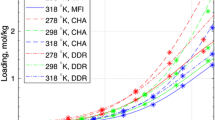
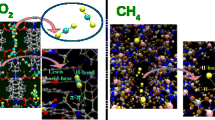
Abstract
Strong gas-mineral interactions or slow adsorption kinetics require a molecular-level understanding of both adsorption and diffusion for these interactions to be properly described in transport models. In this combined molecular simulation and experimental study, noble gas adsorption and mobility is investigated in two naturally abundant zeolites whose pores are similar in size (clinoptilolite) and greater than (mordenite) the gas diameters. Simulated adsorption isotherms obtained from grand canonical Monte Carlo simulations indicate that both zeolites can accommodate even the largest gas (Rn). However, gas mobility in clinoptilolite is significantly hindered at pore-limiting window sites, as seen from molecular dynamics simulations in both bulk and slab zeolite models. Experimental gas adsorption isotherms for clinoptilolite confirm the presence of a kinetic barrier to Xe uptake, resulting in the unusual property of reverse Kr/Xe selectivity. Finally, a kinetic model is used to fit the simulated gas loading profiles, allowing a comparison of trends in gas diffusivity in the zeolite pores.











Similar content being viewed by others
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials, or may be made available through appropriate requests.
References
Ackley, M. W., & Yang, R. T. (1991). Diffusion in ion-exchanged clinoptilolites. AIChE Journal, 37, 1645–1656.
Allen, M. P., & Tildesley, D. J. (1987). Computer simulation of liquids. Clarendon Press.
Aqvist, J. (1990). Ion-water interaction potentials derived from free energy perturbation simulations. Journal of Physical Chemistry, 94, 8021–8024.
Barrer, R. M. (1949). Transient flow of gases in sorbents providing uniform capillary networks of molecular dimensions. Transactions of the Faraday Society, 45, 358–373.
Beerdsen, E., Smit, B., & Dubbeldam, D. (2004). Molecular simulation of loading dependent slow diffusion in confined systems. Physical Review Letters, 93, 248301.
Bourg, I. C., Beckingham, L. E., & DePaolo, D. J. (2015). The nanoscale basis of CO2 trapping for geologic storage. Environmental Science & Technology, 49, 10265–10284.
Buttefey, S., Boutin, A., Mellot-Draznieks, C., & Fuchs, A. H. (2001). A simple model for predicting the Na+ distribution in anhydrous NaY and NaX zeolites. The Journal of Physical Chemistry B, 105, 9569–9575.
Carrigan, C. R., Heinle, R. A., Hudson, G. B., Nitao, J. J., & Zucca, J. J. (1996). Trace gas emissions on geological faults as indicators of underground nuclear testing. Nature, 382, 528–531.
Carrigan, C. R., Sun, Y., & Antoun, T. (2022). Evaluation of subsurface transport processes of delayed gas signatures applicable to underground nuclear explosions. Scientific Reports, 12, 13169.
Coudert, F. X., Cailliez, F., Vuilleumier, R., Fuchs, A. H., & Boutin, A. (2009). Water nanodroplets confined in zeolite pores. Faraday Discussions, 141, 377–398.
Crank, J. (1948). XlV. A diffusion problem in which the amount of diffusing substance is finite. IV. Solutions for small values of the time. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, 39, 362–376.
Crank, J. (1975). The mathematics of diffusion. Oxford University Press.
Dang, L. X. (1995). Mechanism and thermodynamics of ion selectivity in aqueous solutions of 18-crown-6 ether: A molecular dynamics study. Journal of the American Chemical Society, 117, 6954–6960.
Di Lella, A., Desbiens, N., Boutin, A., Demachy, I., Ungerer, P., Bellat, J. P., & Fuchs, A. H. (2006). Molecular simulation studies of water physisorption in zeolites. Physical Chemistry Chemical Physics, 8, 5396–5406.
Dubbeldam, D., Beerdsen, E., Vlugt, T. J. H., & Smit, B. (2005). Molecular simulation of loading-dependent diffusion in nanoporous materials using extended dynamically corrected transition state theory. Journal of Chemical Physics, 122, 224712.
Dutta, R. C., & Bhatia, S. K. (2018). Interfacial barriers to gas transport in zeolites: Distinguishing internal and external resistances. Physical Chemistry Chemical Physics, 20, 26386–26395.
Dutta, R. C., & Bhatia, S. K. (2019). Interfacial barriers to gas transport: Probing solid-gas interfaces at the atomistic level. Molecular Simulation, 45, 1148–1162.
Feldman, J., Paul, M., Xu, G., Rademacher, D. X., Wilson, J., & Nenoff, T. M. (2020). Effects of natural zeolites on field-scale geologic noble gas transport. Journal of Environmental Radioactivity, 220–221, 106279.
Geng, L., Li, G., Zitha, P., Tian, S., Sheng, M., & Fan, X. (2016). A diffusion–viscous flow model for simulating shale gas transport in nano-pores. Fuel, 181, 887–894.
Greathouse, J. A., Hart, D. B., Bowers, G. M., Kirkpatrick, R. J., & Cygan, R. T. (2015). Molecular simulation of structure and diffusion at smectite–water interfaces: Using expanded clay interlayers as model nanopores. Journal of Physical Chemistry C, 119, 17126–17136.
Greathouse, J. A., Cygan, R. T., Fredrich, J. T., & Jerauld, G. R. (2016). Molecular dynamics simulation of diffusion and electrical conductivity in montmorillonite interlayers. The Journal of Physical Chemistry C, 120, 1640–1649.
Green, C. T., Luo, W., Conaway, C. H., Haase, K. B., Baker, R. J., & Andraski, B. J. (2019). Spatial fingerprinting of biogenic and anthropogenic volatile organic compounds in an arid unsaturated zone. Vadose Zone Journal, 18, 190047.
Guo, S. Y., Yu, C. L., Gu, X. H., Jin, W. Q., Zhong, J., & Chen, C. L. (2011). Simulation of adsorption, diffusion, and permeability of water and ethanol in naa zeolite membranes. Journal of Membrane Science, 376, 40–49.
Han, K. N., Bernardi, S., Wang, L. Z., & Searles, D. J. (2017). Water structure and transport in zeolites with pores in one or three dimensions from molecular dynamics simulations. Journal of Physical Chemistry C, 121, 381–391.
Harvey, J. A., & Thompson, W. H. (2015). Thermodynamic driving forces for dye molecule position and orientation in nanoconfined solvents. Journal of Physical Chemistry B, 119, 9150–9159.
Hassanzadeh, A., & Sabzi, F. (2021). Prediction of CO2 and H2 solubility, diffusion, and permeability in MFI zeolite by molecular dynamics simulation. Structural Chemistry, 32, 1641–1650.
Heinemann, N., Alcalde, J., Miocic, J. M., Hangx, S. J. T., Kallmeyer, J., Ostertag-Henning, C., Hassanpouryouzband, A., Thaysen, E. M., Strobel, G. J., Schmidt-Hattenberger, C., Edlmann, K., Wilkinson, M., Bentham, M., Haszeldine, R. S., Carbonell, R., & Rudloff, A. (2021). Enabling large-scale hydrogen storage in porous media - the scientific challenges. Energy & Environmental Science, 14, 853–864.
Ho, T. A., & Wang, Y. F. (2020). Pore size effect on selective gas transport in shale nanopores. Journal of Natural Gas Science and Engineering, 83, 103543.
Ho, C. K., & Webb, S. W. (2006). Gas transport in porous media. Springer.
Hsu, C. N., Tsai, S. C., & Liang, S. M. (1994). Evaluation of diffusion parameters of radon in porous material by flow-through diffusion experiment. Applied Radiation and Isotopes, 45, 845–850.
Inzoli, I., Simon, J.-M., & Kjelstrup, S. (2009). Surface adsorption isotherms and surface excess densities of n-butane in silicalite-1. Langmuir, 25, 1518–1525.
Jayaraman, A., Yang, R. T., Chinn, D., & Munson, C. L. (2005). Tailored clinoptilolites for nitrogen/methane separation. Industrial & Engineering Chemistry Research, 44, 5184–5192.
Jeffroy, M., Boutin, A., & Fuchs, A. H. (2011). Understanding the equilibrium ion exchange properties in faujasite zeolite from Monte Carlo simulations. Journal of Physical Chemistry B, 115, 15059–15066.
Jeffroy, M., Nieto-Draghi, C., & Boutin, A. (2014). Molecular simulation of zeolite flexibility. Molecular Simulation, 40, 6–15.
Jeffroy, M., Nieto-Draghi, C., & Boutin, A. (2017). New molecular simulation method to determine both aluminum and cation location in cationic zeolites. Chemistry of Materials, 29, 513–523.
Jordan, A. B., Stauffer, P. H., Knight, E. E., Rougier, E., & Anderson, D. N. (2015). Radionuclide gas transport through nuclear explosion-generated fracture networks. Scientific Reports, 5(1), 18383.
Karger, J., & Ruthven, D. M. (2016). Diffusion in nanoporous materials: Fundamental principles, insights and challenges. New Journal of Chemistry, 40, 4027–4048.
Karra, S., Makedonska, N., Viswanathan, H. S., Painter, S. L., & Hyman, J. D. (2015). Effect of advective flow in fractures and matrix diffusion on natural gas production. Water Resources Research, 51, 8646–8657.
Kennedy, D. A., & Tezel, F. H. (2018). Cation exchange modification of clinoptilolite - screening analysis for potential equilibrium and kinetic adsorption separations involving methane, nitrogen, and carbon dioxide. Microporous and Mesoporous Materials, 262, 235–250.
Krishna, R. (2012). Diffusion in porous crystalline materials. Chemical Society Reviews, 41, 3099–3118.
Kroutil, O., Nguyen, D. V., Volanek, J., Kucera, A., Predota, M., & Vranova, V. (2021). Clinoptilolite/electrolyte interface probed by a classical molecular dynamics simulations and batch adsorption experiments. Microporous and Mesoporous Materials, 328, 111406.
Lawler, K. V., Sharma, A., Alagappan, B., & Forster, P. M. (2016). Assessing zeolite frameworks for noble gas separations through a joint experimental and computational approach. Microporous and Mesoporous Materials, 222, 104–112.
Li, J. R., Kuppler, R. J., & Zhou, H. C. (2009). Selective gas adsorption and separation in metal-organic frameworks. Chemical Society Reviews, 38, 1477–1504.
Li, J., Ullah, R., Jiao, J., Sun, J. H., & Bai, S. Y. (2020). Ion exchange of cations from different groups with ammonium-modified clinoptilolite and selectivity for methane and nitrogen. Materials Chemistry and Physics, 256, 123760.
Ma, Z. Y., & Ranjith, P. G. (2019). Review of application of molecular dynamics simulations in geological sequestration of carbon dioxide. Fuel, 255, 115644.
Mohammed, S., & Gadikota, G. (2020). Exploring the role of inorganic and organic interfaces on CO2 and CH4 partitioning: Case study of silica, illite, calcite, and kerogen nanopores on gas adsorption and nanoscale transport behaviors. Energy & Fuels, 34, 3578–3590.
Murthy, V., Khosravi, M., & Mackinnon, I. D. R. (2018). Molecular modeling of univalent cation exchange in zeolite n. Journal of Physical Chemistry C, 122, 10801–10810.
Nagumo, R., Takaba, H., & Nakao, S. (2007). A methodology to estimate transport diffusivities in “single-file” permeation through zeolite membranes using molecular simulations. Journal of Chemical Engineering of Japan, 40, 1045–1055.
Neil, C. W., Boukhalfa, H., Xu, H., Ware, S. D., Ortiz, J., Avendaño, S., Harp, D., Broome, S., Hjelm, R. P., Mao, Y., Roback, R., Brug, W. P., & Stauffer, P. H. (2022). Gas diffusion through variably-water-saturated zeolitic tuff: Implications for transport following a subsurface nuclear event. Journal of Environmental Radioactivity, 250, 106905.
Pellenq, R. J. M., & Levitz, P. E. (2002). Capillary condensation in a disordered mesoporous medium: A grand canonical Monte Carlo study. Molecular Physics, 100, 2059–2077.
Perez-Carbajo, J., Dubbeldam, D., Calero, S., & Merkling, P. J. (2018). Diffusion patterns in zeolite MFI: The cation effect. Journal of Physical Chemistry C, 122, 29274–29284.
Perry, J. J., Teich-McGoldrick, S. L., Meek, S. T., Greathouse, J. A., Haranczyk, M., & Allendorf, M. D. (2014). Noble gas adsorption in metal-organic frameworks containing open metal sites. Journal of Physical Chemistry C, 118, 11685–11698.
Plimpton, S. J., Pollock, R., & Stevens, M. (1997). Particle-mesh Ewald and rRESPA for parallel molecular dynamics simulations. Proceedings of the Eighth SIAM Conference on Parallel Processing for Scientific Computing, Minneapolis, MN, USA.
Simoncic, P., & Armbruster, T. (2004). Peculiarity and defect structure of the natural and synthetic zeolite mordenite: A single-crystal x-ray study. American Mineralogist, 89, 421–431.
Simonnin, P., Marry, V., Noetinger, B., Nieto-Draghi, C., & Rotenberg, B. (2018). Mineral- and ion-specific effects at clay-water interfaces: Structure, diffusion, and hydrodynamics. Journal of Physical Chemistry C, 122, 18484–18492.
Slavova, S. O., Sizova, A. A., & Sizov, V. V. (2020). Molecular dynamics simulation of carbon dioxide diffusion in naa zeolite: Assessment of surface effects and evaluation of bulk-like properties. Physical Chemistry Chemical Physics, 22, 22529–22536.
Smart, O. S., Neduvelil, J. G., Wang, X., Wallace, B. A., & Sansom, M. S. P. (1996). Hole: A program for the analysis of the pore dimensions of ion channel structural models. Journal of Molecular Graphics & Modelling, 14, 354–360.
Smirnov, K. S. (2017). A molecular dynamics study of the interaction of water with the external surface of silicalite-1. Physical Chemistry Chemical Physics, 19, 2950–2960.
Smyth, J. R., Spaid, A. T., & Bish, D. L. (1990). Crystal-structures of a natural and a Cs-exchanged clinoptilolite. American Mineralogist, 75, 522–528.
Taghdisian, H., Tasharrofi, S., Firoozjaie, A. G., & Hosseinnia, A. (2019). Loading-dependent diffusion of SO2 in 13x and 5a using molecular dynamics: Effects of extraframework ions and topology. Journal of Chemical and Engineering Data, 64, 3092–3104.
Teleman, O., Jonsson, B., & Engstrom, S. (1987). A molecular dynamics simulation of a water model with intramolecular degrees of freedom. Molecular Physics, 60, 193–203.
Thompson, A. P., Aktulga, H. M., Berger, R., Bolintineanu, D. S., Brown, W. M., Crozier, P. S., in’t Veld, P. J., Kohlmeyer, A., Moore, S. G., Nguyen, T. D., Shan, R., Stevens, M. J., Tranchida, J., Trott, C., & Plimpton, S. J. (2022). LAMMPS - a flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Computer Physics Communications, 271, 108171.
Trucano, P., & Chen, R. (1975). Structure of graphite by neutron diffraction. Nature, 258, 136–137.
Uzunova, E. L., & Mikosch, H. (2013). Cation site preference in zeolite clinoptilolite: A density functional study. Microporous and Mesoporous Materials, 177, 113–119.
van Loef, J. J. (1981). On the thermo-physical properties of liquid rn 222. Physica B & C, 103, 362–364.
Viswanadham, N., & Kumar, M. (2006). Effect of dealumination severity on the pore size distribution of mordenite. Microporous and Mesoporous Materials, 92, 31–37.
Wendling, J., Justinavicius, D., Sentis, M., Amaziane, B., Bond, A., Calder, N. J., & Treille, E. (2019). Gas transport modelling at different spatial scales of a geological repository in clay host rock. Environmental Earth Sciences, 78, 221.
Wilson, A. H. (1948). V. A diffusion problem in which the amount of diffusing substance is finite. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, 39, 48–58.
Yu, H., Xu, H., Fan, J., Wang, F., & Wu, H. (2020). Roughness factor-dependent transport characteristic of shale gas through amorphous kerogen nanopores. The Journal of Physical Chemistry C, 124, 12752–12765.
Yu, Y., Li, X., Min, X., Shang, M., Tao, P., & Sun, T. (2022). Influences of channel morphology and brønsted acidity on ETS-10, ZSM-5, and SSZ-13 for xenon and krypton separation. Journal of Environmental Chemical Engineering, 10, 106982.
Zhang, T., & Sun, S. Y. (2019). A coupled lattice boltzmann approach to simulate gas flow and transport in shale reservoirs with dynamic sorption. Fuel, 246, 196–203.
Acknowledgments
The authors thank Todd R. Zeitler for helpful discussions. This work was fully supported by the LDRD program of Sandia National Laboratories. This paper describes objective technical results and analysis. Any subjective views or opinions that might be expressed in the paper do not necessarily represent the views of the U.S. Department of Energy or the United States Government. This article has been authored by an employee of National Technology & Engineering Solutions of Sandia, LLC under Contract No. DE-NA0003525 with the U.S. Department of Energy (DOE). The employee owns all right, title and interest in and to the article and is solely responsible for its contents. The United States Government retains and the publisher, by accepting the article for publication, acknowledges that the United States Government retains a non-exclusive, paid-up, irrevocable, world-wide license to publish or reproduce the published form of this article or allow others to do so, for United States Government purposes. The DOE will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan https://www.energy.gov/downloads/doe-public-access-plan.
Author information
Authors and Affiliations
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Greathouse, J.A., Paul, M.J., Xu, G. et al. Molecular Dynamics Simulation of Pore-Size Effects on Gas Adsorption Kinetics in Zeolites. Clays Clay Miner. 71, 54–73 (2023). https://doi.org/10.1007/s42860-023-00231-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42860-023-00231-x