Abstract
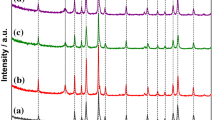
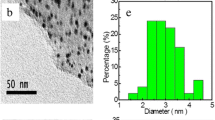
A novel kind of self-assembled graphene quantum dots-Co3O4 (GQDs-Co3O4) nanocomposite was successfully manufactured through a hydrothermal approach and used as an extremely effectual oxygen evolution reaction (OER) electrocatalyst. The characterization of morphology with scanning electron microscopy (SEM) and transmission electron microscopy (TEM) showed that Co3O4 nanosheets combined with graphene quantum dots (GQDs) had a new type of hexagonal lamellar self-assembly structure. The GQDs-Co3O4 electrocatalyst showed enhanced electrochemical catalytic properties in an alkaline solution. The start potential of the OER was 0.543 V (vs SCE) in 1 M KOH solution, and 0.577 V (vs SCE) in 0.1 M KOH solution correspondingly. The current density of 10 mA cm−2 had been attained at the overpotential of 321 mV in 1 M KOH solution and 450 mV in 0.1 M KOH solution. Furthermore, the current density can reach 171 mA cm−2 in 1 M KOH solution and 21.4 mA cm−2 in 0.1 M KOH solution at 0.8 V. Moreover, the GQDs-Co3O4 nanocomposite also maintained an ideal constancy in an alkaline solution with only a small deterioration of the activity (7%) compared with the original value after repeating potential cycling for 1000 cycles.








Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this manuscript.
References
Anwar J (2016) Analysis of energy security, environmental emission and fuel import costs under energy import reduction targets: a case of Pakistan. Renew Sust Energy Rev 65:1065–1078
Miyamoto S, Kollman PA (1993) Absolute and relative binding free energy calculations of the interaction of biotin and its analogs with streptavidin using molecular dynamics/free energy perturbation approaches. Proteins 3:226–245
Warren EL, Mckone JR, Boettcher SW, Mi Q (2010) Solar water splitting. Cells 110:6446–6473
Rioult M, Magnan H, Stanescu D, Barbier A (2014) Single crystalline hematite films for solar water splitting: Ti-doping and thickness effects. J Phys Chem C 118:3007–3014
Niishiro R, Konta R, Kato H, Chun WJ, Asakura K, Kudo A (2007) Photocatalytic O2 evolution of rhodium and antimony-codoped rutile-type TiO2 under visible light irradiation. J Phys Chem C 111:17420–17426
Leonard KC, Nam KM, Lee HC, Kang SH, Park HS, Bard AJ (2013) ZnWO4/WO3 composite for improving photoelectrochemical water oxidation. J Phys Chem C 117:15901–15910
Armaroli N, Balzani V (2007) The future of energy supply: challenges and opportunities. Angew Chem Int Ed 46:52–66
Zhao YF, Zhang YX, Yang ZY, Yan YM, Sun KN (2013) Synthesis of MoS2 and MoO2 for their applications in H2 generation and lithium ion batteries: a review. Sci Technol Adv Mater 14:043–501
Zhang ZH, Dua RB, Zhang LB, Zhu HB, Zhang HN, Wang P (2013) Carbon-layer-protected cuprous oxide nanowire arrays for efficient water reduction. ACS Nano 7:1709–1717
Kim D, Sakimoto KK, Hong D, Yang P (2015) Artificial pohotosynthesis for sustainable fuel and chemical production. Angew Chem Int Ed 127:3259–3266
Yuan WY, Shen PK, Jiang SP (2014) Controllable synthesis of graphene supported MnO2 nanowires via self-assembly for enhanced water oxidation in both alkaline and neutral solutions. J Mater Chem A 2:123–129
Jahan M, Liu ZL, Loh KP (2013) A graphene oxide and copper-centered metal organic framework composite as a tri-functional catalyst for HER, OER, and ORR. Adv Funct Mater 3:363–5372
Bian XJ, Zhu J, Liao L, Scanlon MD, Ge PY, Ji C, Girault HH, Liu BH (2012) Nanocomposite of MoS2 on ordered mesoporous carbon nanospheres: a highly active catalyst for electrochemical hydrogen evolution. Electrochem Commun 22:128–132
Masud J, Swesi AT, Liyanage WPR, Nath M (2016) Cobalt selenide nanostructures: an efficient bifunctional catalyst with high current density at low coverage. ACS Appl Mater Interfaces 8:17292–17302
Dutta A, Samantara AK, Dutta SK, Jena BK, Pradhan N (2016) Surface-oxidized dicobalt phosphide nanoneedles as a nonprecious, durable, and efficient OER catalyst. ACS Energy Lett 1:169–174
Burke MS, Enman LJ, Batchellor AS, Zou S, Boettcher SW (2015) Oxygen evolution reaction electrocatalysis on transition metal oxides and (Oxy)hydroxides: activity trends and design principles. Chem Mater 27:7549–7558
Hong WT, Risch M, Stoerzinger KA, Grimaud A, Jin S, Shao-Horn Y (2015) Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis. Energy Environ Sci 8:1404–1427
Lee Y, Suntivich J, May KJ, Perry EE, Shao-Horn Y (2012) Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions. J Phys Chem Lett 3:399–404
Reier T, Oezaslan M, Strasser P (2012) Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and Pt catalysts: a comparative study of nanoparticles and bulk materials. Acs Catal 2:1765–1772
Lin CC, Guo Y, Vela J (2015) Microstructure effects on the water oxidation activity of Co3O4/porous silica nanocomposites. Acs Catal 5:1037–1044
Zhao YF, Chen SQ, Sun B, Su DW, Huang XD, Liu H, Yan YM, Sun KN, Wang GX (2015) Graphene-Co3O4 nanocomposite as electrocatalyst with high performance for oxygen evolution reaction. Sci Rep 5:7629–7629
Huynh M, Bediako DK, Nocera DG (2014) A functionally stable manganese oxide oxygen evolution catalyst in acid. J Am Chem Soc 136:6002–6010
Mette K, Bergmann A, Tessonnier JP, Hävecker M, Yao L, Ressler T, Schlögl R, Strasser P, Behrens M (2012) Nanostructured manganese oxide supported on carbon nanotubes for electrocatalytic water splitting. ChemCatChem 4:851–862
Song H, Sun J, Zhang QB, Zhang DZ, Zhou ZX, Geng XH (2006) Research on properties of iron-nickel oxide film for OER in KOH electrolyte at different temperatures. J Synth Cryst 35:536
Chi J, Yu HM, Li GF, Fu L, Jia J, Gao XQ, Yi BL, Shao ZG (2016) Nickel/cobalt oxide as a highly efficient OER electrocatalyst in an alkaline polymer electrolyte water electrolyzer. RSC Adv 6:90397–90400
Xie X, Li Y, Liu ZQ, Haruta M, Shen W (2009) Low-temperature oxidation of CO catalysed by Co3O4 nanorods. Nature 458:746–749
Li W, Xu L, Chen J (2005) Co3O4 nanomaterials in lithium-ion batteries and gas sensors. Adv Funct Mater 15:851–857
Li Y, Tan B, Wu Y (2008) Mesoporous Co3O4 nanowire arrays for lithium ion batteries with high capacity and rate capability. Nano Lett 8:265–270
Chen Z, Kronawitter CX, Koel BE (2015) Facet-dependent activity and stability of Co3O4 nanocrystals towards the oxygen evolution reaction. Phys Chem 17:29387–29393
Wang YZ, Tang ZH, Shen SL, Yang JH (2022) The influence of heteroatom doping on the performance of carbon-based electrocatalysts for oxygen evolution reactions. New Carbon Mater 37(2):321–336
Li P, Wang HL (2021) Recent advances in carbon-supported iron group electrocatalysts for the oxygen reduction reaction. New Carbon Mater 36(4):665–682
Kumar K, Canaff C, Rousseau J, Arrii-Clacens S, Napporn TW, Habrioux A, Kokoh KB (2016) Effect of the oxide–carbon heterointerface on the activity of Co3O4/NRGO nanocomposites toward ORR and OER. J Phys Chem C 120:7949–7958
Du G, Liu X, Zong Y, Hor TA, Yu A, Liu Z (2013) Co3O4 nanoparticle-modified MnO2 nanotube bifunctional oxygen cathode catalysts for rechargeable zinc/air batteries. Nanoscale 5:4657–4661
Geim AK, Novoselov KS (2007) The rise of graphene. Nature Mater 6:183–191
Geim AK (2009) Graphene: status and prospects. Science 324:1530–1534
Li RY, Zhang HH, Li ZJ (2022) Switchable two-color graphene quantum dot as a promising fluorescence probe to highly sensitive pH detection and bioimaging. Spectrochim Acta A 275:121028
Li BY et al (2022) Mn, B, N co-doped graphene quantum dots for fluorescence sensing and biological imaging. Arab J Chem 15(7):103856
Kim KS, Zhao Y, Jang H, Lee SY, Kim JM, Kim KS, Ahn J-H, Kim P, Choi J-Y, Hong BH (2009) Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 457:706–710
Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun Z, Slesarev A, Alemany LB, Lu W, Tour JM (2010) Improved synthesis of graphene oxide. ACS Nano 4:4806–4814
Nassar M, Ahmed I (2011) Hydrothermal synthesis of cobalt carbonates using different counter ions: an efficient precursor to nano-sized cobalt oxide (Co3O4). Polyhedron 30:2431–2437
Liu M, Li J (2016) Cobalt phosphide hollow polyhedron as efficient bifunctional electrocatalysts for the evolution reaction of hydrogen and oxygen. ACS Appl Mater Interfaces 8:2158–2165
Wang AL, Lin J, Xu H, Tong YX, Li GR (2016) Ni2P–CoP hybrid nanosheet arrays supported on carbon cloth as an efficient flexible cathode for hydrogen evolution. J Mater Chem A 4:16992–16999
Yang Y, Dang L, Shearer MJ, Sheng H, Li W, Chen J, Xiao P, Zhang Y, Hamers RJ, Jin S (2018) Highly active trimetallic NiFeCr layered double hydroxide electrocatalysts for oxygen evolution reaction. Adv Energy Mater 8:1703189
Bronk H, Röhrs S, Bjeoumikhov AO, Langhoff N, Schmalz J, Wedell R, Gorny H-E, Herold A, Waldschläger U (2001) ArtTAX a new mobile spectrometer for energy-dispersive micro X-ray fluorescence spectrometry on art and archaeological objects. Fresenius J Anal Chem 371:307–316
Shinde V, Mahadik S, Gujar T, Lokhande C (2006) Supercapacitive cobalt oxide (Co3O4) thin films by spray pyrolysis. Appl Surf Sci 252:7487–7492
Stobinski L, Lesiak B, Malolepszy A, Mazurkiewicz M, Mierzwa B, Zemek J, Jiricek P, Bieloshapka I (2014) Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J Electron Spectrosc 195:145–154
Nguyen-Huy C, Kim N, Nguyen-Phan TD, Yoo IK, Shin EW (2014) Adsorptive interaction of bisphenol A with mesoporous titanosilicate reduced graphene oxide nanocomposite materials: FT-IR and Raman analyses. Nanoscale Res Lett 9:1–7
Pfaffeneder-Kmen M, Bausch F, Trettenhahn GN, Kautek W (2016) In situ FTIR and in situ QMB study of the electrochemistry of graphene oxide on platinum. J Phys Chem C 120:15563–15568
Wang R, Xu C, Sun J, Liu Y, Gao L, Lin C (2013) Free-standing and binder-free lithium-ion electrodes based on robust layered assembly of graphene and Co3O4 nanosheets. Nanoscale 5:6960–6967
Gao MR, Cao X, Gao Q, Xu YF, Zheng YR, Jiang J, Yu SH (2014) Nitrogen-doped graphene supported CoSe2 nanobelt composite catalyst for efficient water oxidation. ACS Nano 8:3970–3978
Duan J, Zheng Y, Chen S, Tang Y, Jaroniec M, Qiao S (2013) Mesoporous hybrid material composed of Mn3O4 nanoparticles on nitrogen-doped graphene for highly efficient oxygen reduction reaction. Chem Commun 49:7705–7707
Ferrari AC, Meyer JC, Scardaci V, Casiraghi C, Lazzeri M, Mauri F, Piscanec S, Jiang D, Novoselov KS, Roth S, Geim AK (2006) Raman spectrum of grapheme and grapheme layers. Phys Rev Lett 97:187401–187404
Chou NH, Ross PN, Bell AT, Tilley TD (2011) Comparison of cobalt-based nanoparticles as electrocatalysts for water oxidation. ChemSusChem 4:1566–1569
Tüysüz H, Hwang YJ, Khan SB, Asiri AM, Yang P (2013) Mesoporous Co3O4 as an electrocatalyst for water oxidation. Nano Res 6:47–54
Lu Y, Lu X, Mayers BT, Herricks T, Xia Y (2008) Synthesis and characterization of magnetic Co nanoparticles: a comparison study of three different capping surfactants. J Solid State Chem 181:1530–1538
Yang Y, Zhou M, Guo W, Cui X, Li Y, Liu F, Xiao P, Zhang Y (2015) NiCoO2 nanowires grown on carbon fiber paper for highly efficient water oxidation. Electrochim Acta 174:246–253
Kim MY, Ban HJ, Song YW, Lim J, Park SJ, Kim WJ, Hong Y, Kang B-S, Kim HS (2022) Synthesis and electrochemical properties of nano-composite IrO2/TiO2 anode catalyst for SPE electrolysis cell. Int J Hydrog Energy 47(73):31098–31108
Acknowledgements
This work was funded by Gansu Province Science and Technology Plan Projects, China (Grant No. 22CX8GA137), Northwest Normal University Research Project, China (Grant No. NWNU-LKQN2022-07) and Science and Technology Program of Gansu Province (20YF3GA022), China.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, R., Fang, M., Chen, Q. et al. Self-assembled graphene quantum dots-Co3O4 nanocomposite for highly efficient oxygen evolution reaction electrocatalyst. Carbon Lett. 33, 1591–1600 (2023). https://doi.org/10.1007/s42823-023-00533-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42823-023-00533-z