Abstract
Heat shock transcription factors (HSFs) are widely known as master regulators of the heat shock response. In invertebrates, a single heat shock factor, HSF1, is responsible for the maintenance of protein homeostasis. In vertebrates, seven members of the HSF family have been identified, namely HSF1, HSF2, HSF3, HSF4, HSF5, HSFX, and HSFY, of which HSF1 and HSF2 are clearly associated with heat shock response, while HSF4 is involved in development. Other members of the family have not yet been studied as extensively. Besides their role in cellular proteostasis, HSFs influence a plethora of biological processes such as aging, development, cell proliferation, and cell differentiation, and they are implicated in several pathologies such as neurodegeneration and cancer. This is achieved by regulating the expression of a great variety of genes including chaperones. Here, we review our current knowledge on the function of HSF family members and important aspects that made possible the functional diversification of HSFs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Living organisms are often faced with proteotoxic stresses such as heat, and the consequent denaturation of cellular proteins. Imbalance in protein homeostasis is detrimental to cellular functions and may even cause cell death. Several stress response pathways evolved to protect cells against such insults. The heat shock response (HSR) is one of the most ancient and evolutionarily conserved pathways that has been identified in all kingdoms of life ranging from prokaryotes to humans. The most characteristic feature of HSR is the prompt induction of a transcriptional program by special transcription factors leading to the activation of genes encoding molecular chaperones that protect proteins from denaturation and help to restore proteostasis. The structure of most molecular chaperones is highly conserved during evolution (Yura et al. 1993). For example, the prokaryotic DnaK shares about 60% sequence homology with the eukaryotic Hsp70 proteins, making Hsp70 one of the most highly conserved molecular chaperones (Richter et al. 2010). The transcription factors that control HSR however differ significantly in prokaryotes and eukaryotes.
In Eubacteria, the expression of molecular chaperones is regulated by specific transcription factors (Schumann 2016). In Escherichia coli, this role is fulfilled by the regulatory protein σ32 (Grossman et al. 1984). σ32 is an alternative subunit of the bacterial RNA polymerase. Under normal conditions, the Hsp70 protein DnaK and its cofactor DnaJ keep σ32 in a complex and prime it for degradation, thus reducing its level and as a result preventing the transcription of Hsp encoding genes (Rodriguez et al. 2008). Upon heat shock, these chaperones bind to the increasing numbers of unfolded proteins present in the cell and release σ32 from this complex. A similar model is suggested for the eukaryotic transcription factor HSF1 (see later) (Richter et al. 2010), although HSF1 regulation is more complex than that of σ32, as posttranslational modifications and oligomerization are also involved in the regulation of HSF1 activity (Prahlad and Morimoto 2009).
The Archaea domain includes organisms that inhabit inhospitable environments and therefore have evolved stress responses to survive the extreme heat, pH, and salinity that they encounter. The archaeal heat shock response is in some ways similar to that of other organisms, although it possesses unique features (Conway de Macario and Macario 1994). In Archaea thus far, only two transcriptional regulators of the HSR have been characterized. Phr in Pyrococcus furiosus and HSR1 in Archaeoglobus fulgidus (Kanai et al. 2010; Rohlin et al. 2005; Vierke et al. 2003). So far, no archaeal heat shock factor homologues have been identified, and it is hypothesized that the known regulators are not the sole heat shock regulators in Archaea (Kanai et al. 2010).
In eukaryotes, the evolutionarily conserved heat shock transcription factors (HSFs) are responsible for coordinating the HSR. In yeast and invertebrates, there is only a single HSF called HSF1. In baker yeast S. cerevisiae, fruit fly D. melanogaster and the nematode C. elegans, HSF1 is essential for survival (Jedlicka et al. 1997; Morton and Lamitina 2013; Sorger and Pelham 1988). Recently, it has been shown that depletion of ScHSF1 can be rescued by the simultaneous and constitutive expression of 2 key chaperones, HSP70 and HSP90, and the majority of heat shock induced gene expression is independent of HSF1 function (Solís et al. 2016). These findings suggest that ScHSF1 is required for the basal expression of molecular chaperones. In invertebrates, no such experiment has been published yet, but several studies suggest that basal expression of heat shock proteins is independent of heat shock transcription factor, while heat stress-induced gene expression is mainly controlled by HSF1 (Birch-Machin et al. 2005; Brunquell et al. 2016; Jedlicka et al. 1997; Morton and Lamitina 2013).
In vertebrates, there are at least seven functional HSF paralogues: HSF1, HSF2, HSF3, HSF4, HSF5, HSFX, and HSFY (Joutsen and Sistonen 2019; Roos-Mattjus and Sistonen 2021). These constitute a structurally and functionally diverse gene family (Fig. 1) with members playing role in several physiological and pathological processes. The murine HSF1 is clearly not an essential gene, and it is not required for the basal expression of molecular chaperones (Xiao et al. 1999), which is mediated by other transcription factors such as STAT1 (signal transducer and activator of transcription 1), STAT3, and NF-IL6 (Stephanou and Latchman 2011). In mammals, the primary coordinator of heat shock response is HSF1, however, HSF2 also takes part in activating heat shock gene expression (Pirkkala et al. 2001). HSF3 is a pseudogene in human, but a functional HSF3 protein is present in mouse and chicken. The mHSF3 activates transcription of non-classical heat shock genes such as PDZD2 (PDZ domain-containing protein 2) and PROM2 (Prominin-2), thus protecting cells against proteotoxic stress (Fujimoto et al. 2010). HSF4 plays an important role during eye lens terminal differentiation by activating DNASE2B transcription (Cui et al. 2013). In the lens fiber cell, HSF4 also up-regulates p53/TP53 protein (Gao et al. 2017). Besides the processes mentioned before, HSF4 is also involved in DNA repair through the transcriptional regulation of RAD51 (Cui et al. 2012). Relatively little information is available on the role of HSF5 protein. In zebrafish (Danio rerio), Saju et al. showed that HSF5 is predominantly expressed in testis and the loss of function mutation of the hsf5 gene leads to low sperm counts and several morphological abnormalities in spermatozoa, causing infertility (Saju et al. 2018). Several studies support that HSF5 has a role in testis in mammalian species (Chalmel et al. 2012; Kogo et al. 2010). Altogether these suggests that HSF5 is essential for the progression of meiotic prophase 1 during spermatogenesis. The functions of the sex-chromosomal HSFX and HSFY are not well-understood. It is known that they have transcription factor activity (Gaudet et al. 2011), and are suggested to regulate gametogenesis (Tessari et al. 2004; Widlak and Vydra 2017).
Phylogenetic tree of heat shock factors in vertebrates. The canonical sequences from the UniProt database have been aligned using ClustalW, and the tree was constructed from the resulting multiple sequence alignment using the maximum parsimony method and a bootstrap test of phylogeny with 100 repeats. The numbers represent these bootstrap values
HSF1 regulates the heat shock response
The most extensively studied member of the HSF family is HSF1. HSF1 has a characteristic domain structure consisting of four essential domains (Akerfelt et al. 2010) (Fig. 2a). The winged-turn-helix DNA binding domain (DBD) is required for DNA binding. The hydrophobic heptad repeat domain (HR-A/B) mediates trimerization. The hydrophobic carboxy-terminal heptad repeat (HR-C) inhibits trimerization by interacting with HR-A/B. The C-terminal transactivation domain (TAD) activates transcription of target genes. These domains are present in nearly all HSF family proteins, however in some HSFs certain domains are lacking or additional domains are also present (for details see Fig. 3 and Sect. 4.2). In mammals for example, a regulatory domain (RD) has been also identified in HSF1 that serves as a platform for several posttranslational modifications (Joutsen and Sistonen 2019). In some cases, it is not clear whether a given HSF does not have a certain domain or it has not been identified yet.
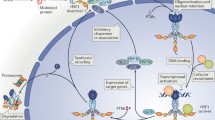
Schematic illustration of the typical, HSF protein domains and the regulation of HSR by HSF1. a Conserved HSF1 protein domains, based on mammalian HSF1. DBD: DNA binding domain, binds the consensus HSE sequence; HR-A/B oligomerization domain: heptad repeat A (HR-A) and heptad repeat B (HR-B) leucine-zipper domains mediate the trimerization of HSF1. HR-C heptad repeat domain keeps HSF1 in a monomeric form, due to its interaction with the HR-A/B domain. TAD: transactivation domain, responsible for transcriptional activation; RD: regulatory domain, modulates TAD activity. b HSF1 is activated by proteotoxic stress (red lightning). The repressive complex releases HSF1, which then trimerizes and translocates to the nucleus and upregulates genes encoding molecular chaperones.
Domain organization of heat shock factor (HSF) family members—based on Joutsen and Sistonen 2019 (modified). In the baker yeast (S. cerevisiae) and in invertebrates—the fruit fly (D. melanogaster) and the nematode (C. elegans)—a single HSF is expressed. Interestingly, the ScHSF1 contains two TADs, one on the N-terminus and another on the C-terminal end of the protein; the DB domain is further away from the N-terminal end, unlike in any other HSFs mentioned. The ScHSF1 does not contain a HR-C heptad repeat domain and such domain has not been identified in CeHSF1 yet. None of the three represented non-vertebrate proteins contain a regulatory domain. There are seven HSFs in mammals (HSF1–5, HSFX, and HSFY), which differ in their expression patterns and biological functions. HSF3 is a pseudogene in humans, in contrast functional HSF3 protein has been identified in mouse (Mus musculus). In human, HSF2 and HSF4 have two TADs, while HSF1 has only one and HSFX and HSFY have none. Human HSF1-2-and 3 contain a single RD, while HSFX and HSFY both lack this domain. mHSF1 is the only murine HSF with a regulatory domain. mHSF1 and 2 both contain a TAD, while the rest of the mouse HSFs do not. The only murine HSFs with a HR-C domain are mHSF1-2 and 3. In mice mHSFY2 is the only HSF that lacks a HR-A/B domain. In chicken (Gallus gallus) four HSFs have been identified, all of which has a similar domain structure, DBD - HR-A/B-HR-C - TAD, with the notable exception of cHSF4, which lacks a HR-C domain. The numbers indicate the number of amino acids that constitute the proteins
Although there are species- and tissue-specific features of HSF1 regulation, the chaperone titration model describes the common mechanism of HSF1 induction in all organisms (Voellmy and Boellmann 2007). In a stress-free environment, HSF1 resides in the cytoplasm in a monomeric form (Fig. 2b). In this state, the two hydrophobic heptad repeats interact with each other and trimerization is inhibited. This interaction is stabilized by several proteins forming a so-called repressive complex. Key components of this complex are the molecular chaperones Hsp70 and Hsp90, but other proteins such as histone deacetylase HDAC6 and TRiC/CCT, a central ATP-dependent chaperonin complex have also been identified to participate (Guo et al. 2001; Neef et al. 2014; Pernet et al. 2014). Upon proteotoxic stress, these components bind to unfolded proteins with high affinity, therefore they are titrated away from HSF1. This permits the trimerization and the activation of HSF1 (Hentze et al. 2016). HSF1 then translocates into the nucleus and induces the expression of molecular chaperons. During HSR heat shock proteins accumulate in the cell. Some of these proteins including HSP70, HSP40, and HSP90 bind to HSF1 and thereby inhibit its transcriptional activation and enable the reformation and stabilization of the repression complex on HSF1 monomers (Akerfelt et al. 2010). Attenuation of HSF1 activity is also facilitated by the ubiquitination and the subsequent proteosomal degradation of trimerized HSF1 (Kourtis et al. 2015). Activity of HSFs is also regulated by several posttranslational modifications such as phosphorylation, SUMOylation and acetylation of specific amino acid residues (Joutsen and Sistonen 2019).
In the nucleus, HSF1 binds to a significantly conserved upstream regulating sequence called heat shock responsive element (HSE). HSE consist of three triplets of inverted repeats of a pentameric nGAAn consensus sequence identified by each DNA binding domains of the active, homotrimerized HSF1 (Amin et al. 1988). Interestingly, it has been shown recently that DB domains of HSF1 and HSF2 differ slightly structurally and functionally giving an explanation how HSFs can regulate the expression of different target genes (Jaeger et al. 2014).
Roles of HSF1 in invertebrates
In invertebrates, HSF1 is the only heat shock transcription factor, and it is required for the induction of heat shock proteins upon proteotoxic stress. Biological roles of HSF1 identified in invertebrates so far are mostly connected with its function in proteostasis. In C. elegans, the role of HSF-1 in aging is also well-established (Hajdu-Cronin et al. 2004; Hsu et al. 2003; Morley and Morimoto 2004; Walker et al. 2003). It seems obvious that CeHSF1 activity influences aging and proteostasis by regulating heat shock proteins; however, there are clues that CeHSF-1 affects aging via HSR independent pathways (Baird et al. 2014; Barna et al. 2012; Kumsta et al. 2017). For example, overexpressing a truncated form of HSF-1 which lacks the putative transactivation domain (TAD) in C. elegans results in increased thermotolerance and longer lifespan (Baird et al. 2014).
Accumulating evidence indicates that HSF1 has an essential function in development (Barna et al. 2018; Kovács et al. 2019; Li et al. 2017). In D. melanogaster, HSF1 is required for larval development as DmHSF1 depleted animals arrest at early larval stages (Jedlicka et al. 1997). However, larvae developed into adults if HSF1 was depleted right after the second larval stage, suggesting that HSF1 plays a critical role in a narrow time window. Moreover, at physiological temperatures, the expression level of heat shock proteins was similar in both wild type and HSF1 mutants during larval development, implying that the developmental role of HSF1 is independent of heat shock proteins. This study also highlighted that DmHSF1 functions in oogenesis, since depleting HSF1 leads to maternal effect sterility (Jedlicka et al. 1997). In worms, the hsf-1(sy441) mutant strain that expresses a truncated form of CeHSF1, lacking the TAD domain, is unable to activate Hsps, but is viable (Hajdu-Cronin et al. 2004). At the same time, the null mutant hsf-1(ok600) animals arrest in L2 larval stage (Morton and Lamitina 2013). CeHSF1 was also shown to regulate the formation of stress resistant dauer larvae (Barna et al. 2012; Walker et al. 2003). A fascinating study from the Morimoto laboratory showed that CeHSF1 cooperates with E2F and DP (dimerization partner) transcription factors to drive a specific transcriptional program during C. elegans larval development (Li et al. 2016).
Functional diversification of HSFs in vertebrates
In vertebrates, there are at least six paralogues of HSFs (Fig. 3). HSFs have undergone significant functional diversification. A plethora of biological functions have been assigned to vertebrate HSFs, especially to the most extensively studied HSF1 and HSF2. These include aging, development, cell proliferation, and metabolism. HSFs were also associated with several pathologies involving neurodegeneration and cancer. These functions were thoroughly discussed in recent reviews (Barna et al. 2018; Joutsen and Sistonen 2019; Puustinen and Sistonen 2020; Syafruddin et al. 2021). Here, we concentrate on the question of how functional specialization of HSFs is achieved.
The family of HSFs was formed most probably by gene duplications. Gene duplications can supply raw material for evolution by creating opportunities for functional divergence of proteins (Innan and Kondrashov 2010). Either one of the duplicates retains its original function, while the other can evolve freely by accumulating changes or both copies evolve through the partitioning of the functions of the original protein. Mechanisms through which HSFs can acquire functional diversification include acquisition of specific spatiotemporal expression patterns, obtaining novel domain structure, being regulated by different posttranslational modifications, interacting functionally with each other or cofactors, and gaining ability to regulate different sets of target genes.
Diverse expression profiles of HSFs
Both HSF1 and HSF2 are ubiquitously expressed in most tissues, but while HSF1 is evenly expressed across tissues during development, HSF2 shows a dynamic spatiotemporal expression (Duchateau et al. 2020; Fiorenza et al. 1995). In contrast to this, increased expression of HSF4 is restricted to some tissues such as muscle tissues, cerebral cortex, midbrain, retina, and pancreas (Nakai et al. 1997; Syafruddin et al. 2021; Tanabe et al. 1999). A good example of how functional diversification is reflected in the expression profile of HSFs comes from studies describing HSF functions in the developing central nervous system (CNS) of mice. mHSF1, mHSF2, and mHSF4 are all expressed in the developing brain; however, while mHSF4 mRNA starts to accumulate only in the postnatal CNS, mHSF1 and mHSF2 exhibit dynamic and distinct expression pattern in both the prenatal and the postnatal brain (Yunhua Chang et al. 2006a, b; Kallio et al. 2002; Rallu et al. 1997).
In line with this, both HSF1 and HSF2 play roles in the developing CNS. Mice lacking HSF1 activity are prone to depression and aggression along with impaired hippocampal spinogenesis and neurogenesis (Uchida et al. 2011). Observations that HSF1 regulates two polysialyltransferase genes, St8siaII and St8siaIV in hippocampus (Homma et al. 2007) and activates Dp71 gene encoding dystrophin (Tan et al. 2015) also support the view that HSF1 activity is required for normal development of CNS. HSF2 null mutant mice exhibit several brain development abnormalities, involving enlarged ventricles, small hippocampus, and mispositioning of neurons (Yunhua Chang et al. 2006a, b; Takaki et al. 2006). These abnormalities may arise from defects in neuronal migration as activity of cyclin-dependent kinase 5 (Cdk5)—which is required for cortical lamination—is altered by HSF2 via regulating p35 and p39 (Yunhua Chang et al. 2006a, b; Tsai et al. 1994). In contrast to this, no roles for HSF4 were identified during the prenatal brain development; however it is clear now that HSF4 influences the development of the lens and the olfactory neuroepithelium in the postnatal brain (Chae et al. 1997; Fujimoto et al. 2004; Takaki et al. 2006). Function of HSF4 in lens development is also supported by reports showing that mutations of HSF4 in humans are linked to severe juvenile cataracts (Anand et al. 2018; Bu et al. 2002).
Different domain structure of HSFs can lead to divergence in regulation and function
Functional domains of the HSF family are highly conserved, though domain structures of HSFs can differ (Fig. 3) (Akerfelt et al. 2010; Gomez-Pastor et al. n.d.; Joutsen and Sistonen 2019). All HSFs have conserved DNA binding domains and N-terminal oligomerization domain (HR-A/B). HR-A/B is required for homo- or heterotrimerization to give rise to the active form of HSFs. Hydrophobic heptad repeats domain (HR-C) is present in all vertebrate HSFs but HSF4. Since this domain inhibits oligomerization and thereby DNA binding of HSFs, HSF4 has the potential to constitutively bind to the promoter of its target genes (Hentze et al. 2016; Nakai et al. 1997). Transcription activation domain (TAD) recruits transcription factors, co-factors, and chromatin-modifying factors to induce transcription of HSF target genes. Importantly, expression of target genes is not necessarily induced by the DNA binding of HSF trimers as PTMs and protein interactors can inhibit transcriptional activity of HSFs (Hensold et al. 1990). Interestingly, HSF4 bears two C-terminal transactivation domains encompassing the regulatory domain (RD) (Syafruddin et al. 2021). TAD can be inhibited or activated by the RD which serves as a platform for posttranslational modifications (PTMs) that fine-tune the transactivation activity of HSFs (Joutsen and Sistonen 2019). It is worth noting that two isoforms of HSF4 have been described having opposing effects on transcription: HSF4a represses, while HSF4b activates transcription of its target genes (Frejtag et al. 2001; Syafruddin et al. 2021; Tanabe et al. 1999). In summary, different domain structure of the HSF family of proteins can lead to significant differences in regulation and function.
Posttranslational regulation of HSF activity
Several amino acid residues of HSFs are subjected to posttranslational modifications that affect the activity of the transcription factor. These include phosphorylation, acetylation, ubiquitination and SUMOylation (Gomez-Pastor et al. 2018; Joutsen and Sistonen 2019; Roos-Mattjus and Sistonen 2021). The different PTMs can contribute to the functional diversification of HSFs, since these can provide an additional level of regulation leading to spatiotemporal differences in activity during stress and development. PTMs were best characterized in case of human and murine HSF1, HSF2, and HSF4 (Fig. 4).
Comparison of the posttranslational modifications of human HSF1, HSF2, and HSF4—based on “Joutsen and Sistonen 2019” and “Roos-Mattjus and Sistonen 2021” (modified). As part of their activation process and attenuation heat shock factors undergo several posttranslational modifications. In case of the DNA-binding domain (DBD) of HSF1, there are several lysine (K) residues that are subjected to both acetylation and SUMOylation and a few that are also ubiquitinylated. The oligomerization domain (HR-A/B) also contains a number of acetylated and SUMOylated K residues, and this is the domain with the most ubiquitinylated lysines. In the regulatory domain (RD), multiple phosphorylated serine (S) and threonine (T) residues can be found. No PTMs have been observed in the HR-C region of the protein. The transactivation domain (TAD) mostly harbors phosphorylated serine (S) residues. In HSF2 the DBD, the HR-A/B and the RD contain several SUMOylated K residues, some of which are also ubiquitinylated. No phosphorylated residues have been identified in HSF2. There are few known PTMs in HSF4. The most notable modified residue is a phosphorylated threonine in the TAD
Hyperphosphorylation of HSF1 at serine and threonine residues happens upon proteotoxic stress, suggesting that phosphorylation enhances HSF1 activity (Cotto et al. 1996; Dayalan Naidu et al. 2016), but HSF1 can be also activated in case of the complete loss of phosphorylation (Budzyński et al. 2015; Zheng et al. 2016). Intriguingly, no phosphorylated residues have been identified in HSF2 (Joutsen and Sistonen 2019; Roos-Mattjus and Sistonen 2021) and only two such residues were identified in HSF4 (Fig. 4). However, it is plausible that phosphorylated residues will be identified also in HSF2 and HSF4 as there are phosphorylated residues in HSF1 (e.g., S13) that are highly conserved in both human and murine HSFs (Online Resource 1).
Acetylation of HSF1 can affect the activity of the protein in several ways. In non-stressed cells, stability of HSF1 is mediated by the acetylation of K208 and K298, while upon chronic stress DNA binding of HSF1 is inhibited by acetylation of K residues in the DB domain (Raychaudhuri et al. 2014; Westerheide et al. 2009). Interestingly, to date, no acetylation has been observed at residues of HSF2 and HSF4; however, there are several conserved K residues such as K62, K80, and K118 in the DBD of HSF2 and HSF4 (Online Resource 1). These conserved lysines however can be also targeted by ubiquitination and SUMOylation. Proteosomal degradation plays an important role in regulating HSF-mediated transcription (Ahlskog et al. 2010; Kourtis et al. 2015; Liao et al. 2015; Raychaudhuri et al. 2014), and several lysine residues of HSF1, HSF2 and HSF4 have been identified at which these proteins can be ubiquitinylated (Akimov et al. 2018; Kim et al. 2007; Wagner et al. 2011). SUMOylation also has been shown to regulate HSF trimerization and DNA binding activity (Hietakangas et al. 2006, 2003), and several lysine residues have been proposed to be SUMOylated in all human HSFs.
Interactions between distinct HSFs contribute to functional diversity
Several studies have reported that distinct HSFs are simultaneously active in the same cells; moreover, it was also showed that HSFs can interact with each other functionally or even physically. These observations provide insight into the complexity of HSF-dependent regulation of gene expression during stress and development.
Binding sites of all HSFs are similar, so it is a plausible scenario that two distinct HSFs present in the same cell can recognize a given HSE. Indeed, such a phenomenon was reported in the case of HSF1 and HSF2 in mitotic cells (Elsing et al. 2014). Here, they showed that HSF2 and HSF1 compete for the same HSE to regulate HSP70 and HSF2 functions as a repressor of HSF1-dependent transcription during mitosis. Cooperative regulation of the same target genes by HSF1 and HSF2 has been also described in mouse testis (Korfanty et al. 2014).
It has been documented that HSF4a is able to repress HSF1 and HSF2 activated gene transcription during the HSR (Kim et al. 2012; Zhang et al. 2001). Moreover, HSF4a has been reported to compete with HSF1 for the promoter of fibroblast growth factor 7 (Fgf-7) during development of lens epithelium (Fujimoto et al. 2004). While HSF1 activates Fgf-7, HSF4a acts as a repressor. Similarly, HSF1 and HSF4 were shown to play antagonistic roles during olfactory neurogenesis in mice (Takaki et al. 2006).
Intramolecular interaction between HSFs has also been described. Several studies showed that HSF1 and HSF2 are able to interact with each other via their HR-A/B domains to form heterotrimers (El Fatimy et al. 2014; Jaeger et al. 2016; Sandqvist et al. 2009; Trinklein et al. 2004). HSF1/2 heterotrimers were shown to be localized to nuclear stress bodies, where they control the stress induced transcription of the noncoding satellite III DNA. Interestingly, overexpression of HSF2 alone induced HSF1-dependent transcription in the absence of stress (Sandqvist et al. 2009). Of note, HSF1/2 heterotrimer was recently reported to govern a specific transcriptional program required for malignancy in diverse types of cancer (Smith et al. 2022). These finding suggest that HSF1/HSF2 heterotrimers are transcriptionally competent and play pivotal role in development and disease by modifying HSF1-mediated transcription.
Cofactors and interactors influence target gene selection of HSFs
Although HSFs recognize similar HSEs in the genome, there are HSF-specific preferences in promoter architecture that make it possible for each HSF to regulate different sets of target genes. These include the number, the length and the orientation of inverted repeats of nGAAn motifs and also the position of HSEs (intergenic or intronic) (Bonner et al. 1994; Jaeger et al. 2014; Somasundaram and Bhat 2000). Interactions of HSFs with other transcription factors can also modulate target gene selection. In C. elegans, adjacent to HSEs a GC-rich motive was identified in the promoters of genes regulated by CeHSF1 during larval development (Li et al. 2016). These GC-rich motives are bound by E2F and DP which function as coactivators for HSF-1 to orchestrate specific transcriptional program required for development.
Similarly, it was reported in human K562 erythroleukemia cells that HSF1 binds to promoters which are primed to be transcribed by transcription factors such as SP2 and GATA, as well as to non-transcribed chromatin bound by CCCTC-binding factor CTCF (Vihervaara et al. 2017). Of note, HSF1 and CTCF have been recently reported to interact in vivo and in vitro, suggesting that CTCF could affect HSF1-mediated transcription (Burchfiel et al. 2021). HSF1 drives a cancer-specific transcriptional program involving many noncanonical target genes that support malignancy and thereby supports the survival of highly malignant human tumors (Mendillo et al. 2012). Interestingly, in human T-cell acute lymphoblastic leukemia cells, oncogenic NOTCH1 and HSF1 were reported to occupy the same promoters to induce HSF1-mediated transcription (Kourtis et al. 2018). In summary, accumulating evidence suggests that the target gene selection of HSFs is context-dependent; promoter architecture and cooperation with other transcription factors can shape the transcriptional program of HSFs.
Conclusions and perspectives
Originally, research of HSFs focused on the role of heat shock factors in stress response. During the last 20 years hundreds of studies revealed that the biological functions of HSFs are broader than previously thought. Now, it is clear that HSFs are crucial factors in regulating development, aging, and age-related diseases such as neurodegeneration and cancer (Fig. 5). The wide range of HSF-related functions raises the question how a single HSF1 in invertebrates or distinct HSFs in vertebrates play role in diverse physiological and pathological processes. In this review, we highlighted five mechanisms that made possible the functional diversification of the HSF family. The most fascinating of these is the finding that cofactors and interactors of HSFs can determine the transcriptional repertoire of HSFs. Identification of general and tissue- or developmental-stage specific interactors of HSFs may elucidate how switching between distinct transcriptional programs of HSFs is achieved.
HSFs plays diverse roles during stress and development. a HSF1 is required for the induction of heat shock proteins upon proteotoxic stress (Akerfelt et al. 2010). HSF1 is also involved in the regulation of other cellular stress responses such as the unfolded protein response (Heldens et al. 2011; Hou et al. 2014) and autophagy (Desai et al. 2013; Kumsta et al. 2017). In C. elegans, HSF-1 also plays a role in aging (Hajdu-Cronin et al. 2004; Hsu et al. 2003; Morley and Morimoto 2004; Walker et al. 2003). HSF1 has an essential function in development (Jedlicka et al. 1997; Li et al. 2016; Morton and Lamitina 2013; Xiao et al. 1999). HSF1 is also associated with several pathologies including cancer (Dai et al. 2007; Mendillo et al. 2012). b HSF2 takes part in activating heat shock gene expression (Pirkkala et al. 2001). It has also been shown to play a role in development (Y Chang et al. 2006a, b; Mezger et al. 1994), cell cycle (Vihervaara et al. 2013; Xing et al. 2005), and spermatogenesis (Wang et al. 2004). c HSF4 is important for a variety of physiological and pathological processes. It plays a role in cell cycle by recruiting Brg1 during the G1 phase (Tu et al. 2006). It is involved in oxidative stress, in lens epithelial cells HSF4 binds to the promoter of HMOX-1, an anti-oxidase (Liao et al. 2018). It is also established that HSF4 mutations cause cataract (Anand et al. 2018). d HSF1/2 heterotrimers localize to nuclear stress bodies, where they regulate the stress induced transcription of the noncoding satellite III DNA (Sandqvist et al. 2009). Of note, HSF1/2 heterotrimers were recently reported to govern a specific transcriptional program required for malignancy in diverse types of cancer (Smith et al. 2022). e HSF4a competes with HSF1 for the promoter of fibroblast growth factor 7 (Fgf-7) during lens development (Fujimoto et al. 2004). HSF1 activates Fgf-7, while HSF4a acts as a repressor. Similarly, HSF1 and HSF4 play antagonistic roles during olfactory neurogenesis in mice (Takaki et al. 2006). f HSFs’ interactions with other transcription factors (purple pentagon) can also modulate transcription. In C. elegans, adjacent HSEs and GC-rich motives were found in the promoters of genes that play a role in larval development (Li et al. 2016). Oncogenic NOTCH1 and HSF1 occupy the same promoters to induce HSF1-mediated transcription in human T-cell acute lymphoblastic leukemia cells (Kourtis et al. 2018)
References
Ahlskog JK, Björk JK, Elsing AN, Aspelin C, Kallio M, Roos-Mattjus P, Sistonen L (2010) Anaphase-promoting complex/cyclosome participates in the acute response to protein-damaging stress. Mol Cell Biol 30:5608–5620. https://doi.org/10.1128/MCB.01506-09
Akerfelt M, Morimoto RI, Sistonen L (2010) Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol 11:545–555. https://doi.org/10.1038/nrm2938
Akimov V, Barrio-Hernandez I, Hansen SVF, Hallenborg P, Pedersen A-K, Bekker-Jensen DB, Puglia M, Christensen SDK, Vanselow JT, Nielsen MM, Kratchmarova I, Kelstrup CD, Olsen JV, Blagoev B (2018) UbiSite approach for comprehensive mapping of lysine and N-terminal ubiquitination sites. Nat Struct Mol Biol 25:631–640. https://doi.org/10.1038/s41594-018-0084-y
Anand D, Agrawal SA, Slavotinek A, Lachke SA (2018) Mutation update of transcription factor genes FOXE3, HSF4, MAF, and PITX3 causing cataracts and other developmental ocular defects. Hum Mutat 39:471–494. https://doi.org/10.1002/humu.23395
Baird NA, Douglas PM, Simic MS, Grant AR, Moresco JJ, Wolff SC, Yates JR, Manning G, Dillin A (2014) HSF-1-mediated cytoskeletal integrity determines thermotolerance and life span. Science 346:360–363. https://doi.org/10.1126/science.1253168
Barna J, Princz A, Kosztelnik M, Hargitai B, Takács-Vellai K, Vellai T (2012) Heat shock factor-1 intertwines insulin/IGF-1, TGF-β and cGMP signaling to control development and aging. BMC Dev Biol 12:32. https://doi.org/10.1186/1471-213X-12-32
Barna J, Csermely P, Vellai T (2018) Roles of heat shock factor 1 beyond the heat shock response. Cell Mol Life Sci 75:2897–2916. https://doi.org/10.1007/s00018-018-2836-6
Birch-Machin I, Gao S, Huen D, McGirr R, White RAH, Russell S (2005) Genomic analysis of heat-shock factor targets in Drosophila. Genome Biol 6:R63. https://doi.org/10.1186/gb-2005-6-7-r63
Bonner JJ, Ballou C, Fackenthal DL (1994) Interactions between DNA-bound trimers of the yeast heat shock factor. Mol Cell Biol 14:501–508. https://doi.org/10.1128/mcb.14.1.501-508.1994
Brunquell J, Morris S, Lu Y, Cheng F, Westerheide SD (2016) The genome-wide role of HSF-1 in the regulation of gene expression in Caenorhabditis elegans. BMC Genomics 17:559. https://doi.org/10.1186/s12864-016-2837-5
Bu L, Jin Y, Shi Y, Chu R, Ban A, Eiberg H, Andres L, Jiang H, Zheng G, Qian M, Cui B, Xia Y, Liu J, Hu L, Zhao G, Hayden MR, Kong X (2002) Mutant DNA-binding domain of HSF4 is associated with autosomal dominant lamellar and Marner cataract. Nat Genet 31:276–278. https://doi.org/10.1038/ng921
Budzyński MA, Puustinen MC, Joutsen J, Sistonen L (2015) Uncoupling stress-inducible phosphorylation of heat shock factor 1 from its activation. Mol Cell Biol 35:2530–2540. https://doi.org/10.1128/MCB.00816-14
Burchfiel ET, Vihervaara A, Guertin MJ, Gomez-Pastor R, Thiele DJ (2021) Comparative interactomes of HSF1 in stress and disease reveal a role for CTCF in HSF1-mediated gene regulation. J Biol Chem 296:100097. https://doi.org/10.1074/jbc.RA120.015452
Chae T, Kwon YT, Bronson R, Dikkes P, Li E, Tsai LH (1997) Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron 18:29–42. https://doi.org/10.1016/s0896-6273(01)80044-1
Chalmel F, Lardenois A, Evrard B, Mathieu R, Feig C, Demougin P, Gattiker A, Schulze W, Jégou B, Kirchhoff C, Primig M (2012) Global human tissue profiling and protein network analysis reveals distinct levels of transcriptional germline-specificity and identifies target genes for male infertility. Hum Reprod 27:3233–3248. https://doi.org/10.1093/humrep/des301
Chang Y, Ostling P, Akerfelt M, Trouillet D, Rallu M, Gitton Y, El Fatimy R, Fardeau V, Le Crom S, Morange M, Sistonen L, Mezger V (2006a) Role of heat-shock factor 2 in cerebral cortex formation and as a regulator of p35 expression. Genes Dev 20:836–847. https://doi.org/10.1101/gad.366906
Chang Y, Östling P, Åkerfelt M, Trouillet D (2006b) Role of heat-shock factor 2 in cerebral cortex formation and as a regulatorof p35 expression. Genes
Conway de Macario E, Macario AJ (1994) Heat-shock response in Archaea. Trends Biotechnol 12:512–518. https://doi.org/10.1016/0167-7799(94)90059-0
Cotto JJ, Kline M., Morimoto RI (1996) Activation of Heat Shock Factor 1 DNA Binding Precedes Stress-induced Serine Phosphorylation: EVIDENCE FOR A MULTISTEP PATHWAY OF…. J Biol Chem
Cui X, Zhang J, Du R, Wang L, Archacki S, Zhang Y, Yuan M, Ke T, Li H, Li D, Li C, Li DW-C, Tang Z, Yin Z, Liu M (2012) HSF4 is involved in DNA damage repair through regulation of Rad51. Biochim Biophys Acta 1822:1308–1315. https://doi.org/10.1016/j.bbadis.2012.05.005
Cui X, Wang L, Zhang J, Du R, Liao S, Li D, Li C, Ke T, Li DW-C, Huang H, Yin Z, Tang Z, Liu M (2013) HSF4 regulates DLAD expression and promotes lens de-nucleation. Biochim Biophys Acta 1832:1167–1172. https://doi.org/10.1016/j.bbadis.2013.03.007
Dai C, Whitesell L, Rogers AB, Lindquist S (2007) Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell 130:1005–1018. https://doi.org/10.1016/j.cell.2007.07.020
Dayalan Naidu S, Sutherland C, Zhang Y, Risco A, de la Vega L, Caunt CJ, Hastie CJ, Lamont DJ, Torrente L, Chowdhry S, Benjamin IJ, Keyse SM, Cuenda A, Dinkova-Kostova AT (2016) Heat shock factor 1 is a substrate for p38 mitogen-activated protein kinases. Mol Cell Biol 36:2403–2417. https://doi.org/10.1128/MCB.00292-16
Desai S, Liu Z, Yao J, Patel N, Chen J, Wu Y, Ahn EE-Y, Fodstad O, Tan M (2013) Heat shock factor 1 (HSF1) controls chemoresistance and autophagy through transcriptional regulation of autophagy-related protein 7 (ATG7). J Biol Chem 288:9165–9176. https://doi.org/10.1074/jbc.M112.422071
Duchateau A, de Thonel A, El Fatimy R, Dubreuil V, Mezger V (2020) The “HSF connection”: Pleiotropic regulation and activities of Heat Shock Factors shape pathophysiological brain development. Neurosci Lett 725:134895. https://doi.org/10.1016/j.neulet.2020.134895
El Fatimy R, Miozzo F, Le Mouël A, Abane R, Schwendimann L, Sabéran-Djoneidi D, de Thonel A, Massaoudi I, Paslaru L, Hashimoto-Torii K, Christians E, Rakic P, Gressens P, Mezger V (2014) Heat shock factor 2 is a stress-responsive mediator of neuronal migration defects in models of fetal alcohol syndrome. EMBO Mol Med 6:1043–1061. https://doi.org/10.15252/emmm.201303311
Elsing AN, Aspelin C, Björk JK, Bergman HA, Himanen SV, Kallio MJ, Roos-Mattjus P, Sistonen L (2014) Expression of HSF2 decreases in mitosis to enable stress-inducible transcription and cell survival. J Cell Biol 206:735–749. https://doi.org/10.1083/jcb.201402002
Fiorenza MT, Farkas T, Dissing M, Kolding D, Zimarino V (1995) Complex expression of murine heat shock transcription factors. Nucleic Acids Res 23:467–474. https://doi.org/10.1093/nar/23.3.467
Frejtag W, Zhang Y, Dai R, Anderson MG, Mivechi NF (2001) Heat shock factor-4 (HSF-4a) represses basal transcription through interaction with TFIIF. J Biol Chem 276:14685–14694. https://doi.org/10.1074/jbc.M009224200
Fujimoto M, Izu H, Seki K, Fukuda K, Nishida T, Yamada S-I, Kato K, Yonemura S, Inouye S, Nakai A (2004) HSF4 is required for normal cell growth and differentiation during mouse lens development. EMBO J 23:4297–4306. https://doi.org/10.1038/sj.emboj.7600435
Fujimoto M, Hayashida N, Katoh T, Oshima K, Shinkawa T, Prakasam R, Tan K, Inouye S, Takii R, Nakai A (2010) A novel mouse HSF3 has the potential to activate nonclassical heat-shock genes during heat shock. Mol Biol Cell 21:106–116. https://doi.org/10.1091/mbc.e09-07-0639
Gao M, Huang Y, Wang L, Huang M, Liu F, Liao S, Yu S, Lu Z, Han S, Hu X, Qu Z, Liu X, Assefa Yimer T, Yang L, Tang Z, Li DW-C, Liu M (2017) HSF4 regulates lens fiber cell differentiation by activating p53 and its downstream regulators. Cell Death Dis 8:e3082. https://doi.org/10.1038/cddis.2017.478
Gaudet P, Livstone MS, Lewis SE, Thomas PD (2011) Phylogenetic-based propagation of functional annotations within the Gene Ontology consortium. Brief Bioinformatics 12:449–462. https://doi.org/10.1093/bib/bbr042
Gomez-Pastor R, Burchfiel ET, Thiele DJ (2018) Regulation of heat shock transcription factors and their roles in physiology and disease. Nat Rev Mol Cell Biol 19:4–19. https://doi.org/10.1038/nrm.2017.73
Grossman AD, Erickson JW, Gross CA (1984) The htpR gene product of E. coli is a sigma factor for heat-shock promoters. Cell 38:383–390. https://doi.org/10.1016/0092-8674(84)90493-8
Hajdu-Cronin YM, Chen WJ, Sternberg PW (2004) The L-type cyclin CYL-1 and the heat-shock-factor HSF-1 are required for heat-shock-induced protein expression in Caenorhabditis elegans. Genetics 168:1937–1949. https://doi.org/10.1534/genetics.104.028423
Heldens L, Hensen SMM, Onnekink C, van Genesen ST, Dirks RP, Lubsen NH (2011) An atypical unfolded protein response in heat shocked cells. PLoS ONE 6:e23512. https://doi.org/10.1371/journal.pone.0023512
Hentze N, Le Breton L, Wiesner J, Kempf G, Mayer MP (2016) Molecular mechanism of thermosensory function of human heat shock transcription factor Hsf1. Elife. https://doi.org/10.7554/eLife.11576
Hietakangas V, Ahlskog JK, Jakobsson AM, Hellesuo M, Sahlberg NM, Holmberg CI, Mikhailov A, Palvimo JJ, Pirkkala L, Sistonen L (2003) Phosphorylation of serine 303 is a prerequisite for the stress-inducible SUMO modification of heat shock factor 1. Mol Cell Biol 23:2953–2968. https://doi.org/10.1128/MCB.23.8.2953-2968.2003
Hietakangas V, Anckar J, Blomster HA, Fujimoto M, Palvimo JJ, Nakai A, Sistonen L (2006) PDSM, a motif for phosphorylation-dependent SUMO modification. Proc Natl Acad Sci USA 103:45–50. https://doi.org/10.1073/pnas.0503698102
Homma S, Jin X, Wang G, Tu N, Min J, Yanasak N, Mivechi NF (2007) Demyelination, astrogliosis, and accumulation of ubiquitinated proteins, hallmarks of CNS disease in hsf1-deficient mice. J Neurosci 27:7974–7986. https://doi.org/10.1523/JNEUROSCI.0006-07.2007
Hou J, Tang H, Liu Z, Österlund T, Nielsen J, Petranovic D (2014) Management of the endoplasmic reticulum stress by activation of the heat shock response in yeast. FEMS Yeast Res 14:481–494. https://doi.org/10.1111/1567-1364.12125
Hsu A-L, Murphy CT, Kenyon C (2003) Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 300:1142–1145. https://doi.org/10.1126/science.1083701
Innan H, Kondrashov F (2010) The evolution of gene duplications: classifying and distinguishing between models. Nat Rev Genet 11:97–108. https://doi.org/10.1038/nrg2689
Jaeger AM, Makley LN, Gestwicki JE, Thiele DJ (2014) Genomic heat shock element sequences drive cooperative human heat shock factor 1 DNA binding and selectivity. J Biol Chem 289:30459–30469. https://doi.org/10.1074/jbc.M114.591578
Jaeger AM, Pemble CW, Sistonen L, Thiele DJ (2016) Structures of HSF2 reveal mechanisms for differential regulation of human heat-shock factors. Nat Struct Mol Biol 23:147–154. https://doi.org/10.1038/nsmb.3150
Jedlicka P, Mortin MA, Wu C (1997) Multiple functions of Drosophila heat shock transcription factor in vivo. EMBO J 16:2452–2462. https://doi.org/10.1093/emboj/16.9.2452
Joutsen J, Sistonen L (2019) Tailoring of Proteostasis Networks with Heat Shock Factors. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a034066
Kallio M, Chang Y, Manuel M, Alastalo T-P, Rallu M, Gitton Y, Pirkkala L, Loones M-T, Paslaru L, Larney S, Hiard S, Morange M, Sistonen L, Mezger V (2002) Brain abnormalities, defective meiotic chromosome synapsis and female subfertility in HSF2 null mice. EMBO J 21:2591–2601. https://doi.org/10.1093/emboj/21.11.2591
Kanai T, Takedomi S, Fujiwara S, Atomi H, Imanaka T (2010) Identification of the Phr-dependent heat shock regulon in the hyperthermophilic archaeon, Thermococcus kodakaraensis. J Biochem 147:361–370. https://doi.org/10.1093/jb/mvp177
Kim HT, Kim KP, Lledias F, Kisselev AF, Scaglione KM, Skowyra D, Gygi SP, Goldberg AL (2007) Certain pairs of ubiquitin-conjugating enzymes (E2s) and ubiquitin-protein ligases (E3s) synthesize nondegradable forked ubiquitin chains containing all possible isopeptide linkages. J Biol Chem 282:17375–17386. https://doi.org/10.1074/jbc.M609659200
Kim S-A, Yoon J-H, Ahn S-G (2012) Heat shock factor 4a (HSF4a) represses HSF2 expression and HSF2-mediated transcriptional activity. J Cell Physiol 227:1–6. https://doi.org/10.1002/jcp.22948
Kogo H, Kowa-Sugiyama H, Yamada K, Bolor H, Tsutsumi M, Ohye T, Inagaki H, Taniguchi M, Toda T, Kurahashi H (2010) Screening of genes involved in chromosome segregation during meiosis I: toward the identification of genes responsible for infertility in humans. J Hum Genet 55:293–299. https://doi.org/10.1038/jhg.2010.26
Korfanty J, Stokowy T, Widlak P, Gogler-Piglowska A, Handschuh L, Podkowiński J, Vydra N, Naumowicz A, Toma-Jonik A, Widlak W (2014) Crosstalk between HSF1 and HSF2 during the heat shock response in mouse testes. Int J Biochem Cell Biol 57:76–83. https://doi.org/10.1016/j.biocel.2014.10.006
Kourtis N, Moubarak RS, Aranda-Orgilles B, Lui K, Aydin IT, Trimarchi T, Darvishian F, Salvaggio C, Zhong J, Bhatt K, Chen EI, Celebi JT, Lazaris C, Tsirigos A, Osman I, Hernando E, Aifantis I (2015) FBXW7 modulates cellular stress response and metastatic potential through HSF1 post-translational modification. Nat Cell Biol 17:322–332. https://doi.org/10.1038/ncb3121
Kourtis N, Lazaris C, Hockemeyer K, Balandrán JC, Jimenez AR, Mullenders J, Gong Y, Trimarchi T, Bhatt K, Hu H, Shrestha L, Ambesi-Impiombato A, Kelliher M, Paietta E, Chiosis G, Guzman ML, Ferrando AA, Tsirigos A, Aifantis I (2018) Oncogenic hijacking of the stress response machinery in T cell acute lymphoblastic leukemia. Nat Med 24:1157–1166. https://doi.org/10.1038/s41591-018-0105-8
Kovács D, Sigmond T, Hotzi B, Bohár B, Fazekas D, Deák V, Vellai T, Barna J (2019) Hsf1base: a comprehensive database of HSF1 (heat shock factor 1) target genes. Int J Mol Sci. https://doi.org/10.3390/ijms20225815
Kumsta C, Chang JT, Schmalz J, Hansen M (2017) Hormetic heat stress and HSF-1 induce autophagy to improve survival and proteostasis in C. elegans. Nat Commun 8:14337. https://doi.org/10.1038/ncomms14337
Li J, Chauve L, Phelps G, Brielmann RM, Morimoto RI (2016) E2F coregulates an essential HSF developmental program that is distinct from the heat-shock response. Genes Dev 30:2062–2075. https://doi.org/10.1101/gad.283317.116
Li J, Labbadia J, Morimoto RI (2017) Rethinking HSF1 in stress, development, and organismal health. Trends Cell Biol 27:895–905. https://doi.org/10.1016/j.tcb.2017.08.002
Liao S, Du R, Wang L, Qu Z, Cui X, Li C, Liu F, Huang M, Wang J, Chen J, Gao M, Yu S, Tang Z, Li DW-C, Jiang T, Liu M (2015) BCAS2 interacts with HSF4 and negatively regulates its protein stability via ubiquitination. Int J Biochem Cell Biol 68:78–86. https://doi.org/10.1016/j.biocel.2015.08.016
Liao S, Qu Z, Li L, Zhou B, Gao M, Huang M, Li D (2018) HSF4 transcriptional regulates HMOX-1 expression in HLECs. Gene 655:30–34. https://doi.org/10.1016/j.gene.2018.02.033
Mendillo ML, Santagata S, Koeva M, Bell GW, Hu R, Tamimi RM, Fraenkel E, Ince TA, Whitesell L, Lindquist S (2012) HSF1 drives a transcriptional program distinct from heat shock to support highly malignant human cancers. Cell 150:549–562. https://doi.org/10.1016/j.cell.2012.06.031
Mezger V, Renard JP, Christians E, Morange M (1994) Detection of heat shock element-binding activities by gel shift assay during mouse preimplantation development. Dev Biol 165:627–638. https://doi.org/10.1006/dbio.1994.1281
Morley JF, Morimoto RI (2004) Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol Biol Cell 15:657–664. https://doi.org/10.1091/mbc.E03-07-0532
Morton EA, Lamitina T (2013) Caenorhabditis elegans HSF-1 is an essential nuclear protein that forms stress granule-like structures following heat shock. Aging Cell 12:112–120. https://doi.org/10.1111/acel.12024
Nakai A, Tanabe M, Kawazoe Y, Inazawa J, Morimoto RI, Nagata K (1997) HSF4, a new member of the human heat shock factor family which lacks properties of a transcriptional activator. Mol Cell Biol 17:469–481. https://doi.org/10.1128/MCB.17.1.469
Pirkkala L, Nykänen P, Sistonen L (2001) Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J 15:1118–1131. https://doi.org/10.1096/fj00-0294rev
Prahlad V, Morimoto RI (2009) Integrating the stress response: lessons for neurodegenerative diseases from C. elegans. Trends Cell Biol 19:52–61. https://doi.org/10.1016/j.tcb.2008.11.002
Puustinen MC, Sistonen L (2020) Molecular mechanisms of heat shock factors in cancer. Cells. https://doi.org/10.3390/cells9051202
Rallu M, Loones M, Lallemand Y, Morimoto R, Morange M, Mezger V (1997) Function and regulation of heat shock factor 2 during mouse embryogenesis. Proc Natl Acad Sci USA 94:2392–2397. https://doi.org/10.1073/pnas.94.6.2392
Raychaudhuri S, Loew C, Körner R, Pinkert S, Theis M, Hayer-Hartl M, Buchholz F, Hartl FU (2014) Interplay of acetyltransferase EP300 and the proteasome system in regulating heat shock transcription factor 1. Cell 156:975–985. https://doi.org/10.1016/j.cell.2014.01.055
Richter K, Haslbeck M, Buchner J (2010) The heat shock response: life on the verge of death. Mol Cell 40:253–266. https://doi.org/10.1016/j.molcel.2010.10.006
Rodriguez F, Arsène-Ploetze F, Rist W, Rüdiger S, Schneider-Mergener J, Mayer MP, Bukau B (2008) Molecular basis for regulation of the heat shock transcription factor sigma32 by the DnaK and DnaJ chaperones. Mol Cell 32:347–358. https://doi.org/10.1016/j.molcel.2008.09.016
Rohlin L, Trent JD, Salmon K, Kim U, Gunsalus RP, Liao JC (2005) Heat shock response of Archaeoglobus fulgidus. J Bacteriol 187:6046–6057. https://doi.org/10.1128/JB.187.17.6046-6057.2005
Roos-Mattjus P, Sistonen L (2021) Interplay between mammalian heat shock factors 1 and 2 in physiology and pathology. FEBS J. https://doi.org/10.1111/febs.16178
Saju JM, Hossain MS, Liew WC, Pradhan A, Thevasagayam NM, Tan LSE, Anand A, Olsson P-E, Orbán L (2018) Heat shock factor 5 is essential for spermatogenesis in zebrafish. Cell Rep 25:3252-3261.e4. https://doi.org/10.1016/j.celrep.2018.11.090
Sandqvist A, Björk JK, Akerfelt M, Chitikova Z, Grichine A, Vourc’h C, Jolly C, Salminen TA, Nymalm Y, Sistonen L (2009) Heterotrimerization of heat-shock factors 1 and 2 provides a transcriptional switch in response to distinct stimuli. Mol Biol Cell 20:1340–1347. https://doi.org/10.1091/mbc.E08-08-0864
Schumann W (2016) Regulation of bacterial heat shock stimulons. Cell Stress Chaperones 21:959–968. https://doi.org/10.1007/s12192-016-0727-z
Smith RS, Takagishi SR, Amici DR, Metz K, Gayatri S, Alasady MJ, Wu Y, Brockway S, Taiberg SL, Khalatyan N, Taipale M, Santagata S, Whitesell L, Lindquist S, Savas JN, Mendillo ML (2022) HSF2 cooperates with HSF1 to drive a transcriptional program critical for the malignant state. Sci Adv 8:6526. https://doi.org/10.1126/sciadv.abj6526
Solís EJ, Pandey JP, Zheng X, Jin DX, Gupta PB, Airoldi EM, Pincus D, Denic V (2016) Defining the essential function of yeast hsf1 reveals a compact transcriptional program for maintaining eukaryotic proteostasis. Mol Cell 63:60–71. https://doi.org/10.1016/j.molcel.2016.05.014
Somasundaram T, Bhat SP (2000) Canonical heat shock element in the αB-crystallin gene shows tissue-specific and developmentally controlled interactions with heat shock factor. J Biol Chem
Sorger PK, Pelham HR (1988) Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell 54:855–864. https://doi.org/10.1016/s0092-8674(88)91219-6
Stephanou A, Latchman DS (2011) Transcriptional modulation of heat-shock protein gene expression. Biochem Res Int 2011:238601. https://doi.org/10.1155/2011/238601
Syafruddin SE, Ling S, Low TY, Mohtar MA (2021) More than meets the eye: revisiting the roles of heat shock factor 4 in health and diseases. Biomolecules. https://doi.org/10.3390/biom11040523
Takaki E, Fujimoto M, Sugahara K, Nakahari T, Yonemura S, Tanaka Y, Hayashida N, Inouye S, Takemoto T, Yamashita H, Nakai A (2006) Maintenance of olfactory neurogenesis requires HSF1, a major heat shock transcription factor in mice. J Biol Chem 281:4931–4937. https://doi.org/10.1074/jbc.M506911200
Tan J, Tan S, Zheng H, Liu M, Chen G, Zhang H, Wang K, Tan S, Zhou J, Xiao X (2015) HSF1 functions as a transcription regulator for Dp71 expression. Cell Stress Chaperones 20:371–379. https://doi.org/10.1007/s12192-014-0558-8
Tanabe M, Sasai N, Nagata K, Liu XD, Liu PC, Thiele DJ, Nakai A (1999) The mammalian HSF4 gene generates both an activator and a repressor of heat shock genes by alternative splicing. J Biol Chem 274:27845–27856. https://doi.org/10.1074/jbc.274.39.27845
Tessari A, Salata E, Ferlin A, Bartoloni L, Slongo ML, Foresta C (2004) Characterization of HSFY, a novel AZFb gene on the Y chromosome with a possible role in human spermatogenesis. Mol Hum Reprod 10:253–258. https://doi.org/10.1093/molehr/gah036
Trinklein ND, Chen WC, Kingston RE, Myers RM (2004) Transcriptional regulation and binding of heat shock factor 1 and heat shock factor 2 to 32 human heat shock genes during thermal stress and differentiation. Cell Stress Chaperones 9:21–28. https://doi.org/10.1379/481.1
Tsai LH, Delalle I, Caviness VS, Chae T, Harlow E (1994) p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature 371:419–423. https://doi.org/10.1038/371419a0
Tu N, Hu Y, Mivechi NF (2006) Heat shock transcription factor (Hsf)-4b recruits Brg1 during the G1 phase of the cell cycle and regulates the expression of heat shock proteins. J Cell Biochem 98:1528–1542. https://doi.org/10.1002/jcb.20865
Uchida S, Hara K, Kobayashi A, Fujimoto M, Otsuki K, Yamagata H, Hobara T, Abe N, Higuchi F, Shibata T, Hasegawa S, Kida S, Nakai A, Watanabe Y (2011) Impaired hippocampal spinogenesis and neurogenesis and altered affective behavior in mice lacking heat shock factor 1. Proc Natl Acad Sci USA 108:1681–1686. https://doi.org/10.1073/pnas.1016424108
Vierke G, Engelmann A, Hebbeln C, Thomm M (2003) A novel archaeal transcriptional regulator of heat shock response. J Biol Chem 278:18–26. https://doi.org/10.1074/jbc.M209250200
Vihervaara A, Sergelius C, Vasara J, Blom MAH, Elsing AN, Roos-Mattjus P, Sistonen L (2013) Transcriptional response to stress in the dynamic chromatin environment of cycling and mitotic cells. Proc Natl Acad Sci USA 110:E3388–E3397. https://doi.org/10.1073/pnas.1305275110
Vihervaara A, Mahat DB, Guertin MJ, Chu T, Danko CG, Lis JT, Sistonen L (2017) Transcriptional response to stress is pre-wired by promoter and enhancer architecture. Nat Commun 8:255. https://doi.org/10.1038/s41467-017-00151-0
Voellmy R, Boellmann F (2007) Chaperone regulation of the heat shock protein response. Adv Exp Med Biol 594:89–99. https://doi.org/10.1007/978-0-387-39975-1_9
Wagner SA, Beli P, Weinert BT, Nielsen ML, Cox J, Mann M, Choudhary C (2011) A proteome-wide, quantitative survey of in vivo ubiquitylation sites reveals widespread regulatory roles. Mol Cell Proteomics 10(M111):013284. https://doi.org/10.1074/mcp.M111.013284
Walker GA, Thompson FJ, Brawley A, Scanlon T, Devaney E (2003) Heat shock factor functions at the convergence of the stress response and developmental pathways in Caenorhabditis elegans. FASEB J 17:1960–1962. https://doi.org/10.1096/fj.03-0164fje
Wang G, Ying Z, Jin X, Tu N, Zhang Y, Phillips M, Moskophidis D, Mivechi NF (2004) Essential requirement for both hsf1 and hsf2 transcriptional activity in spermatogenesis and male fertility. Genesis 38:66–80. https://doi.org/10.1002/gene.20005
Westerheide SD, Anckar J, Stevens SM, Sistonen L, Morimoto RI (2009) Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science 323:1063–1066. https://doi.org/10.1126/science.1165946
Widlak W, Vydra N (2017) The role of heat shock factors in mammalian spermatogenesis. Adv Anat Embryol Cell Biol 222:45–65. https://doi.org/10.1007/978-3-319-51409-3_3
Xiao X, Zuo X, Davis AA, McMillan DR, Curry BB, Richardson JA, Benjamin IJ (1999) HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J 18:5943–5952. https://doi.org/10.1093/emboj/18.21.5943
Xing H, Wilkerson DC, Mayhew CN, Lubert EJ, Skaggs HS, Goodson ML, Hong Y, Park-Sarge O-K, Sarge KD (2005) Mechanism of hsp70i gene bookmarking. Science 307:421–423. https://doi.org/10.1126/science.1106478
Yura T, Nagai H, Mori H (1993) Regulation of the heat-shock response in bacteria. Annu Rev Microbiol 47:321–350. https://doi.org/10.1146/annurev.mi.47.100193.001541
Zhang Y, Frejtag W, Dai R, Mivechi NF (2001) Heat shock factor-4 (HSF-4a) is a repressor of HSF-1 mediated transcription. J Cell Biochem 82:692–703. https://doi.org/10.1002/jcb.1191
Zheng X, Krakowiak J, Patel N, Beyzavi A, Ezike J, Khalil AS, Pincus D (2016) Dynamic control of Hsf1 during heat shock by a chaperone switch and phosphorylation. Elife. https://doi.org/10.7554/eLife.18638
Funding
Open access funding provided by Eötvös Loránd University. This research has been supported by the National Research Development and Innovation Office (NKFIH) through the OTKA Grant FK 131944. J.B. is also supported by the ELKH-ELTE Genetics Research Group (01062). D.K. is also supported by the ÚNKP-21–3 New National Excellence Program of the Ministry of Innovation and Technology from the source of the National Research, Development and Innovation Fund.
Author information
Authors and Affiliations
Contributions
Conceptualization was done by J.B.; software was done by M.K.; writing and original draft preparation were done by J.B., D.K., and S.A.; writing and review and editing were done by D.K., J.B., S.A., and M.K.; visualization was done by D.K and M.K.; funding acquisition was done by J.B.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kovács, D., Kovács, M., Ahmed, S. et al. Functional diversification of heat shock factors. BIOLOGIA FUTURA 73, 427–439 (2022). https://doi.org/10.1007/s42977-022-00138-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42977-022-00138-z