 4] found a significant increase in muscle cross-sectional area (approximately 100%), in muscle mass (260%) and in maximum strength (95%) in animal muscles. Stretching the muscle can be assumed to lengthen the muscle fiber through serial accumulation of sarcomeres [4, 10]. In animal experiments, muscle lengthening of up to 60% depending on the stretch duration was found by Antonio and Gonyea [8] or up to 77% by Antonio and Gonyea [9]. According to Goldspink and Harridge [29], this can lead to a faster contractile capacity of the muscle and thus an increase in fast or explosive power capacity. This hypothesis is confirmed by Medeiros and Lima [39] who identified 14 studies with a positive influence on “muscle performance” through chronic stretching. Muscle performance was recorded in the studies by functional tests such as jumps or sprints or by isometric or isotonic contractions. This is contradicted by data on the change in myosin heavy chain expression in stretched muscle as demonstrated in the animal experiment by Roman and Alway [47]. Myosin isoform SM2 increased from a level of 43.1% (± 1.7%) in the control muscle to 55% (± 1.2%) in the stretched muscle. It was shown that sustained stretching resulted in increased expression of SH2 myosin heavy chains and decreased expression of SH1 myosin heavy chains. Thus, due to the decreased ATPase activity in hypertrophied type I fibers after stretching, a negative effect on muscle contraction speed can be assumed, which was confirmed by Alway [2].
Contraction time increased significantly from 149 ms (± 9 ms) to 162 ms (± 7 ms) in young animals and from 174 ms (± 16 ms) to 215 ms (± 14 ms) in old animals by continuous stretching with 12% of their own body weight. “Overload increased twitch contraction time by 36% in muscles from … birds” [5]. There was a measurable shift from SM1 myosin isoform to SM2 myosin isoform. “Nevertheless, the slowing of V, and Vmax in the ALD was related to the decrease in SMl and slow muscle fibers. The explanation for a shift in fiber type or myosin isoforms is unable to explain all of the 60% decline in shortening velocity, unless ATPase activity also declined in SM1 or slow-p fibers. Our preliminary data suggest that Ca2+ activated ATPase activity was − 20% lower in the SM2 isoform than the SM1 isoform, and ATPase activity decreased in both isoforms after stretch overload” [4]. If these results are transferable to humans, it can be assumed that an increase in the ST-fiber content and thus a reduction in high-speed power output (e.g. jumps, sprints) is due to muscle plasticity and a reduced ATPase activity.
For the investigated parameters MM, FCSA and FN, heterogeneity was relatively large (I2 > 90%), suggesting that moderator variables could explain some of the differences between the true effect sizes of the included individual studies. The forest plots for MCSA, FCSA, FN and FL provide graphical information of which effect sizes differ the most from the weighted averages, but systematic subgroup analyses where studies are grouped with respect to moderators, such as muscle group or fiber distribution within the muscle, gender of the test animal, age of the animal or stretching duration, does not seem feasible due to the (still) relatively small number of effect sizes. Using only birds as experimental animals and including ALD and PAT in the analysis of this meta-analysis, we already tried to account for potential heterogeneity by controlling these variables in the selection of studies (in contrast to Kelley [33].
Practical Implications
Although the results from animal experiments presented here are conclusive, they may not be directly transferable to humans. First evidence that stretching training can induce micro-traumatization in humans if appropriate intensity of the stimulus is given was provided by Smith et al. [55]. Schoenfeld [51, p. 2862] also refers to the possibility to induce sufficient mechanical tension to induce morphological adaptations using stretching training: "Mechanically induced tension produced both by force generation and stretch is considered essential to muscle growth, and the combination of these stimuli appears to have a pronounced additive effect”. Consequently, there are some studies pointing out improvements in sport-specific parameters as jumping and sprinting [34, 44], maximal strength [41, 43, 70] and muscle thickness [44, 53] using stretching durations of up to 6 × 5 min [70] for up to 12 weeks [44]. However, there is still a lack of human studies on the effects of long-lasting stretching interventions for many weeks on muscular hypertrophy, hyperplasia, and force development. Because frequency, magnitude, and especially intensity of stretching appear to play an important role in adaptive responses, further studies need to focus on load controls via these load normatives. Apostolopoulos et al. [11] hypothesized that below the pain threshold stretches in the muscle are compensated via the elastic components and only stretches above the pain threshold lead to inflammation, which is normal after a fatiguing load [32] and/or delayed onset muscle soreness. In addition to intensity, a minimum amount and duration of stretching is essential, as Fowles et al. [24] showed that a single bout of stretching does not seem to be sufficient to increase protein synthesis. In accordance, Freitas et al. [26] pointed out that interventions of less than 8 weeks with a stretching duration of less than 20 min per week would not be expected to produce statistically significant structural changes in humans. Therefore, stretching duration may play an important role, too. Only one study using daily long-lasting stretching training for the plantar flexors could be determined, showing significant increases in maximal strength, muscle thickness and flexibility [64]. Since in animal studies, apparatuses were used to achieve long-lasting stretching durations, stretching devices (as used by Warneke et al. [64]) could also be recommended to achieve long-lasting stretching durations in humans. Otherwise, it can be assumed that stretching durations lasting several hours are not feasible. If a certain degree of transferability to humans is assumed, the studies analyzed here can be seen to have particular relevance in rehabilitation [29], as immobilization due to injury is known to lead to significant muscle atrophy [45]. If the hypertrophy effects from animal studies are assumed to be transferable to humans, aid-based continuous stretching for several hours could counteract atrophy and, if necessary, support muscle mass gain. “The therapeutic applications of stretch should therefore be borne in mind when designing regimens for rehabilitation or improved athletic performance” [29]. Furthermore, if voluntary muscle activation is not possible, stretching intervention would already be applicable. This could minimize muscle atrophy and loss of strength through immobilization due to injuries or illnesses [65, 68].
For an examination of the results in humans, moderator variables should be taken into account to be able to examine their influence.
If transferability of our results to humans is given, we see a high potential in using long-lasting stretching to achieve muscle hypertrophy. But it remains controversial whether hyperplasia effects occur in humans as a result of a training intervention. MacDougall notes, “One possible explanation is that hyperplasia occurs only in response to a significant stretch overload that also causes muscle lengthening, and that conventional resistance training does not impose such a stimulus” [36].
Limitations
In all studies included in the meta-analysis, the control values were provided by non-stretched animals because collecting pre- and post-measures from the same animals is not possible. This is different in studies using human participants. With regard to the conducted quality assessment, an important limitation appears to be the fact that in most studies, the assessors (of the outcome parameters) were not blinded with regard to which animals were assigned to the experimental or control group. Also, visual inspection of the funnel plots performed for each outcome parameter suggested slight deviations from a symmetric distribution in some cases. However, this could be due to the rather small effect sizes and should be interpreted with caution. Furthermore, also due to the rather small number of studies, it was not possible to reliably investigate the potential influence of moderator variables, such as duration of stretching, for instance. Finally, it needs to be highlighted that most studies were performed about 30–40 years ago.
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author upon request.
Notes
In addition, we provide funnel plots for each outcome parameter as supplemental material to illustrate potential publication bias.
Using the formulae.\({SMD}_{i}=\frac{{m}_{1i}-{m}_{2i}}{{s}_{i}}\left(1-\frac{3}{4{N}_{i}-9}\right)\) and \(SE\left\{{SMD}_{i}\right\}=\sqrt{\frac{{N}_{i}}{{n}_{1i}{n}_{2i}}+\frac{{SMD}_{i}^{2}}{2\left({N}_{i}-3.94\right)}}\).
References
Aguilar-Agon KW, Capel AJ, Martin NRW, Player DJ, Lewis MP. Mechanical loading stimulates hypertrophy in tissue-engineered skeletal muscle: molecular and phenotypic responses. J Cell Physiol. 2019;234(12):23547–58. https://doi.org/10.1002/jcp.28923.
Alway SE. Stretch induces non-uniform lsomyosin expression in the quail anterior latissimus dorsi muscle. Anat Rec. 1993;237(1):7.
Alway SE. Contractile properties of aged avian muscle after stretch-overload. Mech Ageing Dev. 1994;73(2):97–112.
Alway SE. Force and contractile characteristics after stretch overload in quail anterior latissimus dorsi muscle. J Appl Physiol. 1994;77(1):135–41.
Alway SE. Attenuation of Ca 21-activated ATPase and shortening velocity in hypertrophied fast twitch skeletal muscle from aged Japanese quail. Exp Gerontol. 2002;37(5):665–78. www.elsevier.com/locate/expgero.
Alway SE, Winchester PK, Davis ME, Gonyea WJ. Regionalized adaptations and muscle fiber proliferation in stretch-induced enlargement. J Appl Physiol. 1989;66(2):771–81.
Alway SE, Gonyea WJ, Davis ME. Muscle fiber formation and fiber hypertrophy during the onset of stretch-overload. Am J Physiol. 1990;259(1 Pt 1):C92–102. https://doi.org/10.1152/ajpcell.1990.259.1.C92.
Antonio J, Gonyea WJ. Role of muscle fiber hypertrophy and hyperplasia in intermittently stretched avian muscle. J Appl Physiol. 1993;259(28):1893–8.
Antonio J, Gonyea WJ. Muscle fiber splitting in stretch enlarged avian muscle. Med Sci Sports Exerc. 1994;26(8):973–7.
Antonio J, Gonyea WJ, Progressive WJG. Progressive stretch overload of skeletal muscle results in hypertrophy before hyperplasia. J Appl Physiol. 1993;75(3):1263–71.
Apostolopoulos N, Metsios GS, Flouris AD, Koutedakis Y, Wyon MA. The relevance of stretch intensity and position—a systematic review. Front Psych. 2015;6:1128. https://doi.org/10.3389/fpsyg.2015.01128.
Ashmore CR. Stretch-induced growth in chicken wing muscles: effects on hereditary muscular dystrophy. Am J Physiol. 1982;242(3):C178–83.
Bacurau RF, Monteiro GA, Ugrinowitsch C, Trikoli V, Cabral LF, Aoki MS. Acute effect of a ballistic and static stretching exercise bout on flexibility and maximal strength. J Strength Cond Res. 2009;23(1):304–8.
Barnett JG, Holly RG, Ashmore CR. Stretch-induced growth in chicken wing muscles: biochemical and morphological characterization. Am J Physiol. 1980;239(1):C39–46. https://doi.org/10.1152/ajpcell.1980.239.1.C39.
Bates GP. The relationship between duration of stimulus per day and the extend of hypertrophy of slow-tonic skeletal muscle in the fowles. Gallus gallus Comp Biochem Physiol. 1993;106A(4):755–8.
Booth FW, Seider MJ. Early change in skeletal muscle protein synthesis after limb immobilisation of rats. J Appl Physiol Respir Environ Exerc Physiol. 1979;47(5):974–7.
Boppart MD, Mahmassani ZS. Integrine signaling: Linking mechanical stimulation to skeletal muscle hypertrophy. Am J Physiol Cell Physiol. 2019;317(4):C629–41.
Brown CR, Palmer WK, Bechtel PJ. Effects of passive stretch on growth and regression of muscle from chichens of various ages. Comp Biochem Physiol. 1986;86(3):443–8.
Carson JA, Alway SE. Stretch overload-induced satellite cell activation in slow tonic muscle from adult and aged Japanese quail. Am J Physiol. 1996;270(2 Pt 1):C578–84. https://doi.org/10.1152/ajpcell.1996.270.2.C578.
Carson JA, Alway SE, Yamaguchi M. Time course of hypertrophic adaptations of the anterior latissimus dorsi muscle to stretch overload in aged Japanese quail. J Gerontol A Biol Sci Med Sci. 1995;50(6):B391–8. https://doi.org/10.1093/gerona/50a.6.b391.
Carson JA, Yamaguchi M, Alway SE, Alway Hypertrophy SE. Hypertrophy and proliferation of skeletal muscle fibers from aged quail. J Appl Physiol. 1995;78(1):293–9.
Collaboration TC. Review manager 5.4.1. 2020. https://training.cochrane.org/online-learning/core-software/revman.
Czerwinski SM, Martin JM, Bechtel PJ. Modulation of IGF mRNA abundance during stretch-induced skeletal muscle hypertrophy and regression. J Appl Physiol. 1994;76(5):2026–30.
Fowles JR, MacDougall JD, Tarnopolsky MA, Sale DG, Roy BD, Yarascheski KE. The effects of acute passive stretch on muscle protein synthesis in humans. Can J Appl Physiol. 2000;25(3):165–80.
Frankeny JR, Holly GR, Ashmore CR. Effects of graded duration of stretch on normal and dystrophic skeletal muscle. Muscle Nerve. 1983;6(4):269–77.
Freitas SR, Mendes B, Le Sant G, Andrade RJ, Nordez A, Milanovic Z. Can chronic stretching change the muscle-tendon mechanical properties? A review. Scand J Med Sci Sports. 2018;28(3):294–306.
Garibotto G, Tessari P, Robaudo C, Zanetti M, Saffioti S, Vettore M, Inchiostro S, Sacco P, Deferrari G, Tizianello A. Protein turnover in the kidney and the whole body in humans. Miner Electrolyte Metab. 1997;23(3–6):185–8.
Goldspink DF, Garlickt PJ, Mcnurlanti MA. Protein turnover measured in vivo and in vitro in muscles undergoing compensatory growth and subsequent denervation atrophy. Biochem J. 1983;210(1):89–98.
Goldspink G, Harridge S. Cellular and Molecular Aspects of Adaptation in Skeletal Muscle. In: Komi PV, editor. Strength and Power in Sport. 2nd ed. New Jersey: Wiley-Blackwell; 2003. pp. 231–51.
Grgic J, Homolak J, Mikulic P, Botella J, Schoenfeld BJ. Inducing hypertrophic effects of type I skeletal muscle fibers: a hypothetical role of time under load in resistance training aimed at muscular hypertrophy. Med Hypotheses. 2018;112:40–2. https://doi.org/10.1016/j.mehy.2018.01.012.
Hooijmans CR, IntHout J, Ritskes-Hoitinga M, Rovers MM. Meta-analyses of animal studies: an introduction of a valuable instrument to further improve healthcare. ILAR J. 2014;55(3):418–26. https://doi.org/10.1093/ilar/ilu042.
Kanda K, Sugama K, Hayashida H, Sakuma J, Kawakami Y, Miura S, Yoshioka H, Mori Y, Suzuki K. Eccentric exercise-induced delayed-onset muscle soreness and changes in markers of muscle damage and inflammation. Exerc Immunol Rev. 2013;19:72–85.
Kelley G. Mechanical overload and skeletal muscle fiber hyperplasia: a meta-analysis. J Appl Physiol. 1996;81(4):1584–8.
Kokkonen J, Nelson AG, Eldredge C, Winchester JB. Chronic static stretching improves exercise performance. Med Sci Sports Exerc. 2007;39(10):1825–31.
Lee J, Alway SE. Adaptations of myonuclei to hypertrophy muscle fibers from aged quail. Mech Ageing Dev. 1996;88(3):185–97.
MacDougall JD. Hypertrophy and Hyperplasia. In: Komi PV, editor. Strength and Power in Sport. 2nd ed. New Jersey: Wiley-Blackwell; 2003. pp. 252–64.
Matthews W, Jenkins RR, Gonyea WJ. Myosin isozyme expression in response to stretch-induced hypertrophy in the Japanese quail. Anat Rec. 1990;228(3):255–61. https://doi.org/10.1002/ar.1092280304.
Medeiros DM, Cini A, Sbruzzi G, Lima CS. Influence of static stretching on hamstring flexibility in healthy young adults: systematic review and meta analysis. Physio Ther Theory Pract. 2016;32(6):438–45.
Medeiros DM, Lima CS. Influence of chronic stretching on muscle performance: systematic review. Hum Mov Sci. 2017;54:220–9.
Medeiros DM, Martini TF. Chronic effect of different types of stretching on ankle dorsiflexion range of motion: systematic review and meta-analysis. Foot (Edinb). 2018;34:28–35.
Mizuno T. Combined effects of static stretching and electrical stimulation on joint range of motion and muscle strength. J Strength Cond Res. 2019;33(10):2694–703.
Nair KS. Muscle protein turnover methodological issues and the effect of aging. J Gerontol A Biol Sci Med Sci. 1995;50:107–12.
Nelson AG, Kokkonen J, Winchester JB, Kalani W, Peterson K, Kenly MS, Arnall DA. A 10-week stretching program increases strength in the contralateral muscle. J Cond Res. 2012;26(3):832–6.
Panidi I, Bogdanis GC, Terzis G, Donti A, Konrad A, Gaspari V, Donti O. Muscle architectural and functional adaptations following 12-weeks of stretching in adolescent female athletes. Front Physiol. 2021;12:701338. https://doi.org/10.3389/fphys.2021.701338
Perkin O, McGuigan P, Thompson D, Stokes K. A reduced activity model: a relevant tool for the study of ageing muscle. Biogerontology. 2016;17(3):435–47.
Riley DA, Van Dyke JM. The effects of active and passive stretching on muscle length. Phys Med Rehabil Clin N Am. 2012;23(1):51–7.
Roman WJ, Alway SE. Stretch-induced transformations in myosin expression of quail anterior latissimus dorsi muscle. Med Sci Sports Exerc. 1995;27(11):1494–9.
Sakuma K, Yamaguchi A. The functional role of calcineurin in hypertrophy, regeneration, and disorders of skeletal muscle. J Biomed Biotechnol. 2010. https://doi.org/10.1155/2010/721219.
Sasai N, Agata N, Inoue-Miyazu M, Kawakami K, Kobayashi K, Sokabe M, Hayakawa K. Involvement of PI3K/Akt/TOR pathway in stretch-induced hypertrophy of myotubes. Muscle Nerve. 2010;41(1):100–6. https://doi.org/10.1002/mus.21473.
Sayegh JF, Lajtha A. In vivo rates of protein synthesis in brain, muscle, and liver of five vertebrate species. Neurochem Res. 1989;11(14):1165–8.
Schoenfeld BJ. The mechanisms of muscle hypertrophy and their application to resistance training. J Strength Cond Res. 2010;24(10):2857–72. https://doi.org/10.1519/JSC.0b013e3181e840f3.
Short KR, Nair KS. Muscle protein metabolism and the sarcopenia of ageing. Int J Sport Nutr Exerc Metab. 2001;11:119–27.
Simpson CL, Kim BDH, Bourcet MR, Jones GR, Jakobi JM. Stretch training induces unequal adaptation in muscle fascicles and thickness in medial and lateral gastrocnemii. Scand J Med Sci Sports. 2017;27(12):1597–604.
Sindhu F, Carpenter L, Seers K. Development of a tool to rate the quality assessment of randomized controlled trials using a Delphi technique. J Adv Nurs. 1997;25(6):1262–8. https://doi.org/10.1046/j.1365-2648.1997.19970251262.x.
Smith JL, Brunetz MH, Chenier TC, McCammon MR, Houmard JA, Franklin ME, Israel RG. The effects of static and ballistic stretching on delayed onset muscle soreness and creatine kinase. Res Q Exerc Sport. 1993;64(1):103–7.
Sparrow MP. Regression of skeletal muscle of chicken wing after stretch-induced hypertrophy. Am J Physiol. 1982;242(5):C333–8. https://doi.org/10.1152/ajpcell.1982.242.5.C333.
Tamaki T, Akatsuka A, Tokunaga M, Ishige K, Uchiyama S, Shiraishi T. Morphological and biochemical evidence of muscle hyperplasia following weight lifting exercise in rats. Am J Physiol. 1997;273(1 Pt 1):C246–56.
Tessari P, Garibotto G, Inchiostro S, Robaudo C, Saffioti S, Vettore M, Zanetti M, Russo R, Deferrari G. Kidney, splanchnic, and leg protein turnover in humans. Inside from leucine and phenylalanine kinetics. J Clin Invest. 1996;98(6):1481–92.
Timur X, Mirzoev M, Tyganov SA, Petrova IO, Shenkman BS. Acute recovery from disuse atrophy: the role of stretch-activated ion channels in the activation of anabolic signaling in skeletal muscle. Am J Physiol Endocrinol Metab. 2019;316:86–95. https://doi.org/10.1152/ajpendo.00261.2018.-The.
Tipton KD. Gender differences in protein metabolism. Curr Opin Clin Nutr Metab Care. 2001;4(6):493–8.
Tyganov S, Mirzoev T, Shenkman B. An anabolic signaling response of rat soleus muscle to eccentric contractions following hindlimb unloading: a potential role of stretch-activated ion channels. Int J Mol Sci. 2019;20(1165):1–15. https://doi.org/10.3390/ijms20051165.
Verhagen A, De Vet H, Bouter L. The delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by delphi consensus fall prevention and quality of life in older people view project to what degree does active cervical rang. J Clin Epidemiol. 1998;51(12):1235–41. https://www.researchgate.net/publication/13203911.
Wackerhage H, Schoenfeld BJ, Hamilton DL, Lehti M, Hulmi JJ. Stimuli and sensors that initiate muscle hypertrophy following resistance exercise. J Appl Physiol. 2019;126(1):30–43.
Warneke K, Brinkmann A, Hillebrecht M, Schiemann S. Influence of long-lasting static stretching on maximal strength. Musc Thick Flex Front Physiol. 2022;13:878955. https://doi.org/10.3389/fphys.2022.878955.
Williams PE. Use of intermittent stretch in the prevention of serial sarcomere loss in immobilised muscle. Ann Rheum Dis. 1990;49(5):316–7.
Williams PE, Catanese T, Lucey EG, Goldspink G. The importance of stretch and contractile activity in the prevention of connective tissue accumulation in muscle. J Anat. 1988;158:109–14.
Williams PE, Goldspink G. The effect of denervation and dystrophy on the adaptation of sarcomere number to the functional length of the muscle in young and adult mice. J Anat. 1976;122:2.
Wilson SJ, Christensen B, Gange K, Todden C, Hatterman-Valenti H, Albrecht JM. Chronic stretching during 2 weeks of immobilization decreases loss of girth, peak torque, and dorsiflexion range of motion. J Sport Rehabil. 2019;28(1):67–71. https://doi.org/10.1123/jsr.2017-0101.
Winchesterand PK, Gonyea WJ. Regional injury and the terminal differentiation of satellite cells in stretched avian slow tonic muscle. Dev Biol. 1992;151(2):459–72.
Yahata K, Konrad A, Sato S, Kiyono R, Yoshida R, Fukaya T, Nunes JP, Nakamura M. Effects of a high-volume static stretching programme on plantar-flexor muscle strength and architecture. Eur J Appl Physiol. 2021;121(4):1159–66.
Young W, Elias G, Power J. Effects of static stretching volume and intensity on plantar flexor explosive force production and range of motion. J Sports Med Phys Fitness. 2006;46(3):403–11.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Consent for publication
All authors have read and agreed to the published version of the manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Warneke, K., Freund, P.A. & Schiemann, S. Long-Lasting Stretching Induces Muscle Hypertrophy: A Meta-Analysis of Animal Studies. J. of SCI. IN SPORT AND EXERCISE 5, 289–301 (2023). https://doi.org/10.1007/s42978-022-00191-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42978-022-00191-z