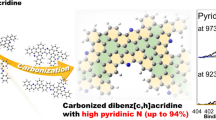
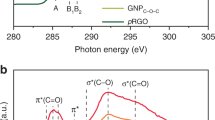
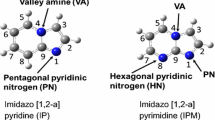
Abstract
Selective doping of pyridinic nitrogen in carbon materials has attracted attention due to its significant properties for various applications such as catalysts and electrodes. However, selective doping of pyridinic nitrogen together with controlling skeletal structure is challenging in the absence of catalysts. In this work, four precursors including four fused aromatic rings and pyridinic nitrogen were simply carbonized in the absence of catalysts in order to attain mass synthesis at low cost and a high percentage of pyridinic nitrogen in carbon materials with controlled edges. Among four precursors, dibenzo[f,h]quinoline (DQ) showed an extremely high percentage of pyridinic nitrogen (96 and 86%) after heat treatment at 923 and 973 K, respectively. Experimental spectroscopic analyses combined with calculated spectroscopic analyses using density functional theory calculations unveiled that the C-H next to the pyridinic nitrogen in DQ generated gulf edge structures with controlled pyridinic nitrogen after carbonization. By comparing the reactivities among the four precursors, three main factors required for maintaining the pyridinic nitrogen in carbon materials with controlled edges, such as (1) high thermal stability of the pyridinic nitrogen, (2) the presence of one pyridinic nitrogen in one ring, and (3) the formation of gulf edges including pyridinic nitrogen to protect the pyridinic nitrogen by the C-H groups on the gulf edges, were revealed.
Graphical abstract
















Similar content being viewed by others
Data and code availability
Not Applicable.
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
References
Su DS, Centi G (2013) A perspective on carbon materials for future energy application. J Energy Chem 22:151–173. https://doi.org/10.1016/S2095-4956(13)60022-4
Greil P (2014) Perspectives of nano-carbon based engineering materials. Adv Eng Mater 17:124–137. https://doi.org/10.1002/adem.201400110
Eletskii AV, Zitserman VY, Kobzev GA (2015) Nanocarbon materials: physicochemical and exploitation properties, synthesis methods, and enegretic applications. High Temp 53:130–150. https://doi.org/10.1134/S0018151X15010034
Balandin A (2011) Thermal properties of graphene and nanostructured carbon materials. Nature Mater 10:569–581. https://doi.org/10.1038/nmat3064
Bianco A, Chen Y, Frackowiak E, Holzinger M, Koratkar N, Meunier V, Mikhailovsky S, Strano M, Tascon JMD, Terrones M (2020) Carbon science perspective in 2020: current research and future challenges. Carbon 161:373–391. https://doi.org/10.1016/j.carbon.2020.01.055
Inagaki M, Toyoda M, Soneda Y, Morishita T (2018) Nitrogen-doped carbon materials. Carbon 132:104–140. https://doi.org/10.1016/j.carbon.2018.02.024
Deng Y, Xie Y, Zou K, Ji X (2016) Review on recent advances in nitrogen-doped carbons: preparations and applications in super-capacitors. J Mater Chem A 4:1144–1173. https://doi.org/10.1039/C5TA08620E
Su H, Hu YH (2021) Recent advances in graphene-based materials for fuel cell applications. Energy Sci Eng 9:958–983. https://doi.org/10.1002/ese3.833
Ma R, Lin G, Zhou Y, Liu Q, Zhang T, Shan G, Yang M, Wang J (2019) A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts. Npj Comput Mater 5:78. https://doi.org/10.1038/s41524-019-0210-3
Shibuya R, Kondo T, Nakamura J (2018) Bottom-up design of nitrogen-containing carbon catalysts for the oxygen reduction reaction. ChemCatChem 10:2019–2023. https://doi.org/10.1002/cctc.201701928
Guo D, Shibuya R, Akiba C, Saji S, Kondo T, Nakamura J (2015) Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 351:1–24. https://doi.org/10.1039/10.1126/science.aad0832
Wu J, Ma L, Yadav RM, Yang Y, Zhang X, Vajtai R, Lou J, Ajayan PM (2015) Nitrogen-doped graphene with pyridinic dominance as a highly active and stable electrocatalyst for oxygen reduction. ACS Appl Mater Interfaces 7:14763–14769. https://doi.org/10.1021/acsami.5b02902
Ning X, Li Y, Ming J, Wang Q, Wang H, Cao Y, Peng F, Yang Y, Yu H (2018) Electronic synergism of pyridinic- and graphitic-nitrogen on N-doped carbons for the oxygen reduction reaction. Chem Sci 10:1589–1596. https://doi.org/10.1039/C8SC04596H
Zheng B, Cai XL, Zhou Y, Xia XH (2016) Pure pyridinic nitrogen-doped single-layer graphene catalyzes two-electron transfer process of oxygen reduction reaction. ChemElectroChem 3:2036–2042. https://doi.org/10.1002/celc.201600130
Li R, Li X, Chen J, Wang J, He H, Huang B, Liu Y, Zhou Y, Yang G (2018) Pyridinic-nitrogen highly doped nanotubular carbon arrays grown on a carbon cloth for high-performance and flexible supercapacitors. Nanoscale 10:3981–3989. https://doi.org/10.1039/C7NR07414J
Lai L, Potts JR, Zhan D, Wang L, Poh CK, Tang C, Gong H, Shen Z, Lin J, Ruoff RS (2012) Exploration of the active center structure of nitrogen-doped graphene-based catalysts for oxygen reduction reaction. Energy Environ Sci 5:7936–7942. https://doi.org/10.1039/C2EE21802J
Khan SM, Kitayama H, Yamada Y, Gohda S, Ono H, Umeda D, Abe K, Hata K, Ohba T (2018) High CO2 sensitivity and reversibility on nitrogen-containing polymer by remarkable CO2 adsorption on nitrogen sites. J Phys Chem C 122:24143–24149. https://doi.org/10.1021/acs.jpcc.8b07420
Saha D, Kienbaum MJ (2019) Role of oxygen, nitrogen and sulfur functionalities on the surface of nanoporous carbons in CO2 adsorption: a critical review. Microporous Mesoporous Mater 287:29–55. https://doi.org/10.1016/j.micromeso.2019.05.051
Tanabe T, Yamada Y, Kim J, Koinuma M, Kubo S, Shimano N, Sato S (2016) Knoevenagel condensation using nitrogen-doped carbon catalysts. Carbon 109:208–220. https://doi.org/10.1016/j.carbon.2016.08.003
Wang X, Li X, Zhang L, Yoon Y, Weber PK, Wang H, Guo J, Dai H (2009) N-doping of graphene through electrothermal reactions with ammonia. Science 324:768–771. https://doi.org/10.1126/science.1170335
Nolan H, Mendoza-Sanchez B, Kumar NA, McEvoy N, O’Brien S, Nicolosi V, Duesberg GS (2014) Nitrogen-doped reduced graphene oxide electrodes for electrochemical supercapacitors. Phys Chem Chem Phys 16:2280–2284. https://doi.org/10.1039/c3cp54877e
Li X, Wang H, Robinson JT, Sanchez H, Diankov G, Dai H (2009) Simultaneous nitrogen doping and reduction of graphene oxide. J Am Chem Soc 131:15939–15944. https://doi.org/10.1021/ja907098f
Schultz BJ, Dennis RV, Aldinger JP, Jaye C, Wang X, Fischer DA, Cartwright AN, Banerjee S (2014) X-ray absorption spectroscopy studies of electronic structure recovery and nitrogen local structure upon thermal reduction of graphene oxide in an ammonia environment. RSC Adv 4:634–644. https://doi.org/10.1039/C3RA45591B
Zhang LS, Liang XQ, Song WG, Wu ZY (2010) Identification of the nitrogen species on N-doped graphene layers and Pt/NG composite catalyst for direct methanolfuelcell. Phys Chem Chem Phys 12:12055–12059. https://doi.org/10.1039/C0CP00789G
Park SH, Chae J, Cho MH, Kim JH, Yoo KH, Cho SW, Kim TG, Kim JW (2014) High concentration of nitrogen doped into graphene using N2 plasma with an aluminum oxide buffer layer. J Mater Chem C 2:933–939. https://doi.org/10.1039/C3TC31773K
Wang CD, Yuen MF, Ng TW, Jha SK, Lu ZZ, Kwok SY, Wong TL, Yang X, Lee CS, Lee ST, Zhang WJ (2012) Plasma-assisted growth and nitrogen doping of graphene films. Appl Phys Lett 100:1–5. https://doi.org/10.1063/1.4729823
Park S, Hu Y, Hwang JO, Lee ES, Casabianca LB, Cai W, Potts JR, Ha H, Chen S, Oh J, Kim SO, Kim YH, Ishii Y, Ruoff RS (2012) Chemical structures of hydrazine-treated graphene oxide and generation of aromatic nitrogen doping. Nat Commun 3:638. https://doi.org/10.1038/ncomms1643
Zhao Y, Zhou Y, O’Hayre R, Shao Z (2013) Electrocatalytic oxidation of methanol on Pt catalyst supported on nitrogen-doped graphene induced by hydrazine reduction. J Phys Chem Solids 74:1608–1614. https://doi.org/10.1016/j.jpcs.2013.06.004
Frackowiak E, Béguin F (2001) Carbon materials for the electrochemical storage of energy in capacitors. Carbon 39:937–950. https://doi.org/10.1016/S0008-6223(00)00183-4
King JA, Klimek DR, Miskioglu I, Odegard GM (2015) Mechanical properties of graphene nanoplatelet/epoxy composites. J Compos Mater 49:659–668. https://doi.org/10.1177/0021998314522
Achaby ME, Arrakhiz FZ, Vaudreuil S, Essassi EM, Qaiss A, Bousmina M (2013) Preparation and characterization of melt-blended graphene nanosheets–poly(vinylidene fluoride) nanocomposites with enhanced properties. J Appl Polym Sci 127:4697–4707. https://doi.org/10.1002/app.38081
Chen XY, Chen C, Zhang ZJ, Xie DH, Deng X (2013) Nitrogen-doped porous carbon prepared from urea formaldehyde resins by template carbonization method for supercapacitors. Ind Eng Chem Res 52:10181–10188. https://doi.org/10.1021/ie400862h
Luo L, Zhang M, Wang P, Wang Y, Wang F (2018) Nitrogen rich carbon nitride synthesized by copolymerization with enhanced visible light photocatalytic hydrogen evolution. New J Chem 42:1087–1091. https://doi.org/10.1039/C7NJ03659K
Yan SC, Li ZS, Zou ZG (2009) Photodegradation performance of g-C3N4 fabricated by directly heating melamine. Langmuir 25:10397–10401. https://doi.org/10.1021/la900923z
Ho W, Zhang Z, Lin W, Huang S, Zhang X, Wang X, Huang Y (2015) Copolymerization with 2,4,6-triaminopyrimidine for the rolling-up the layer structure, tunable electronic properties, and photocatalysis of g-C3N4. ACS Appl Mater Interfaces 7:5497–5505. https://doi.org/10.1021/am509213x
Luo Z, Lim S, Tian Z, Shang J, Lai L, MacDonald B, Fu C, Shen Z, Yu T, Lin J (2011) Pyridinic N doped graphene: synthesis, electronic structure, and electrocatalytic property. J Mater Chem 21:8038–8044. https://doi.org/10.1039/c1jm10845j
Wei D, Liu Y, Wang Y, Zhang H, Huang L, Yu G (2009) Synthesis of N-doped graphene by chemical vapor deposition and its electrical properties. Nano Lett 9:1752–1758. https://doi.org/10.1021/nl803279t
Lv R, Li Q, Botello-Méndez AR, Hayashi T, Wang B, Berkdemir A, Hao Q, Eléas AL, Cruz-Silva R, Gutiérrez HR, Kim YA, Muramatsu H, Zhu J, Endo M, Terrones H, Charlier JC, Pan M, Terrones M (2012) Nitrogen-doped graphene: beyond single substitution and enhanced molecular sensing. Sci Rep 2:586. https://doi.org/10.1038/srep00586
Wu T, Shen H, Sun L, Cheng B, Liu B, Shen J (2012) Nitrogen and boron doped monolayer graphene by chemical vapor deposition using polystyrene, urea and boric acid. New J Chem 36:1385–1391. https://doi.org/10.1039/C2NJ40068E
Yasuda S, Yu L, Kim J, Murakoshi K (2013) Selective Nitrogen doping in graphene for oxygen reduction reactions. Chem Commun 49:9627–9629. https://doi.org/10.1039/C3CC45641B
Dey S, Govindaraj A, Biswas K, Rao CNR (2014) Luminescence properties of boron and nitrogen doped graphene quantum dots prepared from arc-discharge-generated doped graphene samples. Chem Phys Lett 595–596:203–208. https://doi.org/10.1016/j.cplett.2014.02.012
Braghiroli FL, Fierro V, Izquierdo MT, Parmentier J, Pizzi A, Celzard A (2012) Nitrogen-doped carbon materials produced from hydrothermally treated tannin. Carbon 50:5411–5420. https://doi.org/10.1016/j.carbon.2012.07.027
Deng D, Pan X, Yu L, Cui Y, Jiang Y, Qi J, Li WX, Fu Q, Ma X, Xue Q, Sun G, Bao X (2011) Toward N-doped graphene via solvothermal synthesis. Chem Mater 23:1188–1193. https://doi.org/10.1021/cm102666r
Geng D, Hu Y, Li Y, Li R, Sun X (2012) One-pot solvothermal synthesis of doped graphene with the designed nitrogen type used as a Pt support for fuel cells. Electrochem Commun 22:65–68. https://doi.org/10.1016/j.elecom.2012.05.033
He B, Liu F, Yah S (2017) Temperature-directed growth of highly pyridinic nitrogen doped, graphitized, ultra-hollow carbon frameworks as an efficient electrocatalyst for the oxygen reduction reaction. J Mater Chem A 5:18064–18070. https://doi.org/10.1039/C7TA04685E
Lv Q, Si W, He J, Sun L, Zhang C, Wang N, Yang Z, Li X, Wang X, Deng W, Long Y, Huang C, Li Y (2018) Selectively nitrogen-doped carbon materials as superior metal-free catalysts for oxygen reduction. Nat Commun 9:3376. https://doi.org/10.1038/s41467-018-05878-y
Vo TH, Shekhirev M, Kunkel DA, Orange F, Guinei M, Enders A, Sinitskii A (2014) Bottom-up solution synthesis of narrow nitrogen-doped graphene nanoribbons. Chem Commun 50:4172–4174. https://doi.org/10.1039/c4cc00885e
Ohtsubo N, Gohda S, Gotoh K, Sato S, Yamada Y (2023) Bottom-up synthesis of pyridinic nitrogen-containing carbon materials with C-H groups next to pyridinic nitrogen from two-ring aromatics. Carbon, accepted. https://doi.org/10.1016/j.carbon.2023.02.019
Yamada Y, Tanaka H, Tanaka Y, Kubo S, Taguchi T, Sato S (2022) Toward strategical bottom-up synthesis of carbon materials with exceptionally high pyridinic-nitrogen content: development of screening techniques. Carbon 198:411–434. https://doi.org/10.1016/j.carbon.2022.06.069
Kawai R, Yamada Y, Gohda S, Sato S (2022) Bottom-up synthesis of pyridinic nitrogen-doped carbon materials using dibenzacridine isomers with zigzag and armchair edges. J Mater Sci 57:7503–7530. https://doi.org/10.1007/s10853-022-07104-z
Yamada Y, Sato H, Gohda S, Sato S (2023) Toward strategical bottom-up synthesis of carbon materials with exceptionally high basal-nitrogen content: development of screening techniques. Carbon 203:498–522. https://doi.org/10.1016/j.carbon.2022.11.043
Diana N, Yamada Y, Gohda S, Ono H, Kubo S, Sato S (2021) Carbon materials with high pentagon density. J Mater Sci 56:2912–2943. https://doi.org/10.1007/s10853-020-05392-x
Liu L, Liu Y, Zybin SV, Sun H, Goddard WAIII (2011) ReaxFF-lg: correction of the ReaxFF reactive force field for London dispersion, with applications to the equations of state for energetic materials. J Phys Chem A 115:11016–11022. https://doi.org/10.1021/jp201599t
ReaxFF (2016) SCM, Theoretical chemistry, Vrije Universiteit, Amsterdam, The Netherlands. http://www.scm.com. Accessed 27 Feb 2023
Kato T, Yamada Y, Nishikawa Y, Otomo T, Sato H, Sato S (2021) Origins of peaks of graphitic and pyrrolic nitrogen in N1s X-ray photoelectron spectra of carbon materials: quaternary nitrogen, tertiary amine, or secondary amine? J Mater Sci 56:15798–15811. https://doi.org/10.1007/s10853-021-06283-5
Kato T, Yamada Y, Nishikawa Y, Ishikawa H, Sato S (2021) Carbonization mechanisms of polyimide: methodology to analyze carbon materials with nitrogen, oxygen, pentagons, and heptagons. Carbon 178:58–80. https://doi.org/10.1016/j.carbon.2021.02.090
Yamada Y, Kawai M, Yorimitsu H, Otsuka S, Takanashi M, Sato S (2018) Carbon materials with zigzag and armchair edges. ACS Appl Mater Interfaces 10:40710–40739. https://doi.org/10.1021/acsami.8b11022
Senda Y, Yamada Y, Morimoto M, Nono N, Sogabe T, Kubo S, Sato S (2019) Analyses of oxidation process for isotropic pitch-based carbon fiber using model compounds. Carbon 142:311–326. https://doi.org/10.1016/j.carbon.2018.10.026
Gohda S, Yamada Y, Murata M, Saito M, Kanazawa S, Ono H, Sato S (2020) Bottom-up synthesis of highly soluble carbon materials. J Mater Sci 55:11808–11828. https://doi.org/10.1007/s10853-020-04813-1
Kanazawa S, Yamada Y, Gohda S, Sato S (2021) Bottom-up synthesis of oxygen-containing carbon materials using a Lewis acid catalyst. J Mater Sci 56:15698–15717. https://doi.org/10.1007/s10853-021-06284-4
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR et al (2016) Gaussian 16, Revision C.0.1. Gaussian Inc., Wallingford CT
Winmostar V9 (2019) X-Ability Co. Ltd., Tokyo, Japan
Yamada Y, Tanaka H, Kubo S, Sato S (2021) Unveiling bonding states and roles of edges in nitrogen-doped graphene nanoribbon by X-ray photoelectron spectroscopy. Carbon 185:342–367. https://doi.org/10.1016/j.carbon.2021.08.085
Sasaki T, Yamada Y, Sato S (2018) Quantitative analysis of zigzag and armchair edges on carbon materials with and without pentagons using infrared spectroscopy. Anal Chem 90:10724–10731. https://doi.org/10.1021/acs.analchem.8b00949
Yano Y, Mitoma N, Ito H, Itami K (2020) A quest for structurally uniform graphene nanoribbons: synthesis, properties, and applications. J Org Chem 85:4–33. https://doi.org/10.1021/acs.joc.9b02814
Solà M (2013) Forty years of Clar’s aromatic π-sextet rule. Front Chem 1:22. https://doi.org/10.3389/fchem.2013.00022
Batsanov SS (2001) Van der Waals radii of elements. Inorg Mater 37:871–885. https://doi.org/10.1023/A:101162
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number JP21K04773.
Funding
This study was funded by JSPS KAKENHI (Grant Number JP21K04773). Yasuhiro Yamada has received research grants from JSPS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest.
Ethical approval
Not Applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Taguchi, T., Gohda, S., Gotoh, K. et al. Synthesis of carbon materials with extremely high pyridinic-nitrogen content and controlled edges from aromatic compounds with highly symmetric skeletons. Carbon Lett. 33, 1279–1301 (2023). https://doi.org/10.1007/s42823-023-00482-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42823-023-00482-7