Abstract
Agricultural management has a great influence on biodiversity and its services in agroecosystems. In Europe, a relevant proportion of biodiversity is dependent on low-input agriculture. To assess the effects of agricultural management on biodiversity, in this study we surveyed the communities of arable plants, diurnal flying insects, and pollinators in three conventional and in two organic fields of a traditional Elephant garlic (Allium ampeloprasum L.) crop of the Valdichiana area, in Tuscany (central Italy). The sampling was carried out twice during the season: in spring, during crop growing, and in summer, after crop harvesting. We assessed the effects of the different agricultural management on the richness and composition (species occurrence and abundance) of the three communities using univariate and multivariate analyses. Concerning our specific case study, only plant species richness was significantly higher in organic fields (15.7 ± 2.7 species per plot), compared to conventional ones (5.4 ± 2.3 species per plot). Regarding community composition, only pollinators showed a marginally significant difference between conventional and organic fields. Conversely, the effect of specific fields significantly explained differences in composition of all the investigated groups (plants, total insects, and pollinators). The results suggest that, in our case study, the emerged differences in diversity of the investigated communities were mainly attributable to environmental and management factors related to single fields, more than to organic or conventional farming. Such evidence could be partly due to the very local scale of the study, to the heterogeneity of the surveyed fields, and to the reduced number of surveyed fields. Further investigation is therefore needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biodiversity is essential to maintain the functionality of ecosystems, and agricultural areas can have a high biodiversity value (Byrnes et al., 2014; Sutcliffe et al., 2015). In agroecosystems, the services provided by living beings are many and diverse, including for instance recycling of nutrients, regulation of microclimate, soil protection, pest control, and detoxification of noxious chemicals. In the last decades, agricultural ecosystems increased their dependence on external energy inputs due to a reduction in their self-regulation ability, which is largely dependent on the occurring biodiversity (Altieri, 1999). This is increasing the awareness that agricultural management must be set towards sustainable practices (MacLaren et al., 2020; Maraux et al., 2013). Thus, in the European Union, a great attention is being paid to reducing the loss of biodiversity in food production systems (European Commission, 2020a, 2020b).
Arable land is one of the main land use types in agricultural landscapes, and arable plants significantly contribute to biodiversity in these habitats (Marshall et al., 2003). Such species are adapted to the regular disturbance of tillage and other agricultural practices (Holzner, 1978, 1982). Arable vegetation evolved under centuries of interactions between man and nature, acquiring a very characteristic species composition (Oppermann et al., 2012). Balanced and biodiverse arable plant communities are currently acknowledged to provide ecosystem services such as support for pest control, improvement of soil quality, and mitigation of yield losses (Adeux et al., 2019; Blaix et al., 2018; Storkey & Neve, 2018). Agricultural intensification caused a remarkable decline in arable plant diversity in the last decades, especially due to chemical weeding and fertilization, shifts from crop rotation to monoculture, and improved seed cleaning (Fanfarillo, Kasperski, et al., 2019; Meyer et al., 2013; Richner et al., 2015; Storkey et al., 2012).
Plant and insect communities are highly interdependent (Moreira et al., 2016; Nelsen et al., 2018). Insects have strong trophic relationships with vascular plants, that conversely may depend on them for pollination and seed dispersal (Bàrberi et al., 2010; Bronstein et al., 2006; Sakai, 2002), or even for the passive defense against herbivores (Grasso et al., 2015). In arable landscapes, arable plants are particularly important for insects, especially in agroecosystems dominated by wind-pollinated crops, like cereals (Bretagnolle & Gaba, 2015; Kovács-Hostyánszki et al., 2016). They may also support beneficial insects as pest antagonists and pollinators, or act as an alternative trophic resource for phytophagous insects otherwise feeding on crops (Barbercheck & Wallace, 2021; Norris & Kogan, 2000). Like other groups of organisms, insects are experiencing a worldwide decline in agricultural landscapes due to the increasing application of pesticides and fertilizers, to the spread of monocultures, and to the elimination of fragments of natural and semi-natural vegetation (Raven & Wagner, 2021).
Agricultural management can have contrasting effects on biotic communities in different crop types, for instance in annual and perennial crops (Brugisser et al., 2010). At the same time, different taxonomic groups can respond differently to management practices (Lüscher et al., 2014). Organic farming, which tends to be considered more sustainable than conventional farming, showed as well contrasting effects on biodiversity, in relation to different groups of organisms and in different agricultural landscapes (Bengtsson et al., 2005; Brittain et al., 2010; Caprio et al., 2015; Gabriel et al., 2013; Stein-Bachinger et al., 2021). Moreover, the same taxonomic group can be influenced in different ways by organic management regarding species richness, abundance, and composition (Brugisser et al., 2010; Hyvönen & Salonen, 2002; Masoni et al., 2017). Such evidence highlights the importance of assessing the effects of agricultural practices on biotic communities including more than one taxonomic group and exploring different aspects of biodiversity (Brugisser et al., 2010; Batáry et al., 2012; Gabriel et al., 2013; Puig-Monserrat et al., 2017; Raven & Wagner, 2021).
Currently, there is lack of knowledge about the impact of agricultural management on biodiversity in horticultural crops. Some available information is limited to the effects of practices on arable plant species and communities, mostly in oilseed rape, beet, and pea crops (Andreasen & Stryhn, 2008; Fried et al., 2008; Lososová et al., 2004). To fill this gap, in this work we investigated plant and diurnal flying insect communities in three conventional and two organic Valdichiana Elephant garlic (Allium ampeloprasum L.) fields in Tuscany, central Italy, both during crop growing and after harvesting. Our aims were to investigate the effects of i) organic vs conventional managements; ii) local-scale factors summarized through the field identity, and iii) the sampling season, on plant and insect communities in terms of species richness and composition (species occurrence and abundance). Based on past evidence mentioned above, we hypothesize that the lower intensity of agricultural practices in organic management does not necessarily increase species richness or favor more sensitive species in the studied biotic assemblages. Moreover, we speculate that the well-known arable plant species turnover along the season (Fried et al., 2008; Lososová et al., 2004) can be highlighted in our data, with a consequent shift also in insect species composition due to plant–insect interdependence.
Materials and methods
Study area
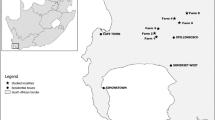
The survey was carried out in five fields in a traditional Elephant garlic crop (Allium ampeloprasum L.) of Tuscany (central Italy), locally known as “Aglione Della Valdichiana”. This traditional Elephant garlic was historically cultivated in the Valdichiana area, between eastern Tuscany and western Umbria, and recently experienced a revival. The Valdichiana is a wide valley that underwent major transformations across the centuries, especially regarding drainages for reclaiming cultivated land. Today, it is characterized by intensive cereal, fruit, and horticultural croplands, and the few remnants of naturalness are represented by residual wetlands (Lastrucci et al., 2010).
The five fields belong to the four farmers that joined the “Vero Aglione Della Valdichiana” project, which aims at a thorough geographic characterization of this traditional crop, e.g., through chemical fingerprinting, and includes the survey and characterization of biodiversity in its fields in relation to agricultural practices (VAV Working Group, 2021; Loppi et al., 2021; Vannini et al., 2021). The location of the surveyed fields and the study area are shown in Fig. 1.
The fields are located between 250 and 500 m a.s.l., in very different landscape contexts that span from intensive agricultural areas dominated by arable land to forested areas, through peri-urban complex mosaics. The bioclimate of the study area is partly Mediterranean and partly Temperate sub-Mediterranean, depending on the duration and intensity of summer drought. Mean annual precipitations range between 730 and 800 mm. Mean annual temperatures are around 13–14 °C (Azzari, 2006; Pesaresi et al., 2017). Soils are mainly alluvial, loamy-clayey, subalkaline to subacid (Hengl et al., 2017). Field size varied between 700 m2 and 9,000 m2.
Collection of management data
Depending on the farm, the “Valdichiana” Elephant garlic is cultivated with conventional or organic techniques. Overall, it is a winter annual crop. Tillage, milling, and bulb sowing are carried out in October, and harvesting takes place in June. Fertilization and mechanical or manual hoeing are usually performed during crop growing, sometimes accompanied by pre-emergence chemical weeding. In the past, the crop was grown with traditional low-intensity methods, which are still widely used nowadays.
All the involved farmers compiled a register where they annotated the date and type of agricultural practices they carried out during the cultivation and, if any, after harvesting, including details on the used products (fertilizers, herbicides—Table 1). Full information on agricultural practices and the used products is available in Supplementary File 1.
Sampling design and method
The sampling was carried out at the end of May 2020 and repeated in the same field at the end of July 2020, regardless of the agricultural succession (fallow, mulching sheets, or summer-annual crop). The resampling after harvesting aimed at catching the influence of the different succession types on biodiversity, given the importance of stubble fields and post-cultural fallows as habitats for arable plants and animals and the seasonal variation in arthropods activity in arable fields (Feber et al., 2015; Kuussaari et al., 2011; Pinke & Pál, 2009). We randomly placed 2 × 2 m plots all over the surface of the fields. The randomized design was built separately once per season, so that summer plots were not the same that were surveyed in spring. The number of surveyed plots was higher in bigger fields, and varied between 3 and 9 per season (field a = 9 plots; fields b,c,d = 3 plots; field e = 4 plots). We surveyed a total number of 44 plots, 22 in May (hereafter “spring plots/data”) and 22 in July (hereafter “summer plots/data”). In each plot, we sampled vascular plants and diurnal flying insects (hereafter “total insects”). Each survey was carried out at least 15 days after the last agricultural operation.
Regarding vascular plants, all the species occurring in the plot were recorded and attributed a cover value, according to the Braun-Blanquet seven-degree scale (Braun-Blanquet, 1964). Plant community data were stored in the “European Weed Vegetation Database” of the European Vegetation Archive (Küzmič et al., 2020). To avoid the influence of disturbance on insects communities, these were sampled before vascular plants, during sunny and windless days. Targeting flying insects, sampling was carried out by sweeping the vegetation ten times all over each plot's surface, using an entomological net (McCravy, 2018). Net sampling was chosen since being more efficient than other methods of catching flying insects, especially pollinators (Popic et al., 2013). After net sampling, we integrated data collection by means of sight hunting and catching, both on the ground and on vegetation, for 15 min per plot. All the entomological samplings were made by the same person. Aphids were not sampled. The number of individuals per each insect taxon collected or observed in the plot was counted. The collected insects were stored in ethanol. Despite the reduced sampling effort, this method allowed to quickly obtain comparable observations on the entomological communities from all the surveyed plots. All the collected samples were identified in the lab at the lowest possible taxonomic level based on Pignatti et al. (2017–2019) for plants, and several sources for insects (Supplementary File 1). The nomenclature of vascular plants was updated according to Bartolucci et al. (2018) and Galasso et al. (2018). The nomenclature of insects follows de Jong et al. (2014). Given the lack of a unique reference, pollinator insects were distinguished through an experience-based approach, i.e., an expert entomologist classified insects into pollinators and non-pollinators based on his knowledge and experience.
Calculations and statistical analyses
Differences in plant, total insect, and pollinator species richness were assessed by means of Generalized Linear Mixed Models (GLMMs) (Bolker et al., 2009) using a Poisson distribution as link function after checking that the data were not overdispersed (function “dispersion test” in the package “AER” of R-project) (Kleiber & Zeileis, 2008; R Core Team, 2020). The GLMMs were run through the function “glmer” in the package “lme4” (Bates et al., 2015). Management type and season were used as fixed factors, while the field was included as a random factor. This model type was chosen after comparing it, through likelihood ratio test, with a more complex one in which the factor “field” was nested in management type. However, compared models did not differ, allowing us to choose the simplest model for all the taxonomic groups.
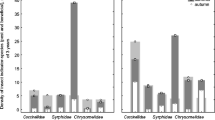
We calculated α, β, and γ diversity to highlight the contribution of diversity components to the total species richness at the plot (α1 and β1), field (β2), and management type (β3) scales, through an additive diversity partitioning analysis (γ = α1 + β1 + β2 + β3; differences were assessed through a randomized model with 99 permutations) (Veech et al., 2002) using the function “adipart” in the package “vegan” (Oksanen et al., 2021).
Permutational Analysis of Variance (PERMANOVA) was performed in the software PRIMER v.6 (Clarke & Gorley, 2006) to test whether management type and the season significantly affected species composition of vascular plants, total insects, and pollinators. One PERMANOVA was performed separately for each of the three groups. PERMANOVA correctly calculates an appropriate pseudo-F statistic for each term in the model for multivariate or univariate datasets. Moreover, the permutation approach is free from many of the assumptions of parametric statistics (Anderson et al., 2008). The effects of management type (2-level fixed effect: organic and conventional), field (5-level random effect nested within the management type factor), and season (2-levels fixed effect: spring and summer) were analyzed, as well as the interactions between these factors. The analyses were based on Bray–Curtis dissimilarities of ln(x + 1)-transformed abundance data (percentage cover for plants and number of individuals for insects). The following settings were used for all the tests:
-
999 permutations of residuals under a reduced model and α = 0.05, which yield the best power and maintain the most accurate type-I error for multi-factorial designs (Anderson & Ter Braak, 2003);
-
type III Sum-of Squares, which is the default option in PERMANOVA and the most conservative one (Anderson et al., 2008).
The differences in community structure (presence and abundance of species) among the management types were visualized by non-metric multidimensional scaling (NMDS) using Bray–Curtis dissimilarity in the program PRIMER v.6. We plotted in the NMDS graphic species with correlations ≥0.4 (Spearman rank correlation).
Results
Description of the surveyed fields
Among the surveyed fields, there was a high variability in the mean cumulative cover of vegetation (sum of the percentage covers of all the plant species occurring in each plot, calculated to provide a measure of the degree of vegetation stratification). Species richness per field varied a lot as well, especially regarding plant communities. Table 2 shows descriptive statistics summarizing the main features of the surveyed fields.
Total plant and insect taxonomic diversity
We detected a total amount of 93 plant species, 97 insect taxa, and 138 insect individuals. Plants were all identified to the species level. Regarding insects, 59 were identified to the species level, 24 to the genus level, 13 to the family level, and one could not be identified at any taxonomic rank. The 38 insect taxa that could not be identified at the species level could anyway be recognized as different from one another so that they could be treated as species in the analyses. Plant richness varied between 1 and 21 species per plot. Insect richness varied between 0 and 14 species per plot. The five most frequent plant species were Echinochloa crus-galli (40% of the plots), Solanum nigrum (34%), Convolvulus arvensis (29%), Erigeron sumatrensis (28%), and Juncus bufonius (26%). The five most frequent insect species were Apis mellifera (13.6% of the plots), Altica sp. (9.1%), Sphaerophoria scripta (9.1%), Adelphocoris lineolatus (6.8%), and Adelphocoris sp. (6.8%).
Effects of agricultural management on plant and insect communities
The results of the GLMMs showed that management type (organic = 15.7 ± 2.7 species per plot, conventional = 5.4 ± 2.3 species per plot) (Fig. 2) and season (spring = 9.5 ± 4.9 species per plot, summer = 6.9 ± 6 species per plot) significantly affected only vascular plant species richness (Table 3). Regarding insect richness, the effect of management type was marginally significant (p = 0.06). The additive diversity partitioning showed significant contributions of α-diversity at the plot level to the total diversity of all the taxonomic groups, while the contribution of β-diversity at the plot level was significant for plants and total insects and marginally significant for pollinators. The contribution of β-diversity at the field level was never significant, while that of β-diversity for management types was significant only for plants (Fig. 3).
The results of PERMANOVA showed that management type was marginally significant only for pollinators (p = 0.07). Factors “Field” and “Season x Field” significantly affected the composition of the three analyzed groups. The effect of “Season” was never significant, as well as the interaction “Season x Management type” (Table 4).
The surveyed communities grouped in the NMDS ordination space only according to the sampling field, especially regarding plant communities. Nevertheless, some plant species like Echinochloa crus-galli and Convolvulus arvensis correlated with conventional fields. Regarding total insects and pollinators, we highlighted a correlation between several species (e.g., Adelphocoris lineolatus, Apis mellifera, Coccinella septempunctata, Sphaerophoria scripta) and the organic field “c”, the one with the highest mean vegetation cover (Fig. 4).
Discussion
The observed differences between the surveyed communities were mostly attributable to the field identity. This is probably due to our study being limited by the very local scale and by the low number of studied fields, which are common issues when studying traditional, non-commercial crops (Latini et al. 2020). Likely, such a low number did not allow to distinguish the effect of management type from that of the environment, or of specific agricultural practices, on the variability of the surveyed communities. However, part of the achieved results is consistent with previous knowledge of the subject.
Our results showed inconsistent effects of conventional and organic management on plant, total insect, and pollinator communities, both in terms of species richness and composition. This is in accordance with previous evidence highlighting that the responses of biotic communities to organic and conventional management are different depending on the taxonomic group, with plants being the most affected organisms (Fuller et al., 2005; Tuck et al., 2014; Rundlöf et al., 2016). We found that the field was the only factor to significantly explain differences in both richness and composition for all the surveyed communities, suggesting that the variability between fields under the same management type is relevant and has a great influence on biotic communities. This strong identity of the fields can be related to several factors, representing the complex system of agricultural and environmental filters that shape biotic communities in arable land. For instance, landscape features were shown to highly affect the composition of plant and insect communities in agroecosystems, being even more important than farm management (Ponce et al., 2011). In the case of local, traditional crops, fields can be very small. This can lead to a high influence of surrounding vegetation on arable plant communities, which are colonized by plant species from adjacent habitats (Fanfarillo et al., 2019). E.g., we found in field e, which is located near a wood edge, plant species from such habitats like Quercus spp. and Xeranthemum cylindraceum. Time from conversion to organic management may also play a role for plant communities, with several years needed to observe shifts in species composition (Weibull & Östman, 2003).
Plant communities were the only ones to show an increased species richness under organic practices, while the richness of total insects and pollinators was not affected by management type. This result is consistent with evidence from the literature. In fact, different taxonomic groups are known to respond differently to agricultural management, with the effects of organic farming being often dependent on single species or functional traits (Fuller et al., 2005). Most of the evidence shows that organic management increases plant species richness (Chamorro et al., 2016; Ponce et al., 2011; Ryan et al., 2010; Stein-Bachinger et al., 2021; Tyšer et al., 2021). However, also low-input, conventional farming is able to maintain a higher number of plant species in arable fields (Berbeć et al., 2020). Insects as well are highly affected by agricultural management, but they strongly respond at the same time to other types of factors. For instance, landscape structure can have relevant effects on insect communities, with simple landscapes reducing their species richness in organic fields (Winqvist et al., 2011, 2012). For this reason, comparisons of entomological communities in organic and conventional farmlands should be done in similar landscape contexts (Masoni et al., 2017). Our fields were instead inserted in very different landscape types (intensive croplands, woodlands, peri-urban), which can be one reason for the marginally significant effect of management on insect richness. The significant decrease in plant species richness in summer is explained by the removal of post-cultural vegetation in the most of the surveyed plots, either by the immediate succession with maize or the use of mulching sheets.
Regarding species composition, only pollinator communities marginally responded to the different management type. The insignificant effect of management type on the composition of plant and total insect communities can be related to the high variability of agricultural practices carried out in different fields under the same management type, a variability that might not have been caught due to the low number of surveyed fields. Conversely, the field identity explained compositional differences in all the three groups of organisms, as well as the interaction between season and field, the latter indicating changes in species composition along the season for some fields, probably related to the different post-cultural management. This suggests once again that the variability of practices within management types is able to mask the effect of organic and conventional farming. In fact, in contrast with our findings, all the surveyed groups of organisms are known to be affected by organic and conventional management in terms of species composition (Andersson et al., 2013; Hyvönen et al., 2003).
The association of certain species to fields could be due to single practices, more than to the overall management type, as suggested by the NMDS results. For instance, fields with recurrent tillage practices and herbicide use resulted to host highly competitive plant species such as Echinochloa crus-galli, Amaranthus retroflexus, and Convolvulus arvensis (Heap, 2014), and to be negatively related to species that can be found also in semi-natural habitats like Cota tinctoria, Filago pyramidata, and Sherardia arvensis (Popic et al., 2013). Frequent mechanical disturbance also unfavoured ground-nesting bees like Adelphocoris lineolatus, Lasioglossum sp., and Halictus scabiosae, which are known to be related to fields with minimum tillage (Kratschmer et al., 2018). A high cover of arable vegetation was instead associated with phytophagous species and their predators, e.g., Adelphocoris spp., Coccinella septempunctata, Pieris rapae, and Sphaerophoria scripta, as already highlighted elsewhere (Sáenz-Romo et al., 2019).
Conclusions
In this work, we found out that the plant, insect, and pollinator communities of the investigated fields differ inconsistently in organic and conventional fields. Our initial hypothesis was partially confirmed since organic management resulted to significantly affect the richness and composition of plant communities only. Shifts in species richness and composition of the surveyed communities seemed to be rather explained by a complex set of other factors, both agronomic and environmental, which are related to the field identity. Several fields being under the same management type showed a variability of the carried-out practices, e.g., during the season, that led to a low affinity between them in terms of management. In order to be caught and interpreted, such variability should be described in a higher number of study sites. The limitations in extent of our research might have led to a partial identification of the differences, in relation to management type, between biotic communities in the investigated crop. Thus, more research is needed to verify possible further differences in richness and composition of the studied communities and their drivers.
References
Adeux, G., Vieren, E., Carlesi, S., Bàrberi, P., Munier-Jolain, N., & Cordeau, S. (2019). Mitigating crop yield losses through weed diversity. Nature, 2, 1018–1026. https://doi.org/10.1038/s41893-019-0415-y
Altieri, M. A. (1999). The ecological role of biodiversity in agroecosystems. Agriculture, Ecosystems & Environment, 74, 19–31. https://doi.org/10.1016/B978-0-444-50019-9.50005-4
Andersson, G. K., Birkhofer, K., Rundlöf, M., & Smith, H. G. (2013). Landscape heterogeneity and farming practice alter the species composition and taxonomic breadth of pollinator communities. Basic and Applied Ecology, 14(7), 540–546. https://doi.org/10.1016/j.baae.2013.08.003
Anderson, M., & Ter Braak, C. T. (2003). Permutation tests for multi-factorial analysis of variance. Journal of Statistical Computation and Simulation, 73(2), 85–113. https://doi.org/10.1080/00949650215733
Anderson, M., Gorley, R., & Clarke, K. (2008). PERMANOVA for PRIMER: guide to software and statistical methods. Primer-e, Plymouth.
Andreasen, C., & Stryhn, H. (2008). Increasing weed flora in Danish arable fields and its importance for biodiversity. Weed Research, 48, 1–9. https://doi.org/10.1111/j.1365-3180.2008.00603.x
Azzari, M. (Ed.). (2006). Atlante Geoambientale Della Toscana. Istituto Geografico De Agostini.
Barbercheck, M. E., & Wallace, J. (2021). Weed-Insect interactions in annual cropping systems. Annals of the Entomological Society of America, 114(2), 276–291. https://doi.org/10.1093/aesa/saab002
Bàrberi, P., Burgio, G., Dinelli, G., Moonen, A. C., Otto, S., Vazzana, C., & Zanin, G. (2010). Functional biodiversity in the agricultural landscape: relationships between weeds and arthropod fauna. Weed Research, 50, 388–401. https://doi.org/10.1111/j.1365-3180.2010.00798.x
Bartolucci, F., Peruzzi, L., Galasso, G., Albano, A., Alessandrini, A., Ardenghi, N. M. G., Astuti, G., Bacchetta, G., Ballelli, S., Banfi, E., Barberis, G., Bernardo, L., Bouvet, D., Bovio, M., Cecchi, L., Di Pietro, R., Domina, G., Fascetti, S., Fenu, G., … Conti, F. (2018). An updated checklist of the vascular flora native to Italy. Plant Biosys, 152(2), 179–303. https://doi.org/10.1080/11263504.2017.1419996
Batáry, P., Holzschuh, A., Orci, K. M., Samu, F., & Tscharntke, T. (2012). Responses of plant, insect and spider biodiversity to local and landscape scale management intensity in cereal crops and grasslands. Agriculture, Ecosystems & Environment, 146(1), 130–136. https://doi.org/10.1016/j.agee.2011.10.018
Bates, D., Maechler, M., Bolker, B., & Walker, S. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–48. https://doi.org/10.18637/jss.v067.i01
Bengtsson, J., Ahnstrom, J., & Weibull, A. C. (2005). The effects of organic agriculture on biodiversity and abundance: A meta-analysis. Journal of Applied Ecology, 42, 261–269. https://doi.org/10.1111/j.1365-2664.2005.01005.x
Berbeć, A. K., Staniak, M., Feledyn-Szewczyk, B., Kocira, A., & Stalenga, J. (2020). Organic but also low-input conventional farming systems support high biodiversity of weed species in winter cereals. Agriculture, 10, 413. https://doi.org/10.3390/agriculture10090413
Blaix, C., Moonen, A. C., Dostatny, D. F., Izquierdo, J., Le Corff, J., Morrison, J., Von Redwitz, C., Schumacher, M., & Westerman, P. R. (2018). Quantification of regulating ecosystem services provided by weeds in annual cropping systems using a systematic map approach. Weed Research, 58, 151–164. https://doi.org/10.1111/wre.12303
Bolker, B. M., Brooks, M. E., Clark, C. J., Geange, S. W., Poulsen, J. R., Stevens, M. H. H., & White, J.-S.S. (2009). Generalized linear mixed models: A practical guide for ecology and evolution. Trends in Ecology & Evolution, 24, 127–135. https://doi.org/10.1016/j.tree.2008.10.008
Braun-Blanquet, J. (1964). Pflanzensoziologie. Springer.
Bretagnolle, V., & Gaba, S. (2015). Weeds for bees? A review. Agronomy for Sustainable Development, 35, 891–909. https://doi.org/10.1007/s13593-015-0302-5
Brittain, C., Bommarco, R., Vighi, M., Setteled, J., & Pottsa, S. G. (2010). Organic farming in isolated landscapes does not benefit flower-visiting insects and pollination. Biological Conservation, 143(8), 1860–1867. https://doi.org/10.1016/j.biocon.2010.04.029
Bronstein, J. L., Alarcón, E., & Geber, M. (2006). The evolution of plant–insect mutualisms. New Phytologist, 172, 412–428. https://doi.org/10.1111/j.1469-8137.2006.01864.x
Bruggisser, O. T., Schmidt-Entling, M. H., & Bacher, S. (2010). Effects of vineyard management on biodiversity at three trophic levels. Biological Conservation, 143(6), 1521–1528. https://doi.org/10.1016/j.biocon.2010.03.034
Byrnes, J. E. K., Gamfeldt, L., Isbell, F., Lefcheck, J. S., Griffin, J. N., Hector, A., Cardinale, B. J., Hooper, D. U., Dee, L. E., & Duffy, J. E. (2014). Investigating the relationship between biodiversity and ecosystem multifunctionality: Challenges and solutions. Methods in Ecology and Evolution, 5(2), 111–124. https://doi.org/10.1111/2041-210X.12143
Caprio, E., Nervo, B., Isaia, M., Allegro, G., & Rolando, A. (2015). Organic versus conventional systems in viticulture: Comparative effects on spiders and carabids in vineyards and adjacent forests. Agricultural Systems, 136, 61–69. https://doi.org/10.1016/j.agsy.2015.02.009
Chamorro, L., Masalles, R. M., & Sans, F. X. (2016). Arable weed decline in Northeast Spain: Does organic farming recover functional biodiversity? Agriculture, Ecosystems & Environment, 223, 1–9. https://doi.org/10.1016/j.agee.2015.11.027
Clarke, K.R., & Gorley, R.N. (2006). PRIMER v6: User Manual/Tutorial (Plymouth Routines in Multivariate Ecological Research). PRIMER-E, Plymouth.
Commission, E. (2020a). EU Biodiversity Strategy for 2030—Bringing Nature Back Into Our Lives. Publication Office of the European Union.
Commission, E. (2020b). A Farm to Fork Strategy for a Fair, Healthy and Environmentally-Friendly Food System. Publication Office of the European Union.
de Jong, Y., Verbeek, M., Michelsen, V., Bjørn, P., Los, W., Steeman, F., Bailly, N., Basire, C., Chylarecki, P., Stloukal, E., Hagedorn, G., Wetzel, F., Glöckler, F., Kroupa, A., Korb, G., Hoffmann, A., Häuser, C., Kohlbecker, A., Müller, A., … Penev, L. (2014). Fauna Europaea – all European animal species on the web. Biodiversity Data Journal, 2, e4034. https://doi.org/10.3897/BDJ.2.e4034
Fanfarillo, E., Kasperski, A., Giuliani, A., & Abbate, G. (2019). Shifts of arable plant communities after agricultural intensification: A floristic and ecological diachronic analysis in maize fields of Latium (central Italy). Botany Letters, 166(3), 356–365. https://doi.org/10.1080/23818107.2019.1638829
Fanfarillo, E., Scoppola, A., Lososová, Z., & Abbate, G. (2019). Segetal plant communities of traditional agroecosystems: A phytosociological survey in central Italy. Phytocoenologia, 49(2), 165–183. https://doi.org/10.1127/phyto/2019/0282
Feber, R. E., Johnson, P. J., Bell, J. R., Chamberlain, D. E., Firbank, L. G., Fuller, R. J., Manley, W., Mathews, F., Norton, L. R., Townsend, M., & Macdonald, D. W. (2015). Organic farming: Biodiversity impacts can depend on dispersal characteristics and landscape context. PLoS ONE, 10(8), e0135921. https://doi.org/10.1371/journal.pone.0135921
Fried, G., Norton, R. L., & Reboud, X. (2008). Environmental and management factors determining weed species composition and diversity in France. Agriculture, Ecosystems & Environment, 128, 68–76. https://doi.org/10.1016/j.agee.2008.05.003
Fried, G., Chauvel, B., & Reboud, X. (2015). Weed flora shifts and specialisation in winter oilseed rape in France. Weed Research, 55, 514–524. https://doi.org/10.1111/wre.12164
Fuller, R. J., Norton, L. R., Feber, R. E., Johnson, P. J., Chamberlain, D. E., Joys, A. C., Mathews, F., Stuart, R. C., Townsend, M. C., Manley, W. J., Wolfe, M. S., Macdonald, D. W., & Firbank, L. G. (2005). Benefits of organic farming to biodiversity vary among taxa. Biology Letters, 1(4), 431–434.
Gabriel, D., Sait, S. M., Kunin, W. E., & Benton, T. G. (2013). Food production vs biodiversity: Comparing organic and conventional agriculture. Journal of Applied Ecology, 50(2), 355–364. https://doi.org/10.1111/1365-2664.12035
Galasso, G., Bartolucci, F., Peruzzi, L., Ardenghi, N. M. G., Albano, A., Alessandrini, A., Bacchetta, G., Ballelli, S., Bandini Mazzanti, M., Barberis, G., Bernardo, L., Blasi, C., Bouvet, D., Bovio, M., Cecchi, L., Del Guacchio, E., Domina, G., Fascetti, S., Gallo, L., … Bartolucci, F. (2018). An updated checklist of the vascular flora alien to Italy. Plant Biosystems, 152(3), 556–592. https://doi.org/10.1080/11263504.2018.1441197
Grasso, D. A., Pandolfi, C., Bazihizina, N., Nocentini, D., Nepi, M., & Mancuso, S. (2015). Extrafloral-nectar-based partner manipulation in plant-ant relationships. AoB PLANTS, 7, 1002. https://doi.org/10.1093/aobpla/plv002
Heap, I. (2014). Global perspective of herbicide-resistant weeds. Pest Management Science, 70, 1306–1315. https://doi.org/10.1002/ps.3696
Hengl, T., Mendes de Jesus, J., Heuvelink, G. B., Ruiperez Gonzalez, M., Kilibarda, M., Blagotic, A., Shangguan, W., Wright, M. N., Geng, X., Bauer-Marschallinger, B., Guevara, M. A., Vargas, R., MacMillan, R. A., Batjes, N. H., Leenaars, J. G. B., Ribeiro, E., Wheeler, I., Mantel, S., & Kempen, B. (2017). SoilGrids250 m: global gridded soil information based on machine learning. PLoS ONE, 12(2), 10169748. https://doi.org/10.1371/journal.pone.0169748
Holzner, W. (1978). Weed species and weed communities. Vegetatio, 38(1), 13–20. https://doi.org/10.1007/BF00141295
Holzner, W. (1982). Concepts, categories and characteristics of weeds. In W. Holzner & M. Numata (Eds.), Biology and ecology of weeds. Springer.
Hyvönen, T., & Salonen, J. (2002). Weed species diversity and community composition in cropping practices at two intensity levels: A six-year experiment. Plant Ecology, 159, 73–81. https://doi.org/10.1023/A:1015580722191
Hyvönen, T., Ketoja, E., Salonen, J., Jalli, H., & Tiainen, J. (2003). Weed species diversity and community composition in organic and conventional cropping of spring cereals. Agriculture, Ecosystems & Environment, 97, 131–149. https://doi.org/10.1016/S0167-8809(03)00117-8
Kleiber, C., & Zeileis, A. (2008). Applied Econometrics with R. Springer.
Kovács-Hostyánszki, A., Földesi, R., Mózes, E., Szirák, Á., Fischer, J., Hanspach, J., & Báldi, A. (2016). Conservation of pollinators in traditional agricultural landscapes – new challenges in Transylvania (Romania) Posed by EU accession and recommendations for future research. PLoS ONE, 11(6), e0151650. https://doi.org/10.1371/journal.pone.0151650
Kratschmer, S., Pachinger, B., Schwantzer, M., Paredes, D., Guernion, M., Burel, F., Nicolai, A., Strauss, P., Bauer, T., Kriechbaum, M., Zaller, J. G., & Winter, D. (2018). Tillage intensity or landscape features: What matters most for wild bee diversity in vineyards? Agriculture, Ecosystems & Environment, 266, 142–152. https://doi.org/10.1016/j.agee.2018.07.018
Kuussaari, M., Hyvönen, T., & Härmä, O. (2011). Pollinator insects benefit from rotational fallows. Agriculture, Ecosystems & Environment, 143, 28–36. https://doi.org/10.1016/j.agee.2011.03.006
Küzmič, F., Šilc, U., Lososová, Z., Mucina, L., Chytrý, M., Knollová, I., Hennekens, S. M., Berg, C., Bergmeier, E., Biurrun, I., Fanfarillo, E., Font, X., Iakushenko, D., Kovačević, Z., Meyer, S., Nagy, K., Pinke, G., & TereshenkoTereshenko, E. S. (2020). European weed vegetation database: A gap-focused vegetation-plot database. Phytocoenologia, 50(1), 93–100. https://doi.org/10.1127/phyto/2019/0337
Lastrucci, L., Landi, M., & Angiolini, C. (2010). Vegetation analysis on wetlands in a Tuscan agricultural landscape (central Italy). Biologia, 65, 54–68. https://doi.org/10.2478/s11756-009-0213-5
Latini, M., Fanfarillo, E., De Luca, E., Iberite, M., & Abbate, G. (2020). The weed vegetation of the bean “Fagiolo Cannellino di Atina” and the red pepper “Peperone di Pontecorvo” PDO crops (Latium central Italy). Plant Sociology, 57(1), 1–10. https://doi.org/10.3897/pls2020571/01
Lososová, Z., Chytrý, M., Cimalová, S., Kropáˇc, Z., Otýpková, Z., Pyšek, P., & Tichý, L. (2004). Weed vegetation of arable land in central Europe: Gradients of diversity and species composition. Journal of Vegetation Science, 15, 415–422. https://doi.org/10.1111/j.1654-1103.2004.tb02279.x
Lüscher, G., Jeanneret, P., Schneider, M. K., Turnbull, L. A., Arndorfer, M., Balázs, K., Báldi, A., Bailey, D., Bernhardt, K. G., Choisis, J. P., Elek, Z., Frank, T., Friedel, J. K., Kainz, M., Kovács-Hostyánszki, A., Oschatz, M. L., Paoletti, M. G., Papaja-Hülsbergen, S., Sarthou, J. P., … Herzog, F. (2014). Responses of plants, earthworms, spiders and bees to geographic location, agricultural management and surrounding landscape in European arable fields. Agriculture, Ecosystems & Environment, 186, 124–134. https://doi.org/10.1016/j.agee.2014.01.020
Loppi, S., Fedeli, R., Canali, G., Guarnieri, M., Biagiotti, S., & Vannini, A. (2021). Comparison of the mineral and nutraceutical profiles of elephant garlic (Allium ampeloprasum L.) grown in organic and conventional fields of valdichiana, a traditional cultivation area of tuscany Italy. Biology, 10, 1058. https://doi.org/10.3390/biology10101058
MacLaren, C., Storkey, J., Menegat, A., Metcalfe, H., & Dehnen-Schmutz, K. (2020). An ecological future for weed science to sustain crop production and the environment: a review. Agronomy for Sustainable Development, 40, 24. https://doi.org/10.1007/s13593-020-00631-6
Maraux, F., Malézieux, É., & Gary, C. (2013). From artificialization to the ecologization of cropping systems. In É. Hainzelin (Ed.), Cultivating biodiversity to transform agriculture (pp. 45–90). Springer.
Marshall, E. J. P., Brown, V. K., Boatman, N. D., Lutman, P. J. W., Squire, G. R., & Ward, L. K. (2003). The role of weeds in supporting biological diversity within crop fields. Weed Research, 43, 77–89. https://doi.org/10.1046/j.1365-3180.2003.00326.x
Masoni, A., Frizzi, F., Brühl, C., Zocchi, N., Palchetti, E., Chelazzi, G., & Santini, G. (2017). Management matters: A comparison of ant assemblages in organic and conventional vineyards. Agriculture, Ecosystems & Environment, 246, 175–183. https://doi.org/10.1016/j.agee.2017.05.036
McCravy, K. W. (2018). A review of sampling and monitoring methods for beneficial arthropods in agroecosystems. Insects, 9(4), 170. https://doi.org/10.3390/insects9040170
Meyer, S., Wesche, K., Krause, B., & Leuschner, C. (2013). Dramatic losses of specialist arable plants in Central Germany since the 1950s/60s: A cross-regional analysis. Diversity and Distributions, 19(9), 1175–1187. https://doi.org/10.1111/ddi.12102
Moreira, X., Abdala-Roberts, L., Rasmann, S., Castagneyrol, B., & Mooney, K. A. (2016). Plant diversity effects on insect herbivores and their natural enemies: Current thinking, recent findings, and future directions. Current Opinion in Insect Science, 14, 1–7. https://doi.org/10.1016/j.cois.2015.10.003
Nelsen, M. P., Ree, R. H., & Moreau, C. S. (2018). Ant–plant interactions evolved through increasing interdependence. PNAS, 115(48), 12253–12258. https://doi.org/10.1073/pnas.1719794115
Norris, R. F., & Kogan, M. (2000). Interactions between weeds, arthropod pests, and their natural enemies in managed ecosystems. Weed Science, 48, 94–158. https://doi.org/10.1614/0043-1745(2000)048[0094:IBWAPA]2.0.CO,2
Oksanen, J., Blanchet, F.G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., Minchin, P.R., O'Hara, R.B., Simpson, G.L., Solymos, P., Stevens, H.M.H., Szoecs, E., & Wagner, H. (2021). vegan: Community Ecology Package. R package version 2.5–6. https://CRAN.R-project.org/package=vegan. Accessed 15 December 2021
Oppermann, R., Beaufoy, G., & Jones, G. (Eds.). (2012). High nature value farming in Europe: 35 European countries - experiences and perspectives. Ubstadt-Weiher: Regionalkultur.
Pesaresi, S., Biondi, E., & Casavecchia, S. (2017). Bioclimates of Italy. Journal of Maps, 13(2), 955–960. https://doi.org/10.1080/17445647.2017.1413017
Pignatti, S., Guarino, R., & La Rosa, M. (eds). (2017–2019). Flora d'Italia, 2nd edn. Edagricole di New Business Media, Milan
Pinke, G., & Pál, R. (2009). Floristic composition and conservation value of the stubble-field weed community, dominated by Stachys annua in western Hungary. Biologia, 64, 279–291. https://doi.org/10.2478/s11756-009-0035-5
Ponce, C., Bravo, C., García de León, D., Magaña, M., & Alonso, J. C. (2011). Effects of organic farming on plant and arthropod communities: A case study in Mediterranean dryland cereal. Agriculture, Ecosystems & Environment, 141(1–2), 193–201. https://doi.org/10.1016/j.agee.2011.02.030
Popic, T. J., Davila, Y. C., & Wardle, G. M. (2013). Evaluation of common methods for sampling invertebrate pollinator assemblages: Net sampling out-perform pan traps. PLoS ONE, 8(6), e66665. https://doi.org/10.1371/journal.pone.0066665
Puig-Monserrat, X., Stefanescu, C., Torre, I., Palet, J., Fàbregas, E., Dantart, J., Arrizabalaga, A., & Flaquer, C. (2017). Effects of organic and conventional crop management on vineyard biodiversity. Agriculture, Ecosystems & Environment, 243, 19–26. https://doi.org/10.1016/j.agee.2017.04.005
R Core Team. (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/. Accessed 15 December 2021.
Raven, P. H., & Wagner, D. L. (2021). Agricultural intensification and climate change are rapidly decreasing insect biodiversity. PNAS, 118(2), e200254811700. https://doi.org/10.1073/pnas.2002548117
Richner, N., Holderegger, R., Linder, H. P., & Walter, T. (2015). Reviewing change in the arable flora of Europe: A meta-analysis. Weed Research, 55, 1–13. https://doi.org/10.1111/wre.12123
Rundlöf, M., Smith, H. G., & Birkhofer, K. (2016). Effects of organic farming on biodiversity. eLS, 14, 1–7. https://doi.org/10.1002/9780470015902.a0026342
Ryan, M. R., Smith, R. G., Mirsky, S. B., Mortensen, D. A., & Seidel, R. (2010). Management filters and species traits: Weed community assembly in long-term organic and conventional systems. Weed Science, 58(3), 265–277. https://doi.org/10.1614/WS-D-09-00054.1
Sáenz-Romo, M. G., Veas-Bernal, A., Martínez-García, H., Campos-Herrera, R., Ibáñez-Pascual, S., Martínez-Villar, E., Pérez-Moreno, I., & Marco-Mancebón, V. S. (2019). Ground cover management in a Mediterranean vineyard: Impact on insect abundance and diversity. Agriculture, Ecosystems & Environment, 283, 106571. https://doi.org/10.1016/j.agee.2019.106571
Sakai, S. (2002). A review of brood-site pollination mutualism: Plants providing breeding sites for their pollinators. Journal of Plant Research, 115, 161–168. https://doi.org/10.1007/s102650200021
Stein-Bachinger, K., Gottwald, F., Haub, A., & Schmidt, E. (2021). To what extent does organic farming promote species richness and abundance in temperate climates? A review. Organic Agriculture, 11, 1–12. https://doi.org/10.1007/s13165-020-00279-2
Storkey, J., & Neve, P. (2018). What good is weed diversity? Weed Research, 58, 239–243. https://doi.org/10.1111/wre.12310
Storkey, J., Meyer, S., Still, K. S., & Leuschner, C. (2012). The impact of agricultural intensification and land-use change on the European arable flora. Proceedings of the Royal Society B, 279, 1421–1429. https://doi.org/10.1098/rspb.2011.1686
Sutcliffe, L. M. E., Batáry, P., Kormann, U., Báldi, A., Dicks, L. V., Herzon, I., Kleijn, D., Tryjanowski, P., Apostolova, I., Arlettaz, R., Aunins, A., Aviron, S., Baležentiene, L., Fischer, C., Halada, L., Hartel, T., Helm, A., Hristov, I., Jelaska, S. D., … Tscharntke, T. (2015). Harnessing the biodiversity value of Central and Eastern European farmland. Diversity and Distribution, 21(6), 722–730. https://doi.org/10.1111/ddi.12288
Tuck, S.L., Winqvist, C., Mota, F., Ahnström, J., Turnbull, L. A., & Bengtsson, J. (2014). Land‐use intensity and the effects of organic farming on biodiversity: A hierarchical meta‐analysis. J Appl Ecol, 51(3), 746–755.https://doi.org/10.1111/1365-2664.12219
Tyšer, L., Kolářová, M., Tulačka, O., & Hamouz, P. (2021). Weed vegetation in conventional and organic farming in West Bohemia (Czech Republic). Plant, Soil and Environment, 67(7), 376–382. https://doi.org/10.17221/6/2021-PSE
Vannini, A., Grattacaso, M., Canali, G., Nannoni, F., Di Lella, L. A., Protano, G., Biagiotti, S., & Loppi, S. (2021). Potentially toxic elements (PTEs) in soils and bulbs of the elephant garlic (Allium ampeloprasum L.) grown in valdichiana, a traditional cultivation area of Tuscany Italy. Applied Sciences, 11, 7023. https://doi.org/10.3390/app11157023
VAV (“Vero Aglione della Valdichiana”) Working Group. (2021). Gli obiettivi del Gruppo Operativo (The aims of the Working Group). https://aglione.ciatoscana.eu/obiettivi/. Accessed 12 August 2021.
Veech, J. A., Summerville, K. S., Crist, T. O., & Gering, J. C. (2002). The additive partitioning of species diversity: Recent revival of an old idea. Oikos, 99, 3–9. https://doi.org/10.1034/j.1600-0706.2002.990101.x
Weibull, A. C., & Östman, Ö. (2003). Species composition in agroecosystems: The effect of landscape, habitat, and farm management. Basic and Applied Ecology, 4, 349–361. https://doi.org/10.1078/1439-1791-00173
Winqvist, C., Bengtsson, J., Aavik, T., Berendse, F., Clement, L. W., Eggers, S., Fischer, C., Flohre, A., Geiger, F., Liira, J., Pärt, T., Thies, C., Tscharntke, T., Weisser, W. W., & Bommarco, R. (2011). Mixed effects of organic farming and landscape complexity on farmland biodiversity and biological control potential across Europe. Journal of Applied Ecology, 48(3), 570–579. https://doi.org/10.1111/j.1365-2664.2010.01950.x
Winqvist, C., Ahnström, J., & Bengtsson, J. (2012). Effects of organic farming on biodiversity and ecosystem services: Taking landscape complexity into account. Annals New York Academy of Sciences, 1249(1), 191–203. https://doi.org/10.1111/j.1749-6632.2011.06413.x
Funding
Open access funding provided by Università degli Studi di Trieste within the CRUI-CARE Agreement. This research was funded by Regione Toscana—Project “Vero Aglione della Valdichiana—VAV” (PS‐GO 45/2017—PSR FEASR 2014–2020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict interest
The authors have no competing interests to declare that are relevant to the content of this article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fanfarillo, E., Calabrese, D., Angiolini, C. et al. Effects of conventional and organic management on plant and insect communities in a traditional elephant garlic crop. COMMUNITY ECOLOGY 23, 417–427 (2022). https://doi.org/10.1007/s42974-022-00091-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42974-022-00091-w