Abstract
Previous research indicated that pain catastrophizing—a negative emotional and cognitive response toward actual or anticipated pain—could contribute to pain intensity and could be associated with depressive symptoms not just in chronic pain patients but in healthy population as well. Accumulated evidence suggests that resting heart rate variability (HRV) as a putative proxy of emotion regulation could moderate the association of self-reported pain catastrophizing and depressed mood. In the present cross-sectional study, we investigated these associations in a healthy young adult sample controlling for the effect of trait rumination. Seventy-two participants (58 females, mean age = 22.2 ± 1.79 years ranging from 19 to 28 years old) completed the Pain Catastrophizing Scale, the Zung Self-Rating Depression Scale and the Ruminative Response Scale. Resting HRV was measured by time domain metric of HRV, the root mean square of successive differences (RMSSD). The results showed that the relationship between pain catastrophizing and depressive symptoms is significantly moderated by resting HRV (indexed by lnRMSSD). Specifically, in participants with higher resting HRV there was no significant relationship between the two investigated variables, while in participants with relatively low or medium HRV pain catastrophizing and depressed mood showed significant positive association. The relationship remained significant after controlling for sex, age and trait rumination. These results might indicate that measuring pain catastrophizing and depressive symptoms is warranted in non-clinical samples as well and higher resting HRV could have a buffer or protective role against depressive symptoms.
Similar content being viewed by others
Introduction
Pain catastrophizing is a negative emotional and cognitive response toward actual or anticipated pain. It encompasses the magnification of the anticipated pain, a feeling of helplessness toward this pain and ruminating about the possibility that this pain will happen and be much worse than it was before (Sullivan et al. 1995). Clinical and experimental research proved that pain catastrophizing might be associated with pain intensity in different diseases where chronic pain is one of the leading symptoms: e.g., low back pain (George et al. 2011), chronic headache (Buenaver et al. 2008), or rheumatic diseases (Edwards et al. 2006) not only in adults but adolescents and children as well (Tran et al. 2015; Feinstein et al. 2017). It is also associated with consequences of chronic pain (e.g., loss of work (Besen et al. 2017) or medication overuse (Martel et al. 2014)) while having a prognostic value in the development of chronic pain (Velly et al. 2011; Theunissen et al. 2012). Preoperative high scores of pain catastrophizing might also be a predictor of postoperative pain (Subedi et al. 2021).
Pain, much like other interoceptive modalities such as thermosensation, comprises both an evaluative and a sensory component, which are sometimes assessed separately. Notably, authors like György Ádám have emphasized the significance of the evaluative aspect, highlighting its importance from a homeostatic perspective as it captures attention (Ádám, 1978, 1998). Moreover, suffering is typically associated with the evaluative component. Thus, it becomes a compelling inquiry to explore which aspect of pain, be it the affective-evaluative or the sensory-discriminative, is more strongly linked to pain catastrophizing. However, it is worth noting that many studies tend to primarily focus on measuring the sensory component.
In line with the findings of a systematic review conducted by Galambos et al. (2019), there is suggestive evidence indicating that pain catastrophizing may indeed be associated with both the affective and intensity-related components of pain. Although the level of evidence in the reviewed studies ranged from moderate to weak, it is reasonable to posit that one of the underlying mechanisms behind catastrophizing cognitions is their ability to amplify the salience of painful stimuli, consequently subjectively intensifying the overall experience.
While in most studies pain catastrophizing is measured on a clinical sample, evidence shows that it can be a predictor of developing chronic pain in a healthy or control population as well (Picavet et al. 2002). Thus, investigation of the correlates of pain catastrophizing is warranted in non-clinical samples as well.
Catastrophizing about pain can be conceptualized in at least two ways. First, as a general tendency, that is considered to refer to a relatively stable, trait-level characteristic, and second, as a situation-specific (i.e., state-level) characteristic, that reflects how the individual responds to current pain (e.g., headache or postoperative pain) or to experimentally induced painful stimulus in the laboratory. Studies using experimentally evoked pain in healthy participants demonstrated that either trait (Beneciuk et al. 2010; Kakon et al. 2021) or state catastrophizing (Procento et al. 2021) was related to subjective pain intensity. In addition, high trait-level pain catastrophizing can boost the impact of high state-level catastrophizing on subjective pain intensity (Procento et al. 2021). Studies that targeted participants with no pain before a medical examination or surgery also found that either trait (Gaffey et al. 2020) or state (e.g., pre- or postoperative (Grosen et al. 2016)) pain catastrophizing was related to subjective pain ratings. Questionnaire studies with healthy samples also found a positive relationship between trait catastrophizing and pain symptoms during the week before answering the questions (Lu et al. 2011). Overall, results suggest that ratings of actual or experimentally induced pain in otherwise healthy people consistently linked to pain catastrophizing; however, the studies differ in the conceptualization of pain catastrophizing.
In addition to pain outcomes, pain catastrophizing is also related to depressive symptoms not only in chronic pain patients but in healthy samples as well (Lu et al. 2011; Keskindag et al. 2022). However, there are studies indicating that trait pain catastrophizing is independent from depressive symptoms in pain-free individuals, but they are linked when pain complaints (e.g., headache) are present (Buenaver et al. 2008). On the one hand, this may mean that the negative correlates of pain catastrophizing can only be detected in a healthy sample if the person is actually exposed to pain (Buenaver et al. 2008). Or alternatively, there may be moderating factors that affect the relationship between pain catastrophizing and mood (or affective distress) in the absence of pain symptoms. These results may also be of interest because studies have demonstrated not only a cross-sectional but also a prospective bi-directional association between depressive symptoms and pain (Bondesson et al. 2018).
This association is not surprising given the theoretical and empirical findings that define pain as an interoceptive signal, a specific behavioral motivation and sensation, and even a homeostatic emotion, which could generate intensive activity in the sympathetic nervous system and trigger a neuroendocrine response (Craig 2003).
Previous research indicated that brain regions responsible for the generation of emotion (e.g., medial prefrontal cortex, insular cortex, anterior temporal cortex, hypothalamus, and amygdala) have extensive connections with brainstem structures involved in pain regulation (periaqueductal gray-PAG and rostral ventral medulla-RVM) (Fields 2000; Rainville et al. 1997). Anticipation of pain, as well as negative emotional states like depression, can activate brain regions such as the anterior cingulate gyrus, intensifying the perception of pain. Depression (due to negative expectancies and neurotransmitter imbalances) might amplify pain signals and might decrease the modulatory effect of the descending pain system (Bair et al. 2003).
Neuroimaging studies show that pain catastrophizing can be connected to structural and functional alterations in the brain (Galambos et al. 2019). Previous results indicate that those brain structures which are involved in the processing of the affective components of pain and pain catastrophizing, the emotional and cognitive inhibitory control (such as anterior cingulate cortex, ventromedial or dorsolateral prefrontal cortex) also have an important role in the regulation of the autonomic nervous system (ANS) (Thayer et al. 2010; Koenig et al. 2016; Galambos et al. 2019). The ANS plays an important role in regulating several involuntary physiologic processes, e.g., respiration, blood pressure and heart rate as well (Waxenbaum et al. 2022).
It is known that the heart is dually innervated, reflecting both sympathetic and parasympathetic influences. However, the results of pharmacological blockade studies indicate that under resting circumstances the heart is mainly under the tonic inhibitory control of the parasympathetic nervous system (Jose and Collison 1970). Therefore, we can propose that the balance of the resting ANS promotes energy conservation by giving priority to the activity of the parasympathetic system over the sympathetic influences (Thayer et al. 2012). The variability in the time interval between heartbeats—the heart rate variability (HRV)—could be a suitable index to measure vagal dominance (although it is worth mentioning that HRV has vagal and non-vagal components as well) (Ritz et al. 2012; Thayer et al. 2012; de Geus et al. 2019). This oscillation in the beat-to-beat changes in heart rate time series was extensively investigated over the past years. For instance, the Neuro Visceral Integration Model states that HRV could reflect the degree to which the brain (especially the prefrontal cortex) is capable of flexibly controlling the periphery, thus also providing a reliable index for top-down self-regulatory and inhibitory control (Park et al. 2014). According to Lane and colleagues (2009), HRV also mirrors the ability to regulate emotions and behavior (Lane et al. 2009; Thayer et al. 2009). Despite that some former studies did not find significant associations between HRV and self-regulation (Blankson et al. 2012), many empirical findings showed reduced HRV in connection with a wide range of mental and somatic problems (such as anxiety, depression, substance abuse and functional somatic symptoms) and emotion- or self-regulation deficits (Ingjaldsson et al. 2003; Rottenberg 2007; Aldao et al. 2013; Capuana et al. 2014; Beauchaine and Thayer 2015; Jarczok et al. 2015; Williams et al. 2015; Chudleigh et al. 2019).
In addition, various studies raised the potential moderator role of HRV (measured both by time and frequency domain indices) in various psychological processes. For instance, investigating healthy participants, Dell’Acqua and colleagues (2021) found that HRV significantly moderated the positive association between depressive rumination and depressed mood (Dell’Acqua et al. 2021). Furthermore, as a putative marker for stress vulnerability, HRV also moderated the relationship between psychological distress and race-related stress, especially among African-American men (Utsey and Hook 2007). All these results indicate that HRV could be interpreted as a proxy for emotion regulation as well and might have a protective or buffer effect from unfavorable outcomes. Indeed, a previous study showed that those young adults who experienced childhood maltreatment were at significant risk for suicidal thoughts through low self-esteem, but for young adults with higher level of HRV (expressed as HF-HRV) the indirect effect from emotional abuse to suicidal ideation through self-esteem was nonsignificant (Duprey et al. 2019).
To the best of our knowledge, only a few studies investigated the association between pain catastrophizing and HRV. While comparing chronic pain patients (suffering from whiplash disorder) and healthy controls, Koenig and colleagues (2016) found that HF-HRV was lower and pain catastrophizing was higher in chronic pain patients than in controls, and pain catastrophizing was associated with lower HF-HRV in both groups. However, they did not find significant differences between the groups in the strength of the association of pain catastrophizing and HRV (Koenig et al. 2016). These findings provide further support to the hypothesis that lower HRV might be an underlying psychophysiological mechanism in pain catastrophizing not just in chronic pain patients but in healthy controls as well.
Considering that people with high pain catastrophizing could be at risk of developing pain-related problems including pain severity, disability or depression (Quartana et al. 2009; Galambos et al. 2021), it is paramount to map those moderator factors that could have a protective effect. According to the above-mentioned results, higher HRV as a putative proxy of emotion regulation could be one of these psychophysiological factors (Koenig et al. 2016; Dell’Acqua et al. 2021). For this reason, in the present study we aimed to test the association of self-reported pain catastrophizing, depressed mood and resting HRV in a healthy young adult sample. We hypothesized that higher pain catastrophizing would be associated with more depressed mood, and HRV (indexed by time domain index of RMSSD) would moderate this association. Since depressive rumination is a widely known correlate of depressed mood (Aldao et al. 2010; Nolen-Hoeksema and Watkins 2011; McLaughlin et al. 2014; Watkins and Nolen-Hoeksema 2014), we aimed to control for its effect in the analysis along with sex and age.
Method
Participants
University students were invited to take part in the present study (for a more detailed description, see (Kocsel et al. 2019)). All the participants were BA psychology students. The recruitment was carried out through internal invitation. Participants were compensated with extra course credits in return. Overall, 90 individuals agreed to participate. Exclusion criteria consisted of smoking, caffeine or alcohol consuming, excessive physical exercising 6 h before the resting HRV recording. Psychotropic or cardiovascular drug use and chronic heart conditions were also assessed in order to evaluate the current state of heart functions. Eighteen volunteers were excluded due to the occurrence of the aforementioned criteria or data quality concerns with their resting HRV registration. The final sample consists of 72 healthy undergraduate students (58 females (80,6%)) aged between 19 and 28 years (mean age = 22.24; SD = 1.79 years).
Procedure
Filling out the questionnaires and measuring resting HRV were completely voluntary and anonymous. Participants were given a consent form to fill out before the examination and received information regarding the purpose and the methodology of the study. Each subject was asked to choose a code which allowed us to combine survey data with data from the resting HRV measurement while still upholding anonymity. 6 h prior to the cardiac activity registration, individuals were instructed not to consume caffeine and alcohol or perform strenuous exercise. Upon arrival at the laboratory, participants were asked to answer demographic questions. After that we registered the participant’s height, weight and physical activity level. Resting HRV measurement was conducted in a quiet room. The subjects were assisted in putting the plastic chest belts on by qualified research assistants. After the belt was attached, each participant underwent a 7-min resting HRV measurement. They were instructed to lie down, keep their eyes closed and were asked to rest and breathe normally. After the measurement, self-reported questionnaires were completed in a different room. The entire duration of the study was approximately 60 min.
The study was approved by the Institutional Review Board of ELTE Eötvös Loránd University, and the work was performed in accordance with the Declaration of Helsinki.
Measurements
Heart rate variability
Resting HRV was registered by the FirstBeat TeamBelt from the FirstBeat Sports Team Pack (developed by FirstBeat Technologies Ltd., Jyväskylä, Finland). Previous research provided evidence for the device’s reliability (Bogdány et al. 2016). Heartbeats were recorded at a sampling rate of 1024 Hz. The measuring device includes a belt that can be attached to the rib cage beneath the pectoral muscles. The belt contains two electrodes, which are used to detect heartbeat. The data are transmitted to the laptop-connected receiver by a wireless unit. Data were collected when subjects were lying horizontally and breathed slowly, naturally for 7 min. The last 5 min of the recordings were used to calculate HRV using the FirstBeat Sports and Kubios 2.0 software (Tarvainen et al. 2014). The FirstBeat Sports Software automatically adjusted outliers in the interbeat intervals (IBIs). According to Saalasti, Seppänen, and Kuusela (2004), the program has an effective artifact correction process for signal noise and irregular beats (Saalasti et al. 2004; Parak and Korhonen 2013).
A number of indicators are determined to operationalize HRV. In this study, the time domain metric of HRV, the root mean square of successive differences (RMSSD), was used (Thayer et al. 2012). RMSSD was log transformed to fit assumptions of linear analyses. According to a previous study, RMSSD is referred as stable (Thayer and Sternberg 2010).
Self-reported measurements
The Zung Self-Rating Depression Scale (ZDS) (Zung 1965; Simon 1998) was used to measure self-reported depression. The 20 items of the questionnaire cover both the psychological and somatic symptoms of depression (e.g., “I am more irritable than usual”). Participants were asked to evaluate each item on a 4-point Likert scale ranging from 1 (a little of the time) to 4 (most of the time). Ten items of the scale are reversed that must be taken into consideration while calculating the final score. The sum of the scores ranged between 20 and 80, and higher score indicated the presence of more depressive symptoms. Cutoff criteria for depression are 48 scores (Simon 1998). In the current analysis, the internal consistency of the scale was high (Cronbach’s alpha = 0.85).
The Pain Catastrophizing Scale (PCS) (Sullivan et al. 1995) was used to evaluate the tendency to exaggerate pain and to feel helpless facing current or anticipated pain. The scale consists of 13 items and three subscales to assess different aspect of pain catastrophizing: Rumination (“I can’t seem to keep it out of my mind”), Magnification (“I become afraid that the pain will get worse”), Helplessness (“I feel I can’t go on”). The respondents were asked to score each item on a 5-point Likert scale from 0 (not at all) to 4 (all the time). A score of 52 is the maximum that can be achieved on scale. In the current study, total score was calculated and used in the further analyses. PCS showed good psychometric properties on a healthy sample (total score’s Cronbach’s alpha = 0.85; Sullivan et al. 1995) and on a clinical sample (total score’s Cronbach’s alpha = 0.87; (Kökönyei 2008)). The scale’s good internal consistency is supported by our study (Cronbach’s alpha = 0.94).
The short form of the Ruminative Response Scale (RRS) (Treynor et al. 2003) measures ruminative tendencies. The 10 items of the questionnaire (e.g., “Go someplace alone to think about your feelings”) reflect two distinct components of the construct: brooding and reflective pondering. Respondents answer on a 4-point Likert scale from 1 (almost never) to 4 (almost always). Greater scores reflect an increased presence of trait ruminative thoughts. The Hungarian version of the scale demonstrated good internal consistency (Cronbach’s alphas were 0.71 and 0.73, respectively) (Kökönyei et al. 2016; Eszlári and Kökönyei 2022)). In the present study, only the total score was calculated, and the scale showed adequate reliability in the current sample (Cronbach’s alpha = 0.70).
Beyond psychological scales, data about age, sex, self-reported height and weight was collected along with self-reported physical fitness (1 = very poor, 5 = excellent).
Statistical analysis
Data analysis and visualization were performed using SPSS (version 28., IBM Chicago, USA) and the PROCESS macro by Hayes (version 4.2) (Hayes 2017). Correlations between variables were tested by Pearson correlation with bootstrapping (BCa 95% CI). In the moderation analysis (Model 1 in the PROCESS macro), we investigated the putative moderator effect of resting HRV (indexed by lnRMSSD) in the relation of pain catastrophizing (measured by the total scores of PCS) and depressed mood (ZDS total scores). In the analysis, sex (coded as 1 = male, 2 = female), age (in years), trait rumination (RRS total scores) and physical fitness were added as covariates. All statistical tests were two two-tailed and were analyzed with a set level of significance of p < 0.05. In the regression model, all continuous variables were mean centered.
Results
As can be seen in Table 1, mean heart rate and BMI were in the normal range. We report mean heart rate in two forms: as HR expressed in beats per minute (bpm) and as interbeat interval or IBI (in ms). Means, medians, standard deviations and internal consistency of psychological scales are also presented along with the cardiac and physiological indices.
Correlation analysis showed a significant, although weak positive association between pain catastrophizing, depressed mood and trait rumination (see Table 2). However, none of the cardiac indices correlated significantly with these psychological scales. As expected, physical fitness correlated negatively with mean HR (r = -0.27, p < 0.05, BCa 95% CI) and positively with mean IBI (r = 0.31, p < 0.05, BCa 95% CI) and lnRMSSD (r = 0.26, p < 0.05, BCa 95% CI).
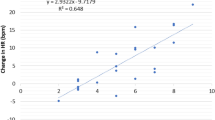
We hypothesized that heart rate variability (lnRMSSD) would significantly moderate the association between pain catastrophizing and depression. The analysis supported this hypothesis: both the main effect of PCS and the interaction of PCS and lnRMSSD significantly explained the variance of ZDS. The significant associations remained after the adjustment of sex, age, physical fitness and trait rumination (for details please, refer to Table 3). Figure 1 also demonstrates that this interplay could have the biggest effect on depressed mood when the level of pain catastrophizing is high and the lnRMSSD is low. When analyzing the slopes of the regression lines we found that they significantly differed from zero at low (t = 3.28; p = 0.002) and medium levels of lnRMSSD (t = 2.65; p = 0.010). The slope at high levels of lnRMSSD does not significantly differed from 0 (t = 0.37; p = 0.712).
The moderator role of resting HRV in the association of pain catastrophizing and depressed mood among healthy participants. Note: lnRMSSD levels were defined as the mean and + / − 1 standard deviation. PCS Pain Catastrophizing Scale, ZDS Zung Depression Scale, lnRMSSD log transformed root mean square of successive differences
One could argue that acute illnesses could influence these associations. For this reason, we also asked our participants whether they had any illness in the last two weeks (e.g., cold, flu, viral or bacterial diarrhea, pneumonia, fever, etc.). When we entered this dichotomous variable in the moderation analysis, the results did not change significantly.
Discussion
The primary goal of the current study was to examine the moderating effect of resting HRV (indexed by lnRMSSD) on the association between pain catastrophizing and depressive symptoms. Our results provided supporting evidence that the relationship between pain catastrophizing and depressive symptoms is significantly moderated by HRV in healthy individuals. In participants with higher resting HRV, there was no significant relationship between the two investigated variables, while in participants with relatively low or medium HRV pain catastrophizing and depressed mood showed significant positive association. In addition, the relationship remained significant after controlling for sex, age and trait rumination. Our findings are consistent with the Neuro Visceral Integration Model which suggests that higher HRV reflects better regulatory functions (Thayer and Lane 2009).
These results indicate that higher vagal activity (indexing by higher HRV) might operate as a protective factor against depressive symptoms. Indeed, the potentially protective role of HRV was found by Batselé and colleagues (2019) in a non-clinical sample as well. They specifically found that at low levels of HRV there was a negative association between trait intrapersonal emotional competence and depression. At the same time, in participants with high levels of HRV, this association was no longer significant (Batselé et al. 2019).
Similarly, at higher levels of HRV, neuroticism resulted to be a less consequential predictor of problematic outcomes than at lower levels of HRV (Ode et al. 2010). Moreover, the interaction between resting HRV and thought suppression also seemed to predict generalized distress symptoms (Gillie et al. 2015). Our results are in line with these empirical findings, highlighting that HRV might serve as a biomarker of self-regulatory mechanisms and might have a protective role against detrimental outcomes such as depression or anxiety.
Interestingly, in our analysis resting HRV did not correlate with pain catastrophizing, trait rumination or depressive symptoms. These results are surprising, as previous studies have shown reduced resting HRV in various forms of psychopathology (Chalmers et al. 2014; Faurholt-Jepsen et al. 2017) in both clinical and experimental studies (McLaughlin and Nolen-Hoeksema 2011; Johnson et al. 2014). However, a recent cross-sectional study (Li et al. 2022) with a large sample of healthy adults found no significant correlation between resting HRV (measured via RMSSD) and trait rumination. This aligns with our previous findings, where we also did not observe a significant association between resting HRV and trait rumination (Kocsel et al. 2019).
Certain features of our study may partially explain the lack of correlation between HRV and the psychological constructs. Although the participants in our dataset showed a diverse range of rumination habits, pain catastrophizing or depressed symptoms, they were all healthy young people without mental illnesses. It is possible that the link between resting HRV and rumination/catastrophizing is stronger in individuals with disrupted parasympathetic control due to prolonged stress exposure or those with stress-related mental disorders (Renna et al. 2022).
Moreover, our results showed a weak, positive correlation between pain catastrophizing and depressive rumination. Despite that depressive rumination and pain catastrophizing have overlapping and similar features, and they are both considered as forms of perseverative cognition (Sullivan et al. 1995; Schütze et al. 2020), generally they are not discussed together. Since rumination about pain is one subscale of the Pain Catastrophizing Scale, we can consider pain catastrophizing as a content-specific form of perseverative cognition. These results shed light on the importance of examining the content-specific repetitive thoughts beyond the general or depressive perseverative thoughts.
As we expected, resting HRV positively correlated with physical fitness, although the magnitude of the correlation was small. This small correlation might be explained by the fact that our participants were not athletes and physical activity was only measured by one item. In addition, it could also be possible that HRV might be greater in active individuals than in sedentary people, but HRV does not increase in a dose-dependent manner with increasing levels of physical activity (Melanson 2000).
Limitations
Our study has certain limitations. First, more women than men participated in the study that might affect our results. In a previous study, sex moderated the relationship of catastrophizing with pain sensitivity and analgesia: it was evident in men, but not in women (Fillingim et al. 2005). Thus, sex might moderate the found associations, but our sample size did not allow to test the three-way interaction. It is important to acknowledge that the modest sample size represents a general limitation that should be taken into account when interpreting the results.
As we did not evoke pain, only trait pain catastrophizing was measured. Interestingly, the mean scores of PCS were around 20, which is slightly higher than in the study by Procento and colleagues (2018) measuring state and trait-level pain catastrophizing among healthy adults. Using the standard instruction, we asked our participants to think of general (past) painful experience as a reference when answering the items. However, Kapoor and colleagues (2015) pointed out that participants often ignore this instruction and recall either a specific pain experience (e.g., a recent injury) or a pain symptom (e.g., a headache), or the worst pain they have ever experienced (Kapoor et al. 2015). These interindividual differences in pain reference might impact the overall mean of PCS in a study. It is tempting to assume that it is easier to ruminate and feel helpless about a more intense and salient pain experience. However, it is worth noting that in the present analysis we applied only the total score of PCS and did not investigate how the subscales (e.g., rumination and helplessness about pain) could be related to depressive symptoms. Regarding the PCS, it is the most frequently used questionnaire for assessing pain catastrophizing, although Crombez and colleagues (2020) have recently raised some concerns about it. They have suggested that the questionnaire measures pain-related worrying rather than catastrophizing. For the present analysis, it means that we investigated the relationship between a content-specific (i.e., pain) perseverative cognition and depressive symptoms, while looking for a potential moderator (Crombez et al. 2020).
We excluded participants who reported chronic conditions, but we did not exclude those who were otherwise healthy but reported the occurrence of acute illnesses in the past two weeks. However, our results did not change when we controlled for this variable. Regarding HRV measurement and analysis, controlling for breathing parameters is advised (Ritz 2009), and however, the device we used did not allow for the measurement of respiratory parameters. However, we aimed to control for its effect, since during heart rate recordings, participants were lying, and we did not use the first 2 min of the recorded 7 min, as it was considered as an adaptation period.
Conclusion
In summary, the current research highlighted that evaluating the relationship between pain catastrophizing and depressive symptoms is important not only in a chronic pain sample, but also in healthy individuals. Our study also points out the cardinal and potentially protective role of resting HRV, which might be an important moderator in the relationship of pain catastrophizing and depressive symptoms. Identifying underlying mechanisms might provide a better understanding of those psychophysiological processes through which HRV influences the relationship between these constructs. According to the findings of Van Den Houte and colleagues (2018), endogenous pain modulation (EPM) might be one of these mechanisms since individuals with higher resting HRV had a more efficient EPM even in the absence of chronic pain (Van Den Houte et al. 2018). Future studies should focus on these mechanisms considering that reduced HRV could contribute to the development of later (chronic) pain.
Conclusions for future biology
Our findings regarding the moderating role of HRV raise questions about the mechanisms through which increased cardiac vagal activity exerts its effects, specifically how it can mitigate the impact of various risk factors on mental health.
HRV is commonly regarded as an indicator of cardiac vagal tone, which signifies the contribution of the parasympathetic nervous system to cardiac regulation. However, as Marmerstein and colleagues (2021) have noted, many studies treat HRV as a representation of overall vagal or parasympathetic activity. In their recent study, they directly measured tonic vagal activity in rats and found no correlation between HRV metrics, average vagal activity and phasic respiratory activity (Marmerstein et al. 2021). Their study concluded that HRV does not reflect the overall vagal activity; instead, it is more likely associated with the activity of a subset of vagal fibers that modulate their activity in response to physiological changes, as evidenced by empirical findings from vagal blockade and vagotomy on HRV. Consequently, HRV does not represent “vagal tone” for all organs innervated by the vagus nerve (Karemaker 2022).
Nonetheless, there is a wealth of empirical evidence concerning the relationship between psychological functioning and mental health in relation to both tonic (resting) and phasic HRV. The interpretation of these findings in psychological research should be informed by insights from biological research.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ádám G (1978) Visceroception, Awareness, and Behavior. In: Schwartz GE, Shapiro D (eds) In: consciousness and self-regulation: advances in research and theory, vol 2. Springer, pp 199–213
Ádám G (1998) Visceral perception: understanding Internal Cognition. Plenum Press. https://doi.org/10.1007/978-1-4757-2903-0
Aldao A, Mennin DS, McLaughlin KA (2013) Differentiating worry and rumination: evidence from heart rate variability during spontaneous regulation. Cognit Ther Res 37:613–619
Aldao A, Nolen-Hoeksema S, Schweizer S (2010) Emotion-regulation strategies across psychopathology: aA meta-analytic review. Clin Psychol Rev 30:217–237. https://doi.org/10.1016/j.cpr.2009.11.004
Bair MJ, Robinson RL, Katon W, Kroenke K (2003) Depression and pain comorbidity: a literature review. Arch Intern Med 163:2433–2445. https://doi.org/10.1001/archinte.163.20.2433
Batselé E, Stefaniak N, Fantini-Hauwel C (2019) Resting heart rate variability moderates the relationship between trait emotional competencies and depression. Personality Individ Differ 138:69–74. https://doi.org/10.1016/j.paid.2018.09.020
Beauchaine TP, Thayer JF (2015) Heart rate variability as a transdiagnostic biomarker of psychopathology. Int J Psychophysiol 98:338–350. https://doi.org/10.1016/j.ijpsycho.2015.08.004
Beneciuk JM, Bishop MD, George SZ (2010) Pain catastrophizing predicts pain intensity during a neurodynamic test for the median nerve in healthy participants. Man Ther 15:370–375. https://doi.org/10.1016/j.math.2010.02.008
Besen E, Gaines B, Linton SJ, Shaw WS (2017) The role of pain catastrophizing as a mediator in the work disability process following acute low back pain. J Appl Biobehav Res 22:e12085. https://doi.org/10.1111/jabr.12085
Blankson AN, O’Brien M, Leerkes EM et al (2012) Differentiating processes of control and understanding in the early development of emotion and cognition. Soc Dev 21:1–20. https://doi.org/10.1111/j.1467-9507.2011.00593.x
Bogdány T, Boros S, Szemerszky R, Köteles F (2016) Validation of the Firstbeat TeamBelt and BodyGuard2 systems. [A Firstbeat TeamBelt és a BodyGuard2 pulzusmérő eszközök validálása]. Magyar Sporttudományi Szemle 17:5–12. https://mstt.hu/wp-content/uploads/MSSZ2016_03.pdf
Bondesson E, Larrosa Pardo F, Stigmar K et al (2018) Comorbidity between pain and mental illness - Evidence of a bidirectional relationship. Eur J Pain 22:1304–1311. https://doi.org/10.1002/ejp.1218
Buenaver LF, Edwards RR, Smith MT et al (2008) Catastrophizing and pain-coping in young adults: associations with depressive symptoms and headache pain. J Pain 9:311–319. https://doi.org/10.1016/j.jpain.2007.11.005
Capuana LJ, Dywan J, Tays WJ et al (2014) Factors influencing the role of cardiac autonomic regulation in the service of cognitive control. Biol Psychol 102:88–97. https://doi.org/10.1016/j.biopsycho.2014.07.015
Chalmers JA, Quintana DS, Abbott MJ-A, Kemp AH (2014) Anxiety disorders are associated with reduced heart rate variability: a meta-analysis. Front Psychiatry 5. https://doi.org/10.3389/fpsyt.2014.00080
Chudleigh C, Savage B, Cruz C et al (2019) Use of respiratory rates and heart rate variability in the assessment and treatment of children and adolescents with functional somatic symptoms. Clin Child Psychol Psychiatry 24:29–39. https://doi.org/10.1177/1359104518807742
Craig AD (2003) A new view of pain as a homeostatic emotion. Trends Neurosci 26:303–307. https://doi.org/10.1016/s0166-2236(03)00123-1
Crombez G, De Paepe AL, Veirman E et al (2020) Let’s talk about pain catastrophizing measures: an item content analysis. PeerJ 8:e8643. https://doi.org/10.7717/peerj.8643
de Geus EJC, Gianaros PJ, Brindle RC et al (2019) Should heart rate variability be “corrected” for heart rate? Biological, quantitative, and interpretive considerations. Psychophysiology 56:e13287. https://doi.org/10.1111/psyp.13287
Dell’Acqua C, Dal Bò E, Messerotti Benvenuti S et al (2021) Depressed mood, brooding rumination and affective interference: The moderating role of heart rate variability. Int J Psychophysiol 165:47–55. https://doi.org/10.1016/j.ijpsycho.2021.03.011
Duprey EB, Oshri A, Liu S (2019) Childhood maltreatment, self-esteem, and suicidal ideation in a low-ses emerging adult sample: the moderating role of heart rate variability. Arch Suicide Res 23:333–352. https://doi.org/10.1080/13811118.2018.1430640
Edwards RR, Bingham CO, Bathon J, Haythornthwaite JA (2006) Catastrophizing and pain in arthritis, fibromyalgia, and other rheumatic diseases. Arthritis Rheum 55:325–332. https://doi.org/10.1002/art.21865
Eszlári N, Kökönyei Gy (2022) Ruminatív Válaszstílus Kérdőív. In: Horváth, Zsolt; Urbán, Róbert; Kökönyei, Gyöngyi; Demetrovics, Zsolt (Eds.), Kérdőíves módszerek a klinikai és egészségpszichológiai kutatásban és gyakorlatban. Medicina, pp 127–132
Faurholt-Jepsen M, Kessing LV, Munkholm K (2017) Heart rate variability in bipolar disorder: A systematic review and meta-analysis. Neurosci Biobehav Rev 73:68–80. https://doi.org/10.1016/j.neubiorev.2016.12.007
Feinstein AB, Sturgeon JA, Darnall BD et al (2017) The effect of pain catastrophizing on outcomes: a developmental perspective across children, adolescents, and young adults with chronic pain. J Pain 18:144–154. https://doi.org/10.1016/j.jpain.2016.10.009
Fields HL (2000) Pain modulation: expectation, opioid analgesia and virtual pain. Prog Brain Res 122:245–253. https://doi.org/10.1016/s0079-6123(08)62143-3
Fillingim RB, Hastie BA, Ness TJ et al (2005) Sex-related psychological predictors of baseline pain perception and analgesic responses to pentazocine. Biol Psychol 69:97–112. https://doi.org/10.1016/j.biopsycho.2004.11.008
Gaffey AE, Burns JW, Aranda F et al (2020) Social support, social undermining, and acute clinical pain in women: Mediational pathways of negative cognitive appraisal and emotion. J Health Psychol 25:2328–2339. https://doi.org/10.1177/1359105318796189
Galambos A, Stoll DP, Bolczár S et al (2021) A bifactor structural model of the Hungarian Pain Catastrophizing Scale and latent classes of a clinical sample. Heliyon 7:e08026. https://doi.org/10.1016/j.heliyon.2021.e08026
Galambos A, Szabó E, Nagy Z et al (2019) A systematic review of structural and functional MRI studies on pain catastrophizing. J Pain Res 12:1155–1178. https://doi.org/10.2147/JPR.S192246
George SZ, Calley D, Valencia C, Beneciuk JM (2011) Clinical investigation of pain-related fear and pain catastrophizing for patients with low back pain. Clin J Pain 27:108–115. https://doi.org/10.1097/AJP.0b013e3181f21414
Gillie BL, Vasey MW, Thayer JF (2015) Individual differences in resting heart rate variability moderate thought suppression success. Psychophysiology 52:1149–1160. https://doi.org/10.1111/psyp.12443
Grosen K, Drewes AM, Pilegaard HK et al (2016) Situational but not dispositional pain catastrophizing correlates with early postoperative pain in pain-free patients before surgery. J Pain 17:549–560. https://doi.org/10.1016/j.jpain.2015.12.016
Hayes AF (2017) Introduction to mediation, moderation, and conditional process analysis. Regression-based approach, second edn. The Guilford Press, New York, NY, USA
Ingjaldsson JT, Laberg JC, Thayer JF (2003) Reduced heart rate variability in chronic alcohol abuse: relationship with negative mood, chronic thought suppression, and compulsive drinking. Biol Psychiatry 54:1427–1436
Jarczok MN, Kleber ME, Koenig J et al (2015) Investigating the associations of self-rated health: heart rate variability is more strongly associated than inflammatory and other frequently used biomarkers in a cross sectional occupational sample. PLoS ONE 10:e0117196. https://doi.org/10.1371/journal.pone.0117196
Johnson JA, Key BL, Routledge FS et al (2014) High trait rumination is associated with blunted nighttime diastolic blood pressure dipping. Ann Behav Med 48:384–391. https://doi.org/10.1007/s12160-014-9617-8
Jose AD, Collison D (1970) The normal range and determinants of the intrinsic heart rate in man. Cardiovasc Res 4:160–167. https://doi.org/10.1093/cvr/4.2.160
Kakon G, Mohamadi A-AK, Levtova N et al (2021) Elevated Heart Rate and Pain During a Cold Pressor Test Correlates to Pain Catastrophizing. Appl Psychophysiol Biofeedback 46:359–366. https://doi.org/10.1007/s10484-021-09520-4
Kapoor S, Thorn BE, Bandy O, Clements KL (2015) Pain referents used to respond to the pain catastrophizing scale. Eur J Pain 19:400–407. https://doi.org/10.1002/ejp.561
Karemaker JM (2022) The multibranched nerve: vagal function beyond heart rate variability. Biol Psychol 172:108378. https://doi.org/10.1016/j.biopsycho.2022.108378
Keskindag B, Karaaziz M, Cirhinlioğlu FG (2022) Dispositional pain catastrophising in non-clinical sample: The role of depression, perceived stress and social support. Curr Psychol 41:4457–4465. https://doi.org/10.1007/s12144-020-00956-1
Kocsel N, Köteles F, Szemenyei E et al (2019) The association between perseverative cognition and resting heart rate variability: a focus on state ruminative thoughts. Biol Psychol. https://doi.org/10.1016/j.biopsycho.2019.04.004
Koenig J, De Kooning M, Bernardi A et al (2016) Lower Resting State Heart Rate Variability Relates to High Pain Catastrophizing in Patients with Chronic Whiplash-Associated Disorders and Healthy Controls. Pain Pract 16:1048–1053. https://doi.org/10.1111/papr.12399
Kökönyei Gy (2008). Érzelemszabályozás krónikus fájdalomban [Emotion regulation in chronic pain]. Doctoral Dissertation. Eötvös Loránd University, Budapest, Hungary
Gy K, Szabó E, Kocsel N et al (2016) Rumination in migraine: Mediating effects of brooding and reflection between migraine and psychological distress. Psychol Health 31:1481–1497. https://doi.org/10.1080/08870446.2016.1235166
Lane RD, McRae K, Reiman EM et al (2009) Neural correlates of heart rate variability during emotion. Neuroimage 44:213–222. https://doi.org/10.1016/j.neuroimage.2008.07.056
Li Z, Pulopulos M, Allaert J, et al (2022) Resting HRV as a trait marker of rumination in healthy individuals? A large cross-sectional analysis. Authorea Preprints. https://doi.org/10.22541/au.167156295.58669034/v1.
Lu Q, Uysal A, Teo I (2011) Need satisfaction and catastrophizing: explaining the relationship among emotional ambivalence, pain, and depressive symptoms. J Health Psychol 16:819–827. https://doi.org/10.1177/1359105310392092
Marmerstein JT, McCallum GA, Durand DM (2021) Direct measurement of vagal tone in rats does not show correlation to HRV. Sci Rep 11:1210. https://doi.org/10.1038/s41598-020-79808-8
Martel MO, Jamison RN, Wasan AD, Edwards RR (2014) The association between catastrophizing and craving in patients with chronic pain prescribed opioid therapy: a preliminary analysis. Pain Med 15:1757–1764. https://doi.org/10.1111/pme.12416
McLaughlin KA, Aldao A, Wisco BE, Hilt LM (2014) Rumination as a transdiagnostic factor underlying transitions between internalizing symptoms and aggressive behavior in early adolescents. J Abnorm Psychol 123:13–23. https://doi.org/10.1037/a0035358
McLaughlin KA, Nolen-Hoeksema S (2011) Rumination as a transdiagnostic factor in depression and anxiety. Behav Res Ther 49:186–193. https://doi.org/10.1016/j.brat.2010.12.006
Melanson EL (2000) Resting heart rate variability in men varying in habitual physical activity. Med Sci Sports Exerc 32:1894–1901. https://doi.org/10.1097/00005768-200011000-00012
Nolen-Hoeksema S, Watkins E (2011) A heuristic for developing transdiagnostic models of psychopathology explaining multifinality and divergent trajectories. Perspect Psychol Sci 6:589–609. https://doi.org/10.1177/1745691611419672
Ode S, Hilmert CJ, Zielke DJ, Robinson MD (2010) Neuroticism’s importance in understanding the daily life correlates of heart rate variability. Emotion 10:536–543. https://doi.org/10.1037/a0018698
Parak J, Korhonen I (2013) Accuracy of FirstBeat BodyGuard2 beat-to-beat heart rate monitor. White paper by FirstBeat Technologies Ltd.
Park G, Vasey MW, Van Bavel JJ, Thayer JF (2014) When tonic cardiac vagal tone predicts changes in phasic vagal tone: the role of fear and perceptual load. Psychophysiology 51:419–426. https://doi.org/10.1111/psyp.12186
Picavet HSJ, Vlaeyen JWS, Schouten JSAG (2002) Pain catastrophizing and kinesiophobia: predictors of chronic low back pain. Am J Epidemiol 156:1028–1034. https://doi.org/10.1093/aje/kwf136
Procento PM, Rand KL, Stewart JC, Hirsh AT (2021) Pain catastrophizing mediates and moderates the link between acute pain and working memory. J Pain 22:981–995. https://doi.org/10.1016/j.jpain.2021.03.138
Quartana PJ, Campbell CM, Edwards RR (2009) Pain catastrophizing: a critical review. Expert Rev Neurother 9:745–758. https://doi.org/10.1586/ERN.09.34
Rainville P, Duncan GH, Price DD et al (1997) Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 277:968–971. https://doi.org/10.1126/science.277.5328.968
Renna ME, Shrout MR, Madison AA et al (2022) Distress disorder histories predict HRV trajectories during and after stress. Psychoneuroendocrinology 135:105575. https://doi.org/10.1016/j.psyneuen.2021.105575
Ritz T (2009) Studying noninvasive indices of vagal control: the need for respiratory control and the problem of target specificity. Biol Psychol 80:158–168. https://doi.org/10.1016/j.biopsycho.2008.08.003
Ritz T, Bosquet Enlow M, Schulz SM et al (2012) Respiratory sinus arrhythmia as an index of vagal activity during stress in infants: respiratory influences and their control. PLoS ONE 7:e52729. https://doi.org/10.1371/journal.pone.0052729
Rottenberg J (2007) Cardiac vagal control in depression: a critical analysis. Biol Psychol 74:200–211. https://doi.org/10.1016/j.biopsycho.2005.08.010
Saalasti S, Seppänen M, Kuusela A (2004) Artefact correction for heart beat interval data. Advanced methods for processing bioelectrical signals. In: Proceedings of the ProBisi meeting, pp 1–10
Schütze R, Rees C, Smith A et al (2020) Metacognition, perseverative thinking, and pain catastrophizing: A moderated-mediation analysis. Eur J Pain 24:223–233. https://doi.org/10.1002/ejp.1479
Simon A (1998) A Zung-féle Önértékelő Depressziós Skála. In: Mérei F, Szakács F (eds) Pszichodiagnosztikai vademecum. Nemzeti Tankönyvkiadó, Budapest, pp 180–184
Subedi A, Pokharel K, Sah BP, Chaudhary P (2021) Association of preoperative pain catastrophizing with postoperative pain after lower limb trauma surgery. J Psychosom Res 149:110575. https://doi.org/10.1016/j.jpsychores.2021.110575
Sullivan MJL, Bishop SR, Pivik J (1995) The pain catastrophizing scale: development and validation. Psychol Assess 7:524–532. https://doi.org/10.1037/1040-3590.7.4.524
Tarvainen MP, Niskanen J-P, Lipponen JA et al (2014) Kubios HRV–heart rate variability analysis software. Comput Methods Prog Biomed 113:210–220. https://doi.org/10.1016/j.cmpb.2013.07.024
Thayer JF, Ahs F, Fredrikson M et al (2012) A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev 36:747–756. https://doi.org/10.1016/j.neubiorev.2011.11.009
Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH (2009) Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann Behav Med 37:141–153. https://doi.org/10.1007/s12160-009-9101-z
Thayer JF, Lane RD (2009) Claude Bernard and the heart–brain connection: further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev 33:81–88. https://doi.org/10.1016/j.neubiorev.2008.08.004
Thayer JF, Sternberg EM (2010) Neural aspects of immunomodulation: focus on the vagus nerve. Brain Behav Immun 24:1223–1228. https://doi.org/10.1016/j.bbi.2010.07.247
Thayer JF, Yamamoto SS, Brosschot JF (2010) The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol 141:122–131. https://doi.org/10.1016/j.ijcard.2009.09.543
Theunissen M, Peters ML, Bruce J et al (2012) Preoperative anxiety and catastrophizing: a systematic review and meta-analysis of the association with chronic postsurgical pain. Clin J Pain 28:819–841. https://doi.org/10.1097/AJP.0b013e31824549d6
Tran ST, Jastrowski Mano KE, Hainsworth KR et al (2015) Distinct influences of anxiety and pain catastrophizing on functional outcomes in children and adolescents with chronic pain. J Pediatr Psychol 40:744–755. https://doi.org/10.1093/jpepsy/jsv029
Treynor W, Gonzalez R, Nolen-Hoeksema S (2003) Rumination reconsidered: a psychometric analysis. Cogn Ther Res 27:247–259. https://doi.org/10.1023/A:1023910315561
Utsey SO, Hook JN (2007) Heart rate variability as a physiological moderator of the relationship between race-related stress and psychological distress in African Americans. Cultur Divers Ethnic Minor Psychol 13:250–253. https://doi.org/10.1037/1099-9809.13.3.250
Van Den Houte M, Van Oudenhove L, Bogaerts K et al (2018) Endogenous pain modulation: association with resting heart rate variability and negative affectivity. Pain Med 19:1587–1596. https://doi.org/10.1093/pm/pnx165
Velly AM, Look JO, Carlson C et al (2011) The effect of catastrophizing and depression on chronic pain–a prospective cohort study of temporomandibular muscle and joint pain disorders. Pain 152:2377–2383. https://doi.org/10.1016/j.pain.2011.07.004
Watkins ER, Nolen-Hoeksema S (2014) A habit-goal framework of depressive rumination. J Abnorm Psychol 123:24–34. https://doi.org/10.1037/a0035540
Waxenbaum JA, Reddy V, Varacallo M (2022) Anatomy, Autonomic Nervous System. In: StatPearls. StatPearls Publishing, Treasure Island (FL)
Williams DP, Cash C, Rankin C, et al (2015) Resting heart rate variability predicts self-reported difficulties in emotion regulation: a focus on different facets of emotion regulation. Front Psychol 6. https://doi.org/10.3389/fpsyg.2015.00261
Zung WW (1965) A self-rating depression scale. Arch Gen Psychiatry 12:63–70
Acknowledgements
We would like to thank Ferenc Köteles, Edina Szabó and Eszter Szemenyei for their contribution in data collection and analysis.
Funding
Open access funding provided by Eötvös Loránd University. This study was supported by the Hungarian National Research, Development and Innovation Office (FK128614, K143764). NK’s contribution was supported by the New National Excellence Program of The Ministry for Culture and Innovation from the source of the National Research, Development and Innovation Fund (ÚNKP-22–4-II-ELTE-751). GK was supported by the Hungarian Brain Research Program (Grants: 2017–1.2.1-NKP-2017–00002) and the Hungarian Brain Research Program 3.0 (NAP2022-I-4/2022).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Ethics approval
The study was approved by the Institutional Review Board of ELTE Eötvös Loránd University (Budapest, Hungary). All participants provided written informed consent before entering the study and the work was carried out in accordance with the Declaration of Helsinki.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kocsel, N., Galambos, A., Szőke, J. et al. The moderating effect of resting heart rate variability on the relationship between pain catastrophizing and depressed mood: an empirical study. BIOLOGIA FUTURA (2023). https://doi.org/10.1007/s42977-023-00190-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42977-023-00190-3