Abstract
Earth harbors unique environments where only microorganisms adapted to extreme conditions, known as extremophiles, can survive. This study focused on a high-altitude meltwater pond, located in the Puna de Atacama, Dry Andes. The extremophilic bacteria of this habitat must adapt to a range of extremities, including cold and dry climate, high UV radiation, high daily temperature fluctuations, low-nutrient availability, and negative water balance. This study aimed to explore the taxonomic diversity of cultivable extremophilic bacteria from sediment samples of a desiccated, high-altitude, meltwater pond using media with different organic matter contents and different incubation temperatures. Based on the 16S rRNA gene sequence analysis, the isolates were identified as members of the phyla Actinobacteria, Proteobacteria, and Firmicutes. The most abundant genera were Arthrobacter and Pseudoarthrobacter. The isolates had oligocarbophilic and psychrotrophic properties, suggesting that they have adapted to the extreme environmental parameters of their natural habitats. The results indicate a positive correlation between nutrient concentration and temperature tolerance.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Permafrost, by definition, is a ground that remains frozen for at least two consecutive years (Everett 1989). The geographical distribution of permafrost is primarily influenced by low temperatures; thus, it can be found either in mountainous regions due to a temperature drop in high altitudes, or in high latitudes where a decrease in solar energy supply causes a cooling effect. In warmer seasons, the upper, active layer of permafrost may undergo freeze–thaw cycles (Dobinski 2011).
Over the last five decades, climate change has triggered permafrost shrinkage. Due to the increased temperature, the active layer of permafrost has thickened both in polar and high-altitude regions. Permafrost degradation has several serious effects, including changing the topographic and hydrological conditions, as well as altering vegetation and wildlife dynamics. In numerous locations along the permafrost border, the soils impacted by permafrost have vanished entirely, while in other areas, the upper layers of permafrost are thawing at a greater depth than previously observed (Streletskiy et al. 2015). The thickened active layer has caused an increase in microbial activity, leading to the release of greenhouse gases, such as methane and carbon dioxide into the atmosphere, exacerbating global warming (Gilichinsky et al. 1995).
Microorganisms living in the permafrost have to tolerate sub-zero temperatures, limited water availability, and low-nutrient stress for extended periods of time (Goordial et al. 2013). Those that can thrive in such harsh environments are called polyextremophiles. These microorganisms can be characterized by their ability to grow optimally within extreme range of many environmental factors (Horikoshi and Bull 2011). Although many microorganisms can only survive these conditions in a persistent or dormant state, there is clear evidence that relatively high diversity and abundance of polyextremophiles exist in the permafrost in viable and active forms (Altshuler et al. 2017). Despite the sub-zero temperature, the active layer of permafrost may contain groundwater, supporting microbial life. This water forms a thin film on the soil/rock particles and around bacterial cells, creating narrow water channels between them. These channels do not support bacterial motility but rather the diffusion of nutrients and waste products (Goordial et al. 2013). Consequently, when the temperature drops below the freezing point, increased water freezing inhibits nutrient transport and bacterial metabolic activity (Rivkina et al. 2000).
Despite the multiple extreme environments, such as sub-zero temperatures and low-nutrient availability, a considerable number of microorganisms ranging from 105 to 109 cell/g were found in the Arctic, 108–1010 cell/g in high-altitude regions, and 103–106 cell/g in Antarctic permafrost samples (Altshuler et al. 2017). Zhang et al. (2013) reported an approximately twofold higher amount of Gram-negative bacteria compared to Gram-positive bacteria when analyzing fatty acids in ancient Siberian permafrost. Several studies aimed at determining the cultivable bacteria of permafrost have found that Proteobacteria, Actinobacteria, Firmicutes, and Bacteroidetes were predominant (Steven et al. 2007; Yang et al. 2008; Belov et al. 2019, 2020).
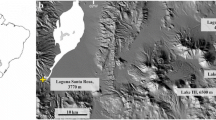
The Ojos del Salado (6893 m above sea level) is the highest volcano on Earth (Fig. 1a), and it rises in the Dry Andes on the border between Chile and Argentina (27°06′33.84′′S, 68°32′29.63′′W). The volcano is currently in a dormant state; therefore, only volcanic accompaniments can be observed. In this region, there is evidence of permafrost above 5250 m a.s.l. (Nagy et al. 2018, 2020). Based on continuous field measurements from 01/12/2015 to 31/12/2019, the daily mean ground temperature at 5830 m a.s.l (Tejos Camp) and at a depth of 10 cm below the surface was − 3.3 °C. Over the course of the four-year period, the lowest temperature recorded was − 20.7 °C, while the highest was 17.2 °C (Fig. 1b). The region shows an annual precipitation below 150–200 mm year−1, with most precipitation occurring during the Australian summer (Garreaud et al. 2003; Messerli et al. 1997) which corresponds to the wet season (Paquis et al. 2023). About 70% of the snow disappears into the atmosphere through sublimation, and the frozen ground limits the infiltration of meltwater. Therefore, in an arid region like Ojos del Salado, the replenishment of water resources is extremely slow (Messerli et al. 1997). Even though Ojos del Salado is the highest mountain of the Puna de Atacama, permanent snow is only observed sporadically. The permanent snow line is around 7000 m because of severe drought and high irradiation (Clapperton 1994). These harsh conditions have made the area a great site for Mars analogue research (Breuer et al. 2020).
a Geographical location of the Ojos del Salado’s highest peak and the sampling sites. b Overall snow coverage, daily mean temperature (dark blue line), hourly temperature fluctuations (light blue line) measured at a depth of − 10 cm, and depth and length of active layer thawing. All data were obtained at 5830 m a.s.l. on the Ojos del Salado (based on Nagy et al. 2018). c In 2014, a shallow high-altitude pond was discovered at 5900 m a.s.l. on the Ojos del Salado. In 2019, the pond dried out. The sampling sites are indicated by arrows
The environment of Ojos del Salado is unique and exposed to multiple extreme conditions that make it challenging to access this area. This environment, however, exhibits remarkable microbial diversity, including rare and unexplored bacterial species. The diversity of bacteria adapted to extreme environments is not yet well understood, despite its potential impact on numerous scientific fields such as biotechnology, medicine, and genetics. This study therefore aimed to explore the composition of extremophilic bacteria from the sediment of a high-altitude dried meltwater pond using cultivation-based techniques including the use of media with different concentrations of organic matter and incubation at different temperatures.
Materials and methods
Description of the sampling site
During the Hungarian Dry Andes Research Program, a shallow high-mountain meltwater pond was discovered at 5900 m a.s.l. in the Ojos del Salado in 2014 (Fig. 1c). The seasonal pond appears in the second half of the Australian summer, lasting for about 6–8 weeks. The pond water originates from the melting of active layer and/or surface precipitation, primarily snow. Major snowfall is not necessary for the pond’s appearance, but it supports the formation by saturating the active layer with melting snow that eventually emerges into the pond basin [Nagy 2024 (unpublished)]. Samples were collected on January 14, 2019, during the Australian summer (Fig. 1c). In that year, the pond dried out completely due to severe drought and cold weather. Samples were collected from 10 cm below the surface into 50-ml sterile Falcon tubes and stored cooled until being processed in the laboratory. Samples were collected from the edge of the dried-out pond (A59-19SE) and from beneath an andesite rock near the pond (A59-19PF).
Cultivation of bacterial isolates
The samples were studied using the combination of dilution-to-extinction spread-plate cultivation method following pre-enrichments. The R2A (DSMZ 830; https://www.dsmz.de) broth and medium (pH 7.0) in three different concentrations (100%, 10%, and 1%) were applied to imitate the oligotrophic conditions of the sampling sites. 1 g of sample was added to each enrichment broth; then, the flasks were incubated for 2–3 weeks in different temperatures (4 °C, 10 °C, and 15 °C). Subsequently, tenfold dilutions were made and spread onto R2A agar of the same composition as the enrichment broths. Incubation time and temperatures remained the same as before. Individual colonies were isolated randomly.
Overall, nine different combinations of medium compositions and temperature values were involved for each sample. The isolate identifiers provide information on the sampling location (Andes, 5900 m), date (2019), sample type (lake sediment or permafrost), R2A medium concentration, and incubation temperature (refer to Table 1), for instance, A59-19SE-F01.
DNA extraction, 16S rRNA gene amplification, and sequencing
For the identification of bacterial isolates, DNA was extracted using a physical disruption method with glass beads (Krett et al. 2017); then, the solutions were stored at − 22 °C until further processes. 16S rRNA genes were amplified by a PCR with bacteria-specific primers: 27F (Lane 1991) and 1401R (Nübel et al. 1996). The PCR mixture composition was described previously by Krett et al. (2017) with a final volume of 25 µl. After a primary denaturation at 95 °C for 5 min, 32 cycles of denaturation (94 °C, 30 s), annellation (52 °C, 45 s), and extension (72 °C, 1 min) were followed, and then a final extension at 72 °C for 10 min.
PCR products were grouped by ARDRA (Amplified rDNA Restriction Analysis) method using Hin6I and BsuRI restriction endonucleases. The premix contained 2 μl restriction enzyme buffer (Tango/R) (Thermo Fisher Scientific, USA), 0.24 μl restriction enzyme (Hin6I/BsuRI) (Thermo Fisher Scientific, USA), 7.76 μl sterile distilled water, and 10 μl of each PCR product. The prepared premixes were placed in a 37 °C water bath for 3 h. The digested PCR products were detected in a 2% agarose gel and grouped by their fingerprints. Representatives of each ARDRA group were sequenced with 27F by LGC Genomics GmbH (Berlin, Germany).
Identification of bacterial isolates
The 16S rRNA gene sequences of the isolates were edited manually using Chromas [version 2.6.6 (2018), Technelysium Pty Ltd, South Brisbane, Queensland, Australia] and identified by the most similar sequences of type strains in the NCBI database (https://www.ncbi.nlm.nih.gov). The phylogenetic tree was constructed in MEGA 11 software version 11.0.13 (Tamura et al. 2021). The evolutionary history was inferred by using the maximum likelihood method and Kimura two-parameter model (Kimura 1980). A discrete Gamma distribution was used to model evolutionary rate differences among sites. The sequences of bacterial isolates are accessible in the GenBank database under accession numbers OM891685-OM891726, OQ254752, and OQ254753.
Environmental tolerances were calculated based on the ARDRA groups. Heatmaps were created in RStudio (RStudio Team 2020) using the dplyr (Wickham et al. 2022) and the ggplot2 (Wickham 2016) packages.
Results and discussion
Taxonomic diversity of cultivable bacteria
In the present study, altogether 127 bacterial isolates were obtained by applying nine combinations of R2A medium concentrations and incubation temperatures. According to the restriction patterns, the isolates were arrayed into 25 different ARDRA groups.
The 16S rRNA gene was sequenced from 36 representatives, 28 originated from the edge of the dried-out pond (A59-19SE) and 8 from the permafrost near the pond (A59-19PF). The isolates were identified as members of the phyla Actinobacteria, Alphaproteobacteria, Betaproteobacteria, and Firmicutes (Fig. 2). Overall, high sequence similarities were obtained between the isolates and the closely related type strains (> 98%). Most of the isolates showed the highest sequence similarity to different species of the genera Arthrobacter (A. bussei, A. flavus, A. ginsengisoli, A. glacialis, A. parietis, and A. psychrochitiniphilus) and Pseudarthrobacter (P. oxydans P. phenanthrenivorans, P. psychrotolerans, and P. siccitolerans). These two genera have recently been separated on the basis of their distinct chemotaxonomic traits, including polar lipid profile, quinone system, and peptidoglycan type (Busse 2016; Busse and Schumann 2019). Nevertheless, the similarity of the 16S rRNA gene regions of these two genera did not allow the separation of the type strains and isolates by genera, as shown in Fig. 2. Previous cultivation-based studies from high-altitude regions indicated that the most abundant phyla represented the high G + C Gram-positives (Actinobacteria), the low G + C Gram-positives (Firmicutes), Proteobacteria, and the Cytophaga–Flavobacterium–Bacteroides (CFB) group (Bai et al. 2006; Zhang et al. 2007b). Both studies revealed that the genus Arthrobacter was predominant. Johnson et al. (2007) reported that Arthrobacter dominated ancient permafrost samples. The study suggested that survival was more reliant on cellular metabolic activity and DNA repair than on dormancy under frozen conditions. Other Ojos del Salado isolates represented the genera Massilia (M. atriviolacea, M. jejuensis, M. polaris, M. psychrophila, and M. soli), Cryobacterium (C. arcticum, C. breve, C. psychrotolerans, and C. soli), and Salinibacterium (S. amurskyense). Only a few isolates belonged into genera Micrococcus (M. yunnanensis), Paenisporosarcina (P. macmurdoensis), and Bosea (B. vestrisii).
Neighbor-joining phylogenetic tree based on the 16S rRNA gene sequences of the Ojos del Salado isolates and their closest phylogenetic relatives. (Only > 50% bootstrap values (n = 500 replications) were indicated at nodes. The analysis involved 60 nucleotide sequences. A total of 913 positions were in the final dataset)
Previously, Aszalós et al. (2020) also studied this high-mountain environment, partly using a similar cultivation method. A nutrient-rich medium (PYG) and a nutrient-poor medium (R2A) were applied for the cultivation process, with an incubation temperature of 8 °C. Representatives of the species Arthrobacter flavus, Arthrobacter ginsengisoli, and Cryobacterium psychrotolerans were identified in both studies. The continuous presence of these extremophiles in the examined habitat suggests that they have successfully adapted to thrive in the harsh environment of Ojos del Salado. Modifications in the cultivation procedure, involving pre-enrichment, different nutrient concentrations of R2A, and more incubation temperatures led to the cultivation of bacterial taxa (e.g., Arthrobacter psychrochitiniphilus, Cryobacterium arcticum, Massilia jejuensis, and Salinibacterium amurskyense) that were previously unidentified in this environment.
Most of the type strains that were most closely related to the cold-adapted isolates from Ojos del Salado have been described from polar and glacial environments. For example, Arthrobacter flavus (Reddy et al. 2000) and Paenisporosarcina macmurdoensis (Reddy et al. 2003) were isolated from cyanobacterial mat samples in McMurdo Dry Valley, Arthrobacter psychrochitiniphilus from Adélie penguin guano (Wang et al. 2009), Pseudarthrobacter psychrotolerans from soil in Antarctica (Shin et al. 2020), while Cryobacterium arcticum (Bajerski et al. 2011) and Massilia polaris (Dahal et al. 2021) were described from soil samples in Arctic regions. Furthermore, Arthrobacter glacialis (Liu et al. 2019), Cryobacterium psychrotolerans (Zhang et al. 2007a), Cryobacterium breve (Liu et al. 2020), and Massilia psychrophila (Guo et al. 2016) were described from distinct Chinese glaciers. The type strain of Pseudarthrobacter siccitolerans (Santacruz-Calvo et al. 2013), however, was isolated from a rhizosphere exposed to seasonal drought. Besides low temperature and desiccation, the limited amount of organic matter may also be a strong growth-limiting factor on Ojos del Salado. Similar to our oligocarbophilic isolates, the type strains of several species were also described from oligotrophic conditions, e.g., Salinibacterium amurskyense (Han et al. 2003) from seawater, Bosea vestrisii (La Scola et al. 2003) from a water supply, and Massilia jejuensis (Weon et al. 2010) from an air sample. The results of the study demonstrate that very similar, extreme habitat characteristics favor the colonization of closely related bacterial taxa even in very distant geographical regions.
Environmental tolerance of cultivable bacteria
All the applied cultivation combination proved to be suitable for the isolation of extremophiles from the studied high-altitude permafrost region (Fig. 3). Most of the type strains with which our isolates showed the highest similarity were psychrotrophs rather than psychrophiles. This distinction is made based on the fact that obligate psychrophiles have an optimal growth temperature of 15 °C or below and cannot grow above 20 °C (Morita 1975), while psychrotrophs are capable of growing at both low and moderate temperatures. Although the psychrophile definition applies only to the type strain of Cryobacterium breve (Liu et al. 2020), some other type strains were also reported to have optimal growth temperature below 20 °C, e.g., Cryobacterium soli (Gong et al. 2020), Massilia polaris (Dahal et al. 2021), Massilia psychrophila (Guo et al. 2016), Paenisporosarcina macmurdoensis (Reddy et al. 2003), and Pseudarthrobacter psychrotolerans (Shin et al. 2020).
The growth pattern of the Ojos del Salado isolates was generally within the temperature range of their closely related type strains; however, it predominantly occurred below their described optimum levels (Supplementary Table 1). For instance, the temperature optimum of the Arthrobacter psychrochitiniphilus type strain is 20 °C according to Wang et al. (2009), but 19 isolates (45%) from the Ojos del Salado identified as A. psychrochitiniphilus preferred 10 °C and 16 isolates (38%) preferred 4 °C. The isolates with the highest sequence similarity to the psychrophilic species of Cryobacterium breve grew optimally at 4 °C and 10 °C, which was also lower than the optimal 10–14°C described for the type strain (Liu et al 2020).
The results of the different cultivation combinations applied in this study showed that bacteria from the Ojos del Salado preferred the 100% R2A medium and 10 °C incubation temperature. Several isolates were, however, detectable on the lowest organic matter content (1% R2A) medium which proved the excellent adaptation of these isolates to the harsh high-altitude environment. Our results suggest that the higher the applied nutrient concentration, the wider the temperature range of the isolates. This effect was observed for isolates of the genera Massilia (Fig. 3c), Cryobacterium (Fig. 3d), and Salinibacterium (Fig. 3e). It may be explained by the fact that the lower the ambient temperature, the higher the energy requirement for survival, which can be facilitated by a more abundant nutrient supply.
Conclusions for future biology
This research, performed using different cultivation methods and incubation temperatures, resulted in the detection of oligocarbophilic and psychrotrophic bacteria previously unknown from the Ojos del Salado area. The examinations confirmed the success of the combined use of novel cultivation methods in the isolation of bacteria adapted to extreme environmental conditions. The results indicate that similar environmental conditions in geographically distant extreme environments favor the occurrence of closely related bacterial taxa. Despite the similar taxonomic compositions of these habitats, the environmental tolerance of the isolates reflects the specific characteristics of their natural habitat. Although the use of cultivation-based methods allows the identification of only a small part of natural communities, it still opens the possibility of describing new species and/or applying the novel isolates for specific industrial–biotechnological purposes.
References
Altshuler I, Goordial J, Whyte LG (2017) Microbial life in permafrost. In: Margesin R (ed) Psychrophiles: from biodiversity to biotechnology, 2nd edn. Springer International Publishing, Cham, pp 1–685
Aszalós JM, Szabó A, Megyes M, Anda D, Nagy B, Borsodi AK (2020) Bacterial diversity of a high-altitude permafrost thaw pond located on Ojos del Salado (Dry Andes, Altiplano-Atacama Region). Astrobiology 20:754–765. https://doi.org/10.1089/ast.2018.2012
Bai Y, Yang D, Wang J, Xu S, Wang X, An L (2006) Phylogenetic diversity of culturable bacteria from alpine permafrost in the Tianshan mountains, northwestern China. Res Microbiol 157:741–751. https://doi.org/10.1016/j.resmic.2006.03.006
Bajerski F, Ganzert L, Mangelsdorf K, Lipski A, Wagner D (2011) Cryobacterium arcticum sp. nov., a psychrotolerant bacterium from an Arctic soil. Int J Syst Evol Microbiol 61:1849–1853. https://doi.org/10.1099/ijs.0.027128-0
Belov AA, Cheptsov VS, Vorobyova EA, Manucharova NA, Ezhelev ZS (2019) Stress-tolerance and taxonomy of culturable bacterial communities isolated from a Central Mojave Desert soil sample. Geosciences 9:166. https://doi.org/10.3390/geosciences9040166
Belov AA, Cheptsov VS, Manucharova NA, Ezhelev ZS (2020) Bacterial communities of novaya zemlya archipelago ice and permafrost. Geosci 10:67. https://doi.org/10.3390/geosciences10020067
Breuer H, Berényi A, Mari L, Nagy B, Szalai Z, Tordai Á, Weidinger T (2020) Analog site experiment in the high Andes-Atacama Region: surface energy budget components on Ojos del Salado from field measurements and WRF simulations. Astrobiology 20:684–700. https://doi.org/10.1089/ast.2019.2024
Busse HJ (2016) Review of the taxonomy of the genus Arthrobacter, emendation of the genus Arthrobacter sensu lato, proposal to reclassify selected species of the genus Arthrobacter in the novel genera Glutamicibacter gen. nov., Paeniglutamicibacter gen. nov., Pseudoglutamicibacter gen. nov., Paenarthrobacter gen. nov. and Pseudarthrobacter gen. nov., and emended description of Arthrobacter roseus. Int J Syst Evol Microbiol 66:9–37. https://doi.org/10.1099/ijsem.0.000702
Busse HJ, Schumann P (2019) Reclassification of Arthrobacter enclensis as Pseudarthrobacter enclensis comb. nov., and emended descriptions of the genus Pseudarthrobacter, and the species Pseudarthrobacter phenanthrenivorans and Pseudarthrobacter scleromae. Int J Syst Evol Microbiol 69:3508–3511. https://doi.org/10.1099/ijsem.0.003652
Clapperton CM (1994) The quaternary glaciation of Chile: a review. Rev Chil Hist Nat 67:369–383
Dahal RH, Chaudhary DK, Kim DU, Kim J (2021) Cold-shock gene cspC in the genome of Massilia polaris sp. nov. revealed cold adaptation. Antonie Van Leeuwenhoek, Int J Gen Mol Microbiol 114:1275–1284. https://doi.org/10.1007/s10482-021-01600-z
Dobinski W (2011) Permafrost. Earth Sci Rev 108:158–169. https://doi.org/10.1016/j.earscirev.2011.06.007
Everett KR (1989) Glossary of permafrost and related ground-ice terms. Arct Alp Res 21:55. https://doi.org/10.2307/1551636
Garreaud R, Vuille M, Clement AC (2003) The climate of the Altiplano: observed current conditions and mechanisms of past changes. Palaeogeogr Palaeoclimatol Palaeoecol 194:5–22. https://doi.org/10.1016/S0031-0182(03)00269-4
Gilichinsky DA, Wagener S, Vishnevetskaya TA (1995) Permafrost Microbiology. Permafr Periglac Process 6:281–291. https://doi.org/10.1002/ppp.3430060402
Gong C, Lai Q, Cai H, Jiang Y, Liao H, Liu Y, Xue D (2020) Cryobacterium soli sp. nov., isolated from forest soil. Int J Syst Evol Microbiol 70:675–679. https://doi.org/10.1099/ijsem.0.003820
Goordial J, Lamarche-Gagnon G, Lay C-Y, Whyte L (2013) Left out in the cold: life in cryoenvironments. Cell Orig Life Extrem Habitats Astrobiol 27:335–363. https://doi.org/10.1007/978-94-007-6488-0_14
Guo B, Liu Y, Gu Z, Shen L, Liu K, Wang N, Xing T, Liu H, Zhou Y, Li J (2016) Massilia psychrophila sp. nov., isolated from an ice core. Int J Syst Evol Microbiol 66:4088–4093. https://doi.org/10.1099/ijsem.0.001315
Han SK, Nedashkovskaya OI, Mikhailov VV, Kim SB, Bae KS (2003) Salinibacterium amurskyense gen. nov., sp. nov., a novel genus of the family Microbacteriaceae from the marine environment. Int J Syst Evol Microbiol 53:2061–2066. https://doi.org/10.1099/ijs.0.02627-0
Horikoshi K, Bull AT (2011) Prologue: definition, categories, distribution, origin and evolution, pioneering studies, and emerging fields of extremophiles. In: Horikoshi K (ed) Extremophiles handbook. Springer, Tokyo, pp 3–15
Johnson SS, Hebsgaard MB, Christensen TR, Mastepanov M, Nielsen R, Munch K, Brand T, Gilbert MTP, Zuber MT, Bunce M, Rønn R, Gilichinsky D, Froese D, Willerslev E (2007) Ancient bacteria show evidence of DNA repair. Proc Natl Acad Sci U S A 104:14401–14405. https://doi.org/10.1073/pnas.0710637104
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120. https://doi.org/10.1007/BF01731581
Krett G, Szabó A, Felföldi T, Márialigeti K, Borsodi AK (2017) The effect of reconstruction works on planktonic bacterial diversity of a unique thermal lake revealed by cultivation, molecular cloning and next generation sequencing. Arch Microbiol 199:1077–1089. https://doi.org/10.1007/s00203-017-1379-9
La Scola B, Mallet MN, Grimont PA, Raoult D (2003) Bosea eneae sp. Nov., Bosea massiliensis sp. Nov. and Bosea vestrisii sp. Nov., isolated from hospital water supplies, and emendation of the genus Bosea. Int J Syst Evol Microbiol 53(1):15–20. https://doi.org/10.1099/ijs.0.02127-0
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, New York, pp 115–175
Liu Q, Liu HC, Zhou YG, Xin YH (2019) Genetic diversity of glacier inhabiting Cryobacterium bacteria in China and description of Cryobacterium zongtaii sp. nov. and Arthrobacter glacialis sp. nov. Syst Appl Microbiol 42:168–177. https://doi.org/10.1016/j.syapm.2018.10.005
Liu Q, Tian J-H, Liu H-C, Zhou Y-G, Xin Y-H (2020) Cryobacterium ruanii sp. nov. and Cryobacterium breve sp. nov., isolated from glaciers. Int J Syst Evol Microbiol 70:1918–1923. https://doi.org/10.1099/ijsem.0.003994
Messerli B, Grosjean M, Vuille M (1997) Water availability, protected areas, and natural resources in the Andean Desert Altiplano. Mt Res Dev 17:229–238. https://doi.org/10.2307/3673850
Morita RY (1975) Psychrophilic bacteria. Bacteriol Rev 39:144–167. https://doi.org/10.1128/mmbr.39.2.144-167.1975
Nagy B, Ignéczi Á, Kovács J, Szalai Z, Mari L (2018) Shallow ground temperature measurements on the highest volcano on Earth, Mt. Ojos del Salado, Arid Andes. Chile Permafr Periglac Process 30:1–16. https://doi.org/10.1002/ppp.1989
Nagy B, Kovács J, Ignéczi Á, Beleznai S, Mari L, Kereszturi Á, Szalai Z (2020) The Thermal behavior of ice-bearing ground: the highest cold, dry desert on Earth as an analog for conditions on Mars, at Ojos del Salado, Puna de Atacama-Altiplano region. Astrobiology 20:701–722. https://doi.org/10.1089/ast.2018.2021
Nagy B (2024) Personnal communication
Nübel U, Engelen B, Felsre A, Snaidr J, Wieshuber A, Amann RI, Ludwig W, Backhaus H (1996) Sequence heterogeneities of genes encoding 16S rRNAs in Paenibacillus polymyxa detected by temperature gradient gel electrophoresis. J Bacteriol 178:5636–5643. https://doi.org/10.1128/jb.178.19.5636-5643.1996
Paquis P, Hengst MB, Florez JZ, Tapia J, Molina V, Pérez V, Pardo-Esté C (2023) Short-term characterization of climatic-environmental variables and microbial community diversity in a high-altitude Andean wetland (Salar de Huasco, Chile). Sci Total Environ 859:160291. https://doi.org/10.1016/j.scitotenv.2022.160291
Reddy GSN, Aggarwal RK, Matsumoto GI, Shivaji S (2000) Arthrobacter flavus sp. nov., a psychrophilic bacterium isolated from a pond in McMurdo Dry Valley. Antarct Int J Syst Evol Microbiol 50:1553–1561. https://doi.org/10.1099/00207713-50-4-1553
Reddy GSN, Matsumoto GI, Shivaji S (2003) Sporosarcina macmurdoensis sp. nov., from a cyanobacterial mat sample from a pond in the McMurdo Dry Valleys. Antarct Int J Syst Evol Microbiol 53:1363–1367. https://doi.org/10.1099/ijs.0.02628-0
Rivkina EM, Friedmann EI, McKay CP, Gilichinsky DA (2000) Metabolic activity of permafrost bacteria below the freezing point. Appl Environ Microbiol 66:3230–3233. https://doi.org/10.1128/AEM.66.8.3230-3233.2000
RStudio Team (2020) RStudio: integrated development for R
Santacruz-Calvo L, González-López J, Manzanera M (2013) Arthrobacter siccitolerans sp. nov., a highly desiccation-tolerant, xeroprotectant-producing strain isolated from dry soil. Int J Syst Evol Microbiol 63:4174–4180. https://doi.org/10.1099/ijs.0.052902-0
Shin Y, Lee B-H, Lee K-E, Park W (2020) Pseudarthrobacter psychrotolerans sp. nov., a cold-adapted bacterium isolated from Antarctic soil. Int J Syst Evol Microbiol 70:6106–6114. https://doi.org/10.1099/ijsem.0.004505
Steven B, Briggs G, McKay CP, Pollard WH, Greer CW, Whyte LG (2007) Characterization of the microbial diversity in a permafrost sample from the Canadian high Arctic using culture-dependent and culture-independent methods. FEMS Microbiol Ecol 59:513–523. https://doi.org/10.1111/j.1574-6941.2006.00247.x
Streletskiy D, Anisimov O, Vasiliev A (2015) Permafrost degradation. Snow and ice-related hazards, risks, and disasters. Elsevier Inc., Amsterdam, pp 303–344
Tamura K, Stecher G, Kumar S (2021) MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38:3022–3027. https://doi.org/10.1093/molbev/msab120
Wang F, Gai Y, Chen M, Xiao X (2009) Arthrobacter psychrochitiniphilus sp. nov., a psychrotrophic bacterium isolated from Antarctica. Int J Syst Evol Microbiol 59:2759–2762. https://doi.org/10.1099/ijs.0.008912-0
Weon HY, Yoo SH, Kim SJ, Kim YS, Anandham R, Kwon SW (2010) Massilia jejuensis sp. nov. and Naxibacter suwonensis sp. nov., isolated from air samples. Int J Syst Evol Microbiol 60:1938–1943. https://doi.org/10.1099/ijs.0.015479-0
Wickham H, François R, Henry L, Müller K (2022) dplyr: A Grammar of Data Manipulation. https://dplyr.tidyverse.org, https://github.com/tidyverse/dplyr
Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York. ISBN 978-3-319-24277-4. https://ggplot2.tidyverse.org
Yang D, Wang J, Bai Y, Xu S, An L (2008) Diversity and distribution of the prokaryotic community in near-surface permafrost sediments in the Tianshan Mountains, China. Can J Microbiol 54:270–280. https://doi.org/10.1139/W08-004
Zhang DC, Wang HX, Cui HL, Yang Y, Liu HC, Dong XZ, Zhou PJ (2007a) Cryobacterium psychrotolerans sp. nov., a novel psychrotolerant bacterium isolated from the China No. 1 glacier. Int J Syst Evol Microbiol 57:866–869. https://doi.org/10.1099/ijs.0.64750-0
Zhang G, Niu F, Ma X, Liu W, Dong M, Feng H, An L, Cheng G (2007b) Phylogenetic diversity of bacteria isolates from the Qinghai-Tibet Plateau permafrost region. Can J Microbiol 53:1000–1010. https://doi.org/10.1139/W07-031
Zhang DC, Brouchkov A, Griva G, Schinner F, Margesin R (2013) Isolation and characterization of bacteria from ancient Siberian permafrost sediment. Biology (basel) 2:85–106. https://doi.org/10.3390/biology2010085
Funding
Open access funding provided by Eötvös Loránd University. This research was supported by the ÚNKP-21–2 New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund (Hungary), and the National Research, Development and Innovation Office, Hungary (Grants NKFIH OTKA K147424).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Faragó, V., Megyes, M., Nagy, B. et al. Taxonomic diversity and environmental tolerance of cultivable extremophilic bacteria from a high-altitude meltwater pond on Ojos del Salado (Chile). BIOLOGIA FUTURA (2024). https://doi.org/10.1007/s42977-024-00229-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42977-024-00229-z