Abstract
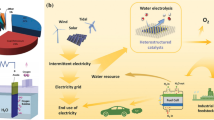
The development of cost-effective and durable hydrogen evolution reaction (HER) electrocatalysts plays a vital role in dealing with the issues related to carbon dioxide emission. Cobalt phosphide-based nanomaterials are evaluated as promising advocates for HER due to high catalytic activities, good stability, and rich defect. This review commences with an exploration of the synthetic pathways of CoP, including solid-phase, solution-phase along with electrochemical methods. Besides, the mechanism of hydrogen formation is expressed thoroughly, after which various integrated strategies of morphology engineering with doping, assisted highly conductive materials, and construction of heterostructure were introduced for HER. Ultimately, burdensome tasks and possible guidance for the advancement of CoP-based nanomaterials were discussed for hydrogen production.

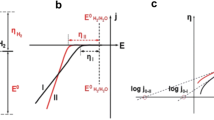
Reproduced with permission from Ref. [47]. Copyright 2016, the Royal Society of Chemistry









Similar content being viewed by others
Data availability
The data generated during and/or analyzed in this article are available from the corresponding author on the reasonable request.
References
Li J, Xu K, Liu F, Li Y, Hu Y, Chen X, Wang H, Xu W, Ni Y, Ding G. Hollow hierarchical Cu2O-derived electrocatalysts steering CO2 reduction to multi-carbon chemicals at low overpotentials. Adv Mater. 2023;35(26):2301127. https://doi.org/10.1002/adma.202301127.
Wang Y, Jiang J, Zou JJ, Mi W. Spin-gapless semiconductors and quantum anomalous hall effects of tetraazanaphthotetraphene-based two-dimensional transition-metal organic frameworks on spintronics and electrocatalysts for CO2 reduction. ACS Appl Electron Mater. 2023;5(2):1243. https://doi.org/10.1021/acsaelm.2c01694.
Chen P, Wu Y, Rufford TE, Wang L, Wang G, Wang Z. Organic molecules involved in Cu-based electrocatalysts for selective CO2 reduction to C2+ products. Mater Today Chem. 2023;27: 101328. https://doi.org/10.1016/j.mtchem.2022.101328.
Jiang D, Bu R, Xia W, Hu Y, Zhou M, Gao E, Asahi T, Yamauchi Y, Tang J. Cobalt phthalocyanine-based conjugated polymer as efficient and exclusive electrocatalyst for CO2 reduction to ethanol. Mater Rep: Energy. 2023;3(1):100176. https://doi.org/10.1016/j.matre.2023.100176.
Liang XD, Tian N, Hu SN, Zhou ZY, Sun SG. Recent advances of bismuth-based electrocatalysts for CO2 reduction: strategies, mechanism and applications. Mater Rep Energy. 2023;3(2):100191. https://doi.org/10.1016/j.matre.2023.100191.
Kuang P, Ni Z, Zhu B, Lin Y, Yu J. Modulating the d-band center enables ultrafine Pt3Fe alloy nanoparticles for pH-universal hydrogen evolution reaction. Adv Mater. 2023;35(41): e2303030. https://doi.org/10.1002/adma.202303030.
Wang Z, Lin Z, Wang Y, Shen S, Zhang Q, Wang J, Zhong W. Nontrivial topological surface states in Ru3Sn7 toward wide pH-range hydrogen evolution reaction. Adv Mater. 2023;35(25):2302007. https://doi.org/10.1002/adma.202302007.
Wang T, Miao L, Zheng S, Qin H, Cao X, Yang L, Jiao L. Interfacial engineering of Ni3N/Mo2N heterojunctions for urea-assisted hydrogen evolution reaction. ACS Catal. 2023;13(7):4091. https://doi.org/10.1021/acscatal.3c00113.
Dogra N, Agrawal P, Pathak S, Saini R, Sharma S. Hydrothermally synthesized MoSe2/ZnO composite with enhanced hydrogen evolution reaction. Int J Hydrog Energy. 2023;48(67):26210. https://doi.org/10.1016/j.ijhydene.2023.03.352.
Bati AS, Zhong YL, Burn PL, Nazeeruddin MK, Shaw PE, Batmunkh M. Next-generation applications for integrated perovskite solar cells. Commun Mater. 2023;4(1):2. https://doi.org/10.1038/s43246-022-00325-4.
Xiao H, Zuo C, Yan K, Jin Z, Cheng Y, Tian H, Xiao Z, Liu F, Ding Y, Ding L. Highly efficient and air-stable inorganic perovskite solar cells enabled by polylactic acid modification. Adv Energy Mater. 2023;13(32):2300738. https://doi.org/10.1002/aenm.202300738.
Bhattarai S, Hossain MK, Pandey R, Madan J, Samajdar D, Rahman MF, Ansari MZ, Amami M. Perovskite solar cells with dual light absorber layers for performance efficiency exceeding 30%. Energy Fuels. 2023;37(14):10631. https://doi.org/10.1021/acs.energyfuels.3c01659.
Do HH, Nguyen THC, Van Nguyen T, Xia C, Nguyen DLT, Raizada P, Singh P, Nguyen VH, Ahn SH, Kim SY. Metal-organic-framework based catalyst for hydrogen production: progress and perspectives. Int J Hydrog Energy. 2022;47(88):37552. https://doi.org/10.1016/j.ijhydene.2022.01.080.
Yao Q, Zhang X, Lu ZH, Xu Q. Metal-organic framework-based catalysts for hydrogen production from liquid-phase chemical hydrides. Coord Chem Rev. 2023;493: 215302. https://doi.org/10.1016/j.ccr.2023.215302.
Paitandi RP, Wan Y, Aftab W, Zhong R, Zou R. Pristine metal–organic frameworks and their composites for renewable hydrogen energy applications. Adv Funct Mater. 2023;33(8):2203224. https://doi.org/10.1002/adfm.202203224.
He J, Yang Q, Song Z, Chang W, Huang C, Zhu Y, Ma X, Wang X. Improving the carbon resistance of iron-based oxygen carrier for hydrogen production via chemical looping steam methane reforming: a review. Fuel. 2023;351: 128864. https://doi.org/10.1016/j.fuel.2023.128864.
Yin Z, Xu H, Chen Y, Zhao T, Wu J. Experimental simulate on hydrogen production of different coals in underground coal gasification. Int J Hydrog Energy. 2023;48(19):6975. https://doi.org/10.1016/j.ijhydene.2022.03.205.
Zhao H, Yuan ZY. Progress and perspectives for solar-driven water electrolysis to produce green hydrogen. Adv Energy Mater. 2023;13(16):2300254. https://doi.org/10.1002/aenm.202300254.
Qian X, Fang J, Xia J, He G, Chen H. Recent progress and perspective on molybdenum-based electrocatalysts for water electrolysis. Int J Hydrog Energy. 2023;48(67):26084. https://doi.org/10.1016/j.ijhydene.2023.03.228.
Wu H, Zheng W, Zhu R, Zhou M, Ren X, Wang Y, Cheng C, Zhou H, Cao S. Modulating coordination structures and metal environments of MOFs-engineered electrocatalysts for water electrolysis. Chem Eng J. 2023;452(3): 139475. https://doi.org/10.1016/j.cej.2022.139475.
Jaimes-Paez CD, Vences-Alvarez E, Salinas-Torres D, Morallón E, Rangel-Mendez JR, Cazorla-Amorós D. Microwave-assisted synthesis of carbon-supported Pt nanoparticles for their use as electrocatalysts in the oxygen reduction reaction and hydrogen evolution reaction. Electrochim Acta. 2023;464: 142871. https://doi.org/10.1016/j.electacta.2023.142871.
Xu S, Niu M, Zhao G, Ming S, Li X, Zhu Q, Ding LX, Kim M, Alothman AA, Mushab MSS. Size control and electronic manipulation of Ru catalyst over B, N co-doped carbon network for high-performance hydrogen evolution reaction. Nano Res. 2023;16(5):6212. https://doi.org/10.1007/s12274-022-5250-1.
Mai HD, Jeong S, Bae GN, Tran NM, Youn JS, Park CM, Jeon KJ. Pd sulfidation-induced 1T-phase tuning in monolayer MoS2 for hydrogen evolution reaction. Adv Energy Mater. 2023;13(23):2300183. https://doi.org/10.1002/aenm.202300183.
Li XX, Liu XC, Liu C, Zeng JM, Qi XP. Co3O4/stainless steel catalyst with synergistic effect of oxygen vacancies and phosphorus doping for overall water splitting. Tungsten. 2023;5(1):100. https://doi.org/10.1007/s42864-022-00144-7.
Ma MY, Yu HZ, Deng LM, Wang LQ, Liu SY, Pan H, Ren JW, Maximov MY, Hu F, Peng SJ. Interfacial engineering of heterostructured carbon-supported molybdenum cobalt sulfides for efficient overall water splitting. Tungsten. 2023;5(4):589. https://doi.org/10.1007/s42864-023-00212-6.
Banerjee S, Kakekhani A, Wexler RB, Rappe AM. Relationship between the surface reconstruction of nickel phosphides and their activity toward the hydrogen evolution reaction. ACS Catal. 2023;13(7):4611. https://doi.org/10.1021/acscatal.2c06427.
Ding X, Yu J, Huang W, Chen D, Lin W, Xie Z. Modulation of the interfacial charge density on Fe2P–CoP by coupling CeO2 for accelerating alkaline electrocatalytic hydrogen evolution reaction and overall water splitting. Chem Eng J. 2023;451(1):138550. https://doi.org/10.1016/j.cej.2022.138550.
Liu SS, Ma LJ, Li JS. Dual-metal-organic-framework derived CoP/MoP hybrid as an efficient electrocatalyst for acidic and alkaline hydrogen evolution reaction. J Colloid Interface Sci. 2023;631:147. https://doi.org/10.1016/j.jcis.2022.10.165.
Di F, Wang X, Farid S, Ren S. CoS2 with carbon shell for efficient hydrogen evolution reaction. Int J Hydrog Energy. 2023;48(47):17758. https://doi.org/10.1016/j.ijhydene.2023.01.287.
Zhang K, Jia J, Tan L, Qi S, Li B, Chen J, Li J, Lou Y, Guo Y. Morphological and electronic modification of NiS2 for efficient supercapacitors and hydrogen evolution reaction. J Power Sour. 2023;577: 233239. https://doi.org/10.1016/j.jpowsour.2023.233239.
Bi X, Xu J, Zhang W, Tang D, Zhang K, Xin S, Zhao Z. Novel WS2/Co9S8 nanoparticles supported on N, S co-doped mesoporous carbon as efficient electrocatalyst for hydrogen evolution reaction. Chem Sel. 2023;8(25): e202300877. https://doi.org/10.1002/slct.202300877.
Bang J, Moon IK, Kim YK, Oh J. Heterostructured Mo2N–Mo2C nanoparticles coupled with N-doped carbonized wood to accelerate the hydrogen evolution reaction. Small Struct. 2023;4(8):2200283. https://doi.org/10.1002/sstr.202200283.
Kong F, Wu A, Wang S, Zhang X, Tian C, Fu H. The “mediated molecular”-assisted construction of MO2N islands dispersed on Co-based nanosheets for high-efficient electrocatalytic hydrogen evolution reaction. Nano Res. 2023;16:10857. https://doi.org/10.1007/s12274-023-5878-5.
Zang Y, Huang S, Yang B, Chen G, Liu X, Zhang N. Constructing collaborative interface between Mo2N and NiS as efficient bifunctional electrocatalysts for overall water splitting. Appl Surf Sci. 2023;611: 155656. https://doi.org/10.1016/j.apsusc.2022.155656.
Jia W, Lu Q, Zheng W, Wang K, Liu X, Yang S, He B. V-doped porous CoP nanoarrays grown on carbon cloth with optimized electronic structure for the hydrogen evolution reaction. Nanoscale Adv. 2023;5(16):4133. https://doi.org/10.1039/d3na00348e.
Liu Z, Wang K, Li Y, Cao Y. Interface-induced electron transfer in sandwich-like hierarchical hollow CoP@NC hybrid for boosted hydrogen evolution reaction in alkaline electrolyte. J Alloys Compd. 2023;956: 170315. https://doi.org/10.1016/j.jallcom.2023.170315.
Wang Z, Chi K, Yang S, Xiao J, Xiao F, Zhao X, Wang S. Optimizing the electronic structure of atomically dispersed Ru sites with CoP for highly efficient hydrogen evolution in both alkaline and acidic media. Small. 2023;19(28):2301403. https://doi.org/10.1002/smll.202301403.
Zhang Y, Diao Z, Wei J, Luo M, Xie L, Huang L, Ren J, Ai N, Li L, Guo H. Bifunctional synergistic CoP/coral-like g-C3N4 catalyst: boosting the photocatalytic water splitting hydrogen evolution and appreciation of anisalcohol at same time. Appl Surf Sci. 2023;614: 156187. https://doi.org/10.1016/j.apsusc.2022.156187.
Zhang XY, Zhu YR, Chen Y, Dou SY, Chen XY, Dong B, Guo BY, Liu DP, Liu CG, Chai YM. Hydrogen evolution under large-current-density based on fluorine-doped cobalt-iron phosphides. Chem Eng J. 2020;399: 125831. https://doi.org/10.1016/j.cej.2020.125831.
Gao WK, Yang M, Chi JQ, Zhang XY, Xie JY, Guo BY, Wang L, Chai YM, Dong B. In situ construction of surface defects of carbon-doped ternary cobalt-nickel-iron phosphide nanocubes for efficient overall water splitting. Sci China Mater. 2019;9(62):1285. https://doi.org/10.1007/s40843-019-9434-7.
Lu SS, Zhang LM, Dong YW, Zhang JQ, Yan XT, Sun DF, Shang X, Chi JQ, Chai YM, Dong B. Tungsten-doped Ni–Co phosphides with multiple catalytic sites as efficient electrocatalysts for overall water splitting. J Mater Chem A. 2019;7(28):16859. https://doi.org/10.1039/C9TA03944A.
Dong B, Li MX, Shang X, Zhou YN, Hu WH, Chai YM. Surface reconstruction through cathodic activation of first-row transition metal phosphides for enhanced hydrogen evolution. J Mater Chem A. 2022;10(34):17477. https://doi.org/10.1039/D2TA05293H.
Zhou YN, Hu WH, Zhen YN, Dong B, Dong YW, Fan RY, Liu B, Liu DP, Chai YM. Metallic MoOx layer promoting high-valence Mo doping into CoP nanowires with ultrahigh activity for hydrogen evolution at 2000 mA·cm-2. Appl Catal B: Environ. 2022;309: 121230. https://doi.org/10.1016/j.apcatb.2022.121230.
Xie Y, Chen M, Cai M, Teng J, Huang H, Fan Y, Barboiu M, Wang D, Su CY. Hollow cobalt phosphide with N-doped carbon skeleton as bifunctional electrocatalyst for overall water splitting. Inorg Chem. 2019;58(21):14652. https://doi.org/10.1021/acs.inorgchem.9b02333.
Huang Z, Chen Z, Chen Z, Lv C, Humphrey MG, Zhang C. Cobalt phosphide nanorods as an efficient electrocatalyst for the hydrogen evolution reaction. Nano Energy. 2014;9:373. https://doi.org/10.1016/j.nanoen.2014.08.013.
Jiang N, You B, Sheng M, Sun Y. Electrodeposited cobalt-phosphorous-derived films as competent bifunctional catalysts for overall water splitting. Angew Chem. 2015;127(21):6349. https://doi.org/10.1002/anie.201501616.
Hu G, Tang Q, Jiang D. CoP for hydrogen evolution: implications from hydrogen adsorption. Phys Chem Chem Phys. 2016;18(34):23864. https://doi.org/10.1039/C6CP04011J.
Nørskov JK, Bligaard T, Logadottir A, Kitchin J, Chen JG, Pandelov S, Stimming U. Trends in the exchange current for hydrogen evolution. J Electrochem Soc. 2005;152(3):J23. https://doi.org/10.1149/1.1856988.
Jin Z, Li P, Xiao D. Metallic Co2P ultrathin nanowires distinguished from CoP as robust electrocatalysts for overall water-splitting. Green Chem. 2016;18(6):1459. https://doi.org/10.1039/C5GC02462E.
Chen Z, Wu H, Li J, Wang Y, Guo W, Cao C, Chen Z. Defect enhanced CoP/Reduced graphene oxide electrocatalytic hydrogen production with Pt-like activity. Appl Catal B: Environ. 2020;265: 118576. https://doi.org/10.1016/j.apcatb.2019.118576.
Zhou G, Li M, Li Y, Dong H, Sun D, Liu X, Xu L, Tian Z, Tang Y. Regulating the electronic structure of CoP nanosheets by O incorporation for high-efficiency electrochemical overall water splitting. Adv Funct Mater. 2020;30(7):1905252. https://doi.org/10.1002/adfm.201905252.
Cao E, Chen Z, Wu H, Yu P, Wang Y, Xiao F, Chen S, Du S, Xie Y, Wu Y. Boron-induced electronic-structure reformation of CoP nanoparticles drives enhanced pH-universal hydrogen evolution. Angew Chem Int Ed. 2020;59(10):4154. https://doi.org/10.1002/anie.201915254.
Shi Y, Zhang B. Recent advances in transition metal phosphide nanomaterials: synthesis and applications in hydrogen evolution reaction. Chem Soc Rev. 2016;45(6):1529. https://doi.org/10.1039/C5CS00434A.
Kibsgaard J, Tsai C, Chan K, Benck JD, Nørskov JK, Abild-Pedersen F, Jaramillo TF. Designing an improved transition metal phosphide catalyst for hydrogen evolution using experimental and theoretical trends. Energy Environ Sci. 2015;8(10):3022. https://doi.org/10.1039/C5EE02179K.
Saadi FH, Carim AI, Verlage E, Hemminger JC, Lewis NS, Soriaga MP. CoP as an acid-stable active electrocatalyst for the hydrogen-evolution reaction: electrochemical synthesis, interfacial characterization and performance evaluation. J Phys Chem C. 2014;118(50):29294. https://doi.org/10.1021/jp5054452.
Jiang P, Liu Q, Ge C, Cui W, Pu Z, Asiri AM, Sun X. CoP nanostructures with different morphologies: synthesis, characterization and a study of their electrocatalytic performance toward the hydrogen evolution reaction. J Mater Chem A. 2014;2(35):14634. https://doi.org/10.1039/C4TA03261F.
Ha DH, Han B, Risch M, Giordano L, Yao KP, Karayaylali P, Shao-Horn Y. Activity and stability of cobalt phosphides for hydrogen evolution upon water splitting. Nano Energy. 2016;29:37. https://doi.org/10.1016/j.nanoen.2016.04.034.
Wang P, Wang B. C-O-Co bond-stabilized CoP on carbon cloth toward hydrogen evolution reaction. Int J Hydrog Energy. 2022;47(15):9209. https://doi.org/10.1016/j.ijhydene.2021.12.264.
Ren Y, Li Z, Deng B, Ye C, Zhang L, Wang Y, Li T, Liu Q, Cui G, Asiri AM. Superior hydrogen evolution electrocatalysis enabled by CoP nanowire array on graphite felt. Int J Hydrog Energy. 2022;47(6):3580. https://doi.org/10.1016/j.ijhydene.2021.11.039.
Wang X, Fei Y, Chen J, Pan Y, Yuan W, Zhang LY, Guo CX, Li CM. Directionally in situ self-assembled, high-density, macropore-oriented, CoP-impregnated, 3D hierarchical porous carbon sheet nanostructure for superior electrocatalysis in the hydrogen evolution reaction. Small. 2022;18(2):2103866. https://doi.org/10.1002/smll.202103866.
Zhao H, Gao G, Wang Y, Chen R, Du Y, Wang M, Li Z, Liu Y, Wang L. Growth of carbon nanotubes coated CoP as electrocatalyst for hydrogen evolution reaction under acidic and alkaline solutions. J Alloys Compd. 2022;927: 167057. https://doi.org/10.1016/j.jallcom.2022.167057.
Yu M, Guo X, Chang X, Ma X, Zhang M. Assembled cobalt phosphide nanoparticles on carbon nanofibers as a bifunctional catalyst for hydrogen evolution reaction and oxygen evolution reaction. Sustain Energy Fuels. 2022;6(21):5000. https://doi.org/10.1039/D2SE01128J.
Jiao L, Zhou YX, Jiang HL. Metal–organic framework-based CoP/reduced graphene oxide: high-performance bifunctional electrocatalyst for overall water splitting. Chem Sci. 2016;7(3):1690. https://doi.org/10.1039/C5SC04425A.
Yang Z, He R, Wu H, Ding Y, Mei H. Needle-like CoP/rGO growth on nickel foam as an efficient electrocatalyst for hydrogen evolution reaction. Int J Hydrog Energy. 2021;46(15):9690. https://doi.org/10.1016/j.ijhydene.2020.07.114.
Guan X, Ma J, Li K, Liang J, Li Z, Peng W, Zhang G, Fan X, Zhang F, Li Y. Multilevel N-doped carbon nanotube/graphene supported cobalt phosphide nanoparticles for electrocatalytic hydrogen evolution reaction. Int J Hydrog Energy. 2019;44(57):30053. https://doi.org/10.1016/j.ijhydene.2019.08.163.
Xu S, Qi Y, Lu Y, Sun S, Liu Y, Jiang D. Fe-doped CoP holey nanosheets as bifunctional electrocatalysts for efficient hydrogen and oxygen evolution reactions. Int J Hydrog Energy. 2021;46(52):26391. https://doi.org/10.1016/j.ijhydene.2021.05.151.
Zhang L, Zhang J, Fang J, Wang XY, Yin L, Zhu W, Zhuang Z. Cr-doped CoP nanorod arrays as high-performance hydrogen evolution reaction catalysts at high current density. Small. 2021;17(28):2100832. https://doi.org/10.1002/smll.202100832.
Wang X, Chen Y, Yu B, Wang Z, Wang H, Sun B, Li W, Yang D, Zhang W. Hierarchically porous W-doped CoP nanoflake arrays as highly efficient and stable electrocatalyst for pH-universal hydrogen evolution. Small. 2019;15(37):1902613. https://doi.org/10.1002/smll.201902613.
Zhang Y, Hui ZX, Zhou HY, Zai SF, Wen Z, Li JC, Yang CC, Jiang Q. Ga doping enables superior alkaline hydrogen evolution reaction performances of CoP. Chem Eng J. 2022;429: 132012. https://doi.org/10.1016/j.cej.2021.132012.
Wang Z, Meng C, Wang J, Song Z, Yu R. Optimizing electronic and geometrical structure of vanadium doped cobalt phosphides for enhanced electrocatalytic hydrogen evolution. Eur J Inorg Chem. 2023;26(13): e202300014. https://doi.org/10.1002/ejic.202300014.
Gao Y, Qian S, Wang H, Yuan W, Fan Y, Cheng N, Xue H, Jiang T, Tian J. Boron-doping on the surface mediated low-valence Co centers in cobalt phosphide for improved electrocatalytic hydrogen evolution. Appl Catal B: Environ. 2023;320: 122014. https://doi.org/10.1016/j.apcatb.2022.122014.
Xu W, Fan G, Zhu S, Liang Y, Cui Z, Li Z, Jiang H, Wu S, Cheng F. Electronic structure modulation of nanoporous cobalt phosphide by carbon doping for alkaline hydrogen evolution reaction. Adv Funct Mater. 2021;31(48):2107333. https://doi.org/10.1002/adfm.202107333.
Men Y, Li P, Zhou J, Cheng G, Chen S, Luo W. Tailoring the electronic structure of Co2P by N doping for boosting hydrogen evolution reaction at all pH values. ACS Catal. 2019;9(4):3744. https://doi.org/10.1021/acscatal.9b00407.
Li B, Li J, Liu X, Guo J, Sun Y, Zhang X. Se-doped CoP nanoneedle arrays grown on carbon cloth for an efficient hydrogen evolution reaction. Energy Fuels. 2022;36(21):13212. https://doi.org/10.1021/acs.energyfuels.2c03124.
Li MX, Ma Y, Xiao B, Zhou YN, Yu WL, Zhai XJ, Lv RQ, Chai YM, Dong BS, Fe dual doped and precisely regulated CoP porous nanoneedle arrays for efficient hydrogen evolution at 3 A·cm-2. Chem Eng J. 2023;470:144081. https://doi.org/10.1016/j.cej.2023.144081.
Chen J, Li X, Ma B, Zhao X, Chen Y. CoP@Ni core-shell heterostructure nanowire array: a highly efficient electrocatalyst for hydrogen evolution. J Colloid Interface Sci. 2023;637:354. https://doi.org/10.1016/j.jcis.2023.01.108.
Zhou Q, Sun R, Ren Y, Tian R, Yang J, Pang H, Huang K, Tian X, Xu L, Tang Y. Reactive template-derived interfacial engineering of CoP/CoO heterostructured porous nanotubes towards superior electrocatalytic hydrogen evolution. Carbon Energy. 2023;5(1): e273. https://doi.org/10.1002/cey2.273.
Yu R, Du YX, Zhao HF, Cao FF, Lu WT, Zhang G. Crystalline/amorphous CoP/MnOx heterostructure derived from phase separation for electrochemical catalysis of alkaline hydrogen evolution reaction. Int J Hydrog Energy. 2023;48(7):2593. https://doi.org/10.1016/j.ijhydene.2022.10.049.
Cai J, Zhang X, Pan Y, Kong Y, Lin S. MoS2||CoP heterostructure loaded on N, P-doped carbon as an efficient trifunctional catalyst for oxygen reduction, oxygen evolution, and hydrogen evolution reaction. Int J Hydrog Energy. 2021;46(69):34252. https://doi.org/10.1016/j.ijhydene.2021.07.220.
Boppella R, Park J, Yang W, Tan J, Moon J. Efficient electrocatalytic proton reduction on CoP nanocrystals embedded in microporous P, N Co-doped carbon spheres with dual active sites. Carbon. 2020;156:529. https://doi.org/10.1016/j.carbon.2019.09.082.
Boppella R, Tan J, Yang W, Moon J. Homologous CoP/NiCoP heterostructure on N-doped carbon for highly efficient and pH-universal hydrogen evolution electrocatalysis. Adv Funct Mater. 2019;29(6):1807976. https://doi.org/10.1002/adfm.201807976.
Boppella R, Park J, Lee H, Jang G, Moon J. Hierarchically structured bifunctional electrocatalysts of stacked core–shell CoS1−xPx heterostructure nanosheets for overall water splitting. Small Methods. 2020;4(7):2000043. https://doi.org/10.1002/smtd.202000043.
Liu SS, Zhu JY, Xu X, Li JS. Three-dimensional graphene-supported CoP-RuP2 with artificial heterointerfaces for an enhanced universal-pH hydrogen evolution reaction. Cryst Growth Des. 2022;22(9):5607. https://doi.org/10.1021/acs.cgd.2c00698.
Gao X, Lu K, Chen J, Min J, Zhu D, Tan M. NiCoP–CoP heterostructural nanowires grown on hierarchical Ni foam as a novel electrocatalyst for efficient hydrogen evolution reaction. Int J Hydrog Energy. 2021;46(45):23205. https://doi.org/10.1016/j.ijhydene.2021.03.155.
Wei Y, Li W, Li D, Yi L, Hu W. Amorphous-crystalline cobalt-molybdenum bimetallic phosphide heterostructured nanosheets as Janus electrocatalyst for efficient water splitting. Int J Hydrog Energy. 2022;47(12):7783. https://doi.org/10.1016/j.ijhydene.2021.12.106.
Tan L, He R, Shi A, Xue L, Wang Y, Li H, Song X. Heterostructured CoFeP/CoP as an electrocatalyst for hydrogen evolution in alkaline media. Inorg Chem. 2023;62(25):9964. https://doi.org/10.1021/acs.inorgchem.3c01186.
Yang L, Cao X, Wang X, Wang Q, Jiao L. Regulative electronic redistribution of CoTe2/CoP heterointerfaces for accelerating water splitting. Appl Catal B: Environ. 2023;329: 122551. https://doi.org/10.1016/j.apcatb.2023.122551.
Putri LK, Ng BJ, Yeo RYZ, Ong WJ, Mohamed AR, Chai SP. Engineering nickel phosphides for electrocatalytic hydrogen evolution: a doping perspective. Chem Eng J. 2023;461: 141845. https://doi.org/10.1016/j.cej.2023.141845.
Zhao G, Rui K, Dou SX, Sun W. Heterostructures for electrochemical hydrogen evolution reaction: a review. Adv Funct Mater. 2018;28(43):1803291. https://doi.org/10.1002/adfm.201803291.
Zou W, Dou K, Jiang Q, Xiang J, Kaun CC, Tang H. Nearly spherical CoP nanoparticle/carbon nanosheet hybrids: a high-performance trifunctional electrocatalyst for oxygen reduction and water splitting. RSC adv. 2019;9(68):39951. https://doi.org/10.1039/C9RA07334E.
Men Y, Li P, Zhou J, Chen S, Luo W. Trends in alkaline hydrogen evolution activity on cobalt phosphide electrocatalysts doped with transition metals. Cell Rep Phys Sci. 2020;1(8): 100136. https://doi.org/10.1016/j.xcrp.2020.100136.
Men Y, Li P, Yang F, Cheng G, Chen S, Luo W. Nitrogen-doped CoP as robust electrocatalyst for high-efficiency pH-universal hydrogen evolution reaction. Appl Catal B Environ. 2019;253:21. https://doi.org/10.1016/j.apcatb.2019.04.038.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mai, T.D., Do, H.H. Integrated strategies on cobalt phosphides-based electrocatalysts for efficient hydrogen evolution reaction. Tungsten (2024). https://doi.org/10.1007/s42864-024-00263-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42864-024-00263-3