Abstract
Arsenic (As) detoxification in polluted soils by iron-based materials can be mediated by the endogenous soil organic matter (SOM), nevertheless the mechanisms remain unclear. Herein, endogenous SOM in a paddy soil was substantially removed to understand its roles on As immobilization by biochar-supported zero-valent iron (ZVI/BC). The results demonstrated that ZVI/BC application significantly decreased As bioavailability by 64.2% compared with the control soil under the anaerobic condition. XPS and HR-TEM suggested As immobilization by ZVI/BC mainly invoked the formation of ternary complexes (i.e., As-Fe-SOM). However, SOM depletion compromised the efficacy of ZVI/BC for As immobilization by 289.8%. This is likely because SOM depletion increased the fulvic acid and OH− contents in soils. Besides, ZVI/BC increased the proportion of As(III) in available As fraction, but SOM depletion altered the mechanisms associated with As(V) reduction. That is, As(V) reduction resulted from the reductive capacity of ZVI in the pristine soil, but the As(V)-reducing bacteria contributed greater to As(V) reduction in the SOM-depleted soil. Additionally, SOM depletion boosted the abundances of Fe(III)- and As(V)-reducing bacteria such as Bacillus and Ammoniphilus in soils, which enhanced the dissimilatory arsenate reduction. Thus, this work highlighted the importance of SOM in the remediation of As-contaminated soils by ZVI/BC.
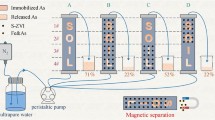
Graphical Abstract

Highlights
-
Endogenous soil organic matter (SOM) was removed to understand its roles in arsenic (As) immobilization by ZVI/BC.
-
SOM depletion compromised the efficacy of ZVI/BC for As immobilization.
-
As(V)-reducing bacteria contributed greater to As reduction in the SOM-depleted soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Arsenic (As), a metalloid, exists mainly as a highly toxic inorganic anionic arsenate (As(V)) or arsenite (As(III)) in soil environments (Song et al. 2022). The elevated arsenic (As) levels in soils are often encountered resulting from the weathering of naturally-occurring As-bearing minerals and anthropogenic activities such as mining and industrial processes. Long-term exposure to As contaminated soils and ingestion of As-enriched foods are associated with acute and chronic human health problems (Kaur et al. 2023). The remediation of As-contaminated paddy soils is challenged by the fluctuating redox status, because As bioavailability is usually enhanced by low redox potential at the flooding stage (Wen et al. 2021; Yang et al. 2023). Thus, enormous effects are devoted to seek for efficient practices to decontaminate As-contaminated paddy soils.
In decades, biochar, as a porous carbon-enriched material produced by thermal decomposition of biomass under anoxic condition, has been widely used for soil detoxification (Yang et al. 2023), soil quality improvement (Nascimento et al. 2023; Yuan et al. 2023), and greenhouse gas mitigation (Medaiyese et al. 2023). However, biochar may enhance As mobility in flooded paddy soils by mediating reductive transformation of iron (Fe) oxides and As in soils (Medaiyese et al. 2023). To improve As immobilization efficacy, biochar is often decorated with nanoscale zero-valent iron (NZVI) with excellent affinity to As that retains As oxyanions by corrosively-formed Fe oxides invoking such mechanisms as electrostatic attraction, surface complexation, reduction/oxidization, and (co)precipitation (Song et al. 2022). The electron shuttling capacity and electrical conductivity of biochar favor electron transfer and confer improved reactivity of ZVI (Fan et al. 2020). Further, the recent works found that biochar enhanced the reductive ability of ZVI which promoted the reduction of As(V) to As(III) under the aerobic condition (Fan et al. 2020; Hu et al. 2023).
Soil organic matter (SOM) comprised small-molecular-size labile organic carbon and persistent humic substances such as fulvic acid and humic acid. Chemical composition and the abundance of SOM have exerted profound influences on As bioavailability. Overall, addition of SOM with high contents of labile aliphatic carbon and organic acids can accelerate the dissolution of As-bearing compounds in soils after flooding possibly resulting from the competitive desorption of negatively-charged functional groups, but humic substance may inhibit As release via As(III) immobilization (Fang et al. 2022; Marzi et al. 2022). Besides, exogeneous humic substance can mediate the change in redox conditions, which may promote the reoxidation of As(III) to As(V) (Peng et al. 2022). On the other hand, SOM can catalyze phase and compositional transformation of Fe oxides, leading to As biogeochemical transformation and immobilization because As immobilization is mainly associated with Fe oxides in soils. In a recent work, it was observed that exogenous SOM increased As release during the first 15–20 days, while a decreased tendency was observed in the later-period, ascribing to the formation of secondary Fe precipitates (Feng et al. 2023). In the anaerobic condition, the reduced SOM moieties facilitated the reductive transformation of Fe oxides and accelerated mobilization of Fe oxide-bound As (Tran et al. 2023). Moreover, the transformation of As-bearing ferrihydrite to lepidocrocite and goethite resulted in As release owing to the decreased surface sites in spite of As sequestration into the newly-formed secondary Fe mineral phases (Stolze et al. 2019). In the soil system, Fe oxides existed mainly as organo-mineral complexes, and this association can enhance As retention by the formation of SOM-Fe oxide-As triple complexes (Liu et al. 2011; Cai et al. 2022). However, recent research focuses on introduction of exogenous SOM, which may confound the role of endogenous SOM, because exogenous SOM would surely result in alteration of endogenous carbon and Fe elemental cycling and soil microbial communities (Meng et al. 2022b; Qiu et al. 2022). In this work, endogenous SOM was removed by oxidation to mimic the carbon mineralization and decomposition in the harsh environmental conditions, which was not considered in previous studies. As a result, organic matter and iron oxide complexes may be disrupted which can expose more iron oxides as substrate, shifting the microbial community composition such as As(V) and Fe(III)-reducing bacteria (Cai et al. 2021) and hence influencing the redox transformation of As and Fe in soils. To date, few attempts have been available concerning the change of As availability as a function of altered soil properties induced by carbon removal in soils.
Besides, ZVI can regulate the dynamics of endogenous carbon and Fe oxides. The Fe oxides originated from ZVI corrosion could alter the nucleation and dissolution of Fe oxides and thus inevitably affect the transformation of endogenous Fe oxides (Hu et al. 2023). Reversely, Fe oxides such as goethite and ferrihydrite can change the mineralization and chemical composition of SOM (Liu et al. 2022). To the best of our knowledge, how the depletion of SOM and the correspondingly changed soil properties and microbial community affect As immobilization and transformation in the presence of ZVI/BC under the anaerobic condition remains unknown. Hence, the present study aims at: 1) investigating the changes of major soil physiochemical properties (pH, carbon content, Fe oxides, and soil microbes) and soil As bioavailability as SOM was partially oxidized; and 2) elucidating the effects and mechanisms of SOM on As immobilization and redox transformation by ZVI/BC in As-spiked soils with different SOM contents. This work can enhance the understanding of the roles of SOM and microorganisms in ZVI/BC-induced As transformation in paddy soils.
2 Materials and methods
2.1 Preparation of ZVI/BC and As-contaminated soil
5 g of pinewood sawdust powder (20 mesh) was mixed with 1 g of hematite in a beaker with a volume of 100 mL and dispersed in 50 mL deionized water. The used hematite (α-Fe2O3) was purchased from Shanghai Aladdin Biochemical Technology Co., LTD. The mixture was placed in an oven and dried for 24 h at 60 ℃ after the ultrasound (the power of 100 W) for 1 h. The purpose of ultrasound was to make pinewood powder and hematite mixed evenly. The dried mixture was pyrolyzed in a nitrogen atmosphere (the flow rate of 40 mL min−1) at 700 ℃ for 2 h. In detail, the mixture was heated for 35 min to achieve 200 ℃ at the speed of 5 ℃ min−1, maintained at 200 ℃ for 1 h, heated for 100 min to reach 700 ℃ (i.e., the speed of 5 ℃ min−1), and kept at 700 ℃ for 2 h (Zhang et al. 2023b). The obtained material was labeled as ZVI/BC. The individual pinewood sawdust powder was pyrolyzed at the same condition, and the obtained material was labeled as BC. The samples were washed for 3 times by deionized (DI) water and ethanol (volume ratio of 1:1) and vacuum dried for 24 h in the oven at 60 ℃.
The experimental soil was collected from the experimental field of Yangzhou University, Jiangsu Province, China. Na2HAsO4·7H2O was added into the soil and aged for 1 month to obtain the As-contaminated paddy soil. The As concentration in the contaminated soil was 400 mg kg−1. The soil pH value was 6.82, and the cation exchange capacity (CEC) was 21.15 cmol kg−1, and the water holding capacity was 18.89%. The soil organic matter (SOM) concentration was quantified using the titration of FeSO4 after the oxidation by K2Cr2O7-H2SO4. The SOM concentration before and after being aged for 1 month was 36.38 and 36.03 g kg−1, respectively.
2.2 Experimental design
The SOM was removed using 30% H2O2 to obtain the OM-reduced contaminated soil (Molamahmood et al. 2020). The SOM content of the obtained OM-reduced soil was 8.01 g kg−1, and the removal rate of SOM was 77.8%. Arsenic was added into the soil before the removal of SOM. 0.25 g of BC or ZVI/BC was added into the polyethylene bottle with 25 g of the pristine contaminated soil or the SOM-reduced soil (i.e., 1% dosage). The mixture was mixed homogeneously, and incubated for 30 days. The two soils without any treatment served as the control groups. 37.5 g of DI water was supplemented into the polyethylene bottle to keep 150% moisture content of the soil to mimic the flooded anaerobic environment. There was no external microorganisms to be added into the soil during the entire incubation process. A previous study indicated that the bacterial community in the soil significantly changed after 7 days, since biochar was added into the soil (Chen et al. 2017). The soil moisture content was maintained by the weighing method every day. The experiment consisted of six treatments with three replicates of each treatment. For the pristine contaminated soil, the control group and treatments were marked as CK, BC, and ZVI/BC, respectively. Correspondingly, they were marked as RCK, RBC, and RZVI/BC, respectively for the OM-reduced soil. After the incubation, a part of the soil was air-dried and passed through 100 mesh to analyze the soil physicochemical properties including pH, dissolved organic carbon (DOC), fulvic acid carbon, humic acid carbon, ferrous iron, amorphous iron oxides, free iron oxides, available As, As fractionation, and the propotion of As(V) and As(III) in available As. About 3 g of the soil from every treatment was stored in a − 80 ℃ refrigerator to analyze the soil microbial community.
2.3 Analytical method of samples
The (NH4)2SO4 solution (0.05 M) was used to extract the non-specific adsorbed fraction As and characterized as the available As in the soil (Fan et al. 2020). The Wenzel sequential extraction method was used to extract the different species of As in soils (Zhou et al. 2022). The detailed extraction procedure is shown in Additional file 1: Table S1. The As concentrations in the extracts were measured using an inductively coupled plasma mass spectrometry (ICP-MS). The As(V) and As(III) concentrations in the (NH4)2SO4 extract were analyzed using a high performance liquid chromatography-mass spectrometry (HPLC–MS) (Fang et al. 2022). The Fe(II) concentration in the soil was extracted using HCl solution (0.5 M) and measured by the 1,10-phenanthroline spectrometric method (Zhang et al. 2023c). The amorphous iron oxides in the soil was extracted darkly using ammonium oxalate solution (0.2 M) and measured using the spectrometric method (Chen et al. 2020). The free iron oxide in the soil was extracted using sodium dithionite-sodium citrate-sodium bicarbonate (DCB) solutions and determined by the spectrometric method (Liu et al. 2023a).
Soil pH was measured with a pH meter based on DI water to soil ratio of 2.5:1 (Zhang et al. 2023c). The DOC was extracted according to the DI water to soil ratio of 5:1 and measured by the TOC analyzer after passing through a 0.45 μm filter membrane (Zhang et al. 2023a). The fulvic acid (FA) and humic acid (HA) in the soil were extracted according to the following method (Kou et al. 2022). Briefly, 1.0 g of soil sample was weighed and transferred into a 50 mL centrifuge tube, and 12 mL DI water was added. After shaking for 1 h in a water bath at 70 ℃, the soil sample was centrifuged and the supernatant was discarded, and then washed with 20 mL DI water. Afterwards, 20 mL of 0.1 mol L−1 Na4P2O7 and 0.1 mol L−1 NaOH mixtures were added into a centrifuge tube with the washed soil, shaken for 5 min and left for 14 h. After filtration, the filtrate pH value was adjusted to 1.0–1.5 using HCl solution, and the obtained acidified solution was kept in a water bath at 70 °C for 2 h before standing overnight. Then, filtration was performed and the resulted solution was FA and the precipitate was HA. The HA concentration was determined by the potassium dichromate oxidation-spectrophotometric method. The FA concentration was determined using a TOC analyzer.
The X-ray photoelectron spectroscopy (XPS) (ESCALAB250Xi, USA) was used to analyze the O and As binding form of the samples before and after adsorption of arsenate by ferrihydrite in the presence of humic acid (Xue et al. 2019). The high resolution-transmission electron microscopy (HR-TEM) (Tecnai 12, Netherlands) was used to analyze the micro-regional elemental distribution (C, O, Fe, and As) of the resultant product of arsenate adsorbed by ferrihydrite in the presence of humic acid (Zhang et al. 2023c).
2.4 Bacterial community analysis
The bacterial community composition in different soil samples was analyzed using the high-throughput sequencing technology (Zhang et al. 2023a). First, an E.Z.N.A.® soil DNA kit was used to extract the bacterial DNA in soils based on the manufacturer’s instructions. The bacterial V3-V4 region of the 16S rRNA gene was amplified with the primers of 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) via polymerase chain reaction (PCR) thermocycler. The PCR amplification of 16S rRNA genes and MiSeq sequencing were finished in Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China). The microbial-related figures were completed on the cloud platform provided by Majorbio Bio-Pharm Technology Co. Ltd.
2.5 Statistical analysis
The data were presented as mean ± standard deviation. One-way analysis of variance analysis (ANOVA) was used to assess the differences between different treatments using the SPSS 25.0 software, and the significance level was set at 0.05. Operational taxonomic units (OTUs) were defined at a 97% sequence identity.
3 Results
3.1 Characterization of materials
The specific surface areas, total pore volumes, and pore sizes of the obtained BC and ZVI/BC were shown in Additional file 1: Table S2. The specific surface area of BC (372.6 m2 g−1) was higher than that of ZVI/BC (198.1 m2 g−1) which can be attributed to the blockage of BC’s channel by the generated ZVI particles. The previous study also achieved the analogous result (Zhang et al. 2023c). Similarly, the total pore volume of ZVI/BC (0.11 cm3 g−1) was lower than that of BC (0.16 cm3 g−1). The XRD spectrum of the obtained BC and ZVI/BC was indicated in Additional file 1: Fig. S1. The characteristic diffraction peaks at 2θ = 44.7° and 65.2° were observed in the XRD spectrum of ZVI/BC, which indicated the successful synthesis of ZVI. The generation of ZVI mainly depended on the reaction of reductant gas (CO, H2, and CH4) from the pyrolysis of biomass and hematite at the high temperatures (700–850 °C for CO, 700–825 °C for CH4, and 200–500 °C for H2) (Liu et al. 2023b) and the phase transformation order of α-Fe2O3 is α-Fe2O3-Fe3O4-FeO-ZVI (Zhang et al. 2022). Also, the characteristic peak at 2θ = 24.8° in the XRD spectrum of BC indicated the amorphous carbon structure of pristine BC which agreed with the previous study (Zhang et al. 2022). In the SEM image of BC, the obvious pore structure was observed (Additional file 1: Fig. S2A). In the SEM image of ZVI/BC, the ZVI particles were mainly distributed at the surface of BC (Additional file 1: Fig. S2B). Additionally, the size of ZVI particles in ZVI/BC was lower than 1.0 μm according to the SEM image of ZVI/BC. Overall, the above-mentioned result indicated that ZVI/BC was successfully prepared.
3.2 Availability and fractionation of As in soil
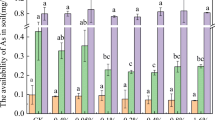
The change in concentration of available As in the soil and the fraction of As for different treatments are shown in Fig. 1. Obviously, the available As concentrations in the OM-reduced soils (RCK, RBC, and RZVI/BC) were significantly higher than those in the pristine soils (CK, BC, and ZVI/BC) no matter immobilizers were added or not (Fig. 1A). Moreover, biochar addition significantly increased available As concentrations in two soils compared with the respective control soils (P < 0.05) (Fig. 1A). On the contrary, available As concentrations in the soil were significantly reduced by 64.2% and 5.1%, respectively for the pristine and OM-reduced soils treated by ZVI/BC (i.e., ZVI/BC and RZVI/BC treatments) compared to the respective control group (CK and RCK) (P < 0.05). Further, adding biochar had different effects on the redox transformation of As in the available As extracted from the two soils. In the OM-reduced soil, biochar addition (RBC treatment) improved the proportion of As(V) from 49.7% (control) to 89.7%, whereas its proportion slightly decreased for the pristine soil (BC treatment). Differently, ZVI/BC addition significantly increased the proportion of As(III) as available As in the soil regardless of soil type. Specifically, the proportion of As(III) increased from 50.3% to 79.9% for the OM-reduced soil (RZVI/BC treatment) and from 38.4% to 81.9% for the pristine soil (ZVI/BC treatment).
(A) Changes in available As concentration and (B) the fractions (F1, F2, F3, F4, and F5) of As in two soils without treatment (CK) and treated by biochar (BC) and biochar-supported zero valent iron (ZVI/BC). RCK, RBC, and RZVI/BC represent the respective soils (CK, BC, and ZVI/BC) with the organic matter being removed. Different lowercase letters represent significant differences between treatments (P < 0.05). F1 means the non-specifically adsorbed As. F2 means the specifically adsorbed As. F3 means the amorphous and poorly-crystalline hydrous oxides of Fe and Al bonded As. F4 means the well-crystallized hydrous oxides of Fe and Al bonded As. F5 means the residual As
The results of Wenzel sequential extraction indicated that the non-specifically adsorbed As (F1) in the soil slightly increased for the two soils treated with biochar (BC and RBC treatments) in comparison to the respective control group (CK and RCK) (Fig. 1B). By contrast, ZVI/BC addition decreased the proportion of non-specifically adsorbed As (F1) in the two soils (ZVI/BC and RZVI/BC treatments) compared with the respective control group (CK and RCK). Correspondingly, the specifically-adsorbed As (F2) decreased from 52.8% to 49.9% for the pristine soil (BC treatment) and the amorphous and poorly-crystalline hydrous oxides of Fe and Al bonded As (F3) descended from 34.1% to 31.2% for the OM-reduced soil (RBC treatment) after the supplementation of biochar. Regarding ZVI/BC addition, the proportion of F3 portion increased from 29.9% to 42.5% for the pristine soil (ZVI/BC treatment), and the proportion of well-crystallized hydrous oxides of Fe and Al bonded As (F4 portion) increased from 5.7% to 6.7% for the OM-reduced soil (RZVI/BC treatment).
3.3 Changes in iron species in the soil
Amorphous Fe (oxyhydr) oxide is an important bonding site to As in soils. Figure 2 shows the changes in Fe(II), free Fe oxide, and amorphous iron oxide concentrations in two soils under different treatments. To be specific, biochar or ZVI/BC addition had no effect on the HCl-extractable Fe(II) concentration in the OM-reduced soil. However, ZVI/BC addition significantly increased the HCl-extractable Fe(II) concentration in the pristine soils in comparison to the control soil (Fig. 2A). In addition, biochar supplementation significantly improved the free Fe oxides concentration in the OM-reduced soil (P < 0.05), whereas it had no significant effect on that in the pristine soil (P > 0.05). Differently, ZVI/BC addition significantly improved the free Fe oxides concentration from 20.1 to 36.5 g kg−1 for the pristine soil and from 19.6 to 36.8 g kg−1 for the OM-removed soil (Fig. 2B). Similar to the free Fe oxides in the soil, the amorphous iron oxide contents in the soil significantly increased from 13.6 to 27.8 g kg−1 for the pristine soil and from 17.3 to 27.1 g kg−1 for the OM-reduced soil after ZVI/BC was added (Fig. 2C). Nevertheless, the individual biochar application showed no significant effect on the amorphous iron oxide contents in the two soils compared to the respective control soil (P > 0.05).
(A) Changes in concentrations of Fe(II), (B) free Fe oxide, and (C) amorphous Fe oxide in the soils without treatment (CK) and treated with biochar (BC) and biochar-supported zero valent iron (ZVI/BC). RCK, RBC, and RZVI/BC represent the respective soils (CK, BC, and ZVI/BC) with organic matter being removed. Different lowercase letters represent significant differences between treatments (P < 0.05)
3.4 Changes in soil pH and organic carbon in soils
The different carbon contents of the two soils and the change in soil pH are shown in Fig. 3. The addition of biochar and ZVI/BC had no significant effect on soil pH compared to the corresponding control group. The removal of organic matter significantly increased soil pH (Fig. 3A). In addition, compared with the corresponding control soil, the application of biochar into the pristine soil reduced the DOC content in the pristine soil, but increased the DOC content in the OM-reduced soil (Fig. 3B). ZVI/BC addition significantly reduced the DOC content in the pristine soil (P < 0.05), while it had no significant effect on that in the OM-reduced soil (P < 0.05). The application of biochar and ZVI/BC had no significant effect on the FA carbon content in the pristine soil. The removal of soil organic matter significantly increased the FA carbon content in the soil (P < 0.05). Furthermore, the FA carbon content in the OM-reduced soil significantly increased with the application of biochar and ZVI/BC (P < 0.05). The individual BC addition had better effect on enhancing the FA carbon content than the ZVI/BC addition, which can be related to the higher dosage of biochar (Fig. 3C). Both removal of OM and stabilizers addition had no significant effect on the HA carbon contents in the two soils (P > 0.05).
(A) Changes in soil pH, (B) dissolved organic carbon (DOC) content, (C) fulvic acid carbon content, and (D) humic acid carbon content in the soil treated by biochar (BC) and biochar-supported zero valent iron (ZVI/BC). RCK, RBC, and RZVI/BC represent the respective soil with organic matter being removed. Different lowercase letters represent significant differences between treatments (P < 0.05)
3.5 Microbial community characteristics
The abundances of soil bacterial communities on the different taxonomic levels (phylum, class, and genus) and the beta diversity of soil bacteria communities are shown in Fig. 4. The result on the phylum level indicated that the composition of bacterial communities was very susceptible to SOM (Fig. 4A). Specifically, the reduction of SOM increased the relative abundance of Proteobacteria (from 8.95% to 11.3%), Firmicutes (from 8.95% to 11.3%), and Spirochaetota (from zero to 4.15%) in the soil. On the contrary, the abundance of Cyanobacteria, Actinobacteriota, and Bacteroidota decreased. In the pristine soil, both biochar and ZVI/BC addition increased the relative abundance of Cyanobacteria, but decreased the abundance of Bacteroidota. For the OM-reduced soils, the abundances of Proteobacteria and Actinobacteriota increased after biochar and ZVI/BC were applied. To be specific, the abundance of Proteobacteria increased from 11.3% to 51.69% for biochar and 61.8% for ZVI/BC. The abundance of Actinobacteriota increased from 1.59% to 10.63% for biochar and 10.92% for ZVI/BC. However, the abundance of Firmicutes decreased by 2.4 and 3.3 times, respectively, compared to the control group.
(A) Relative abundance of soil bacteria for different treatments on phylum level, (B) class level, (C) genus level, and (D) the principal component analysis (PCA) of soil bacterial community based on the phylum level. CK means control group, and BC means the soil treated by biochar (BC), and ZVI/BC means the soil treated by biochar-supported zero valent iron (ZVI/BC). RCK, RBC, and RZVI/BC represent the respective soil with the organic matter being removed
The relative abundances of soil bacterial communities on the class level indicated that Bacilli and Clostridium were the most abundant in the OM-reduced control soil (Fig. 4B). Both Bacilli and Clostridium belong to the phylum Firmicutes (Li et al. 2020b). Moreover, the relative abundances of Cyanobacteriia and Actinobacteria in the pristine soil were higher than those in the OM-reduced soil. In addition, the abundances of Gammaproteobacteria, Alphaproteobacteria, and Actinobacteria were significantly increased when biochar and ZVI/BC were applied to the OM-reduced soil. These results were consistent with the taxonomic results on the phylum level. Furthermore, the abundances of class Desulfitobacteriia and Desulfotomaculia associated with sulfate reduction were the highest in the OM-reduced soil treated by ZVI/BC. However, these two classes were not detected in the pristine soil treated by ZVI/BC. This can be attributed to the fact that the high FA carbon concentration in the OM-reduced soil can serve as microbial carbon source.
In addition, the organic matter removal caused some shifts to the microorganisms at the genus level (Fig. 4C). Specifically, the relative abundances of Synechococcus_CC9902, Candidatus_Aquiluna, Delftia, and cyanobium_PSC-6307 decreased. Synechococcus_CC9902 was reported to own the assimilation function of nitrite at high ammonium content. Candidatus_Aquiluna can participate in the organic matter degradation and anaerobic ammonia-oxidation process (Lukwambe et al. 2020). A previous study indicated that Delftia, as a denitrifying phosphate-accumulating organism, possessed high removal efficiencies of NO3−-N and PO43−-P (Li et al. 2020a). However, the relative abundance of Pseudomonas, Bacillus, Symbiobacterium, and Arthorbacter increased after the removal of organic matter. In the OM-reduced soil, ZVI/BC addition made the relative abundance of Pseudomonas increase from 9.3% (control) to 36.6% (RZVI/BC treatment).
The results of principal component analysis (PCA) at the phylum level showed that the first two principal components accounted for 84.38% of the total variation (Fig. 4D). Further, the distance between samples in the pristine soil group was smaller than that between the samples in the OM-reduced soil group, indicating that the removal of SOM treatment had a significant effect on the microbial community in the soil. In addition, the addition of biochar and ZVI/BC significantly affected the soil microbial community structure for the OM-reduced soil.
3.6 Correlations of environmental characteristics with microbial communities
The results of RDA and Pearson correlation analysis between environmental factors and the relative abundance of bacteria on phylum level are shown in Fig. 5. According to the RDA results (Fig. 5A), the content of available As in soils was positively correlated with soil pH and FA content, but negatively correlated with HA and Fe(II) contents. Available As, soil pH, and FA mainly affected the bacterial community in the OM-reduced soil. Moreover, according to the results of correlation analysis (Additional file 1: Table S3), the amorphous Fe oxide concentration was significantly negatively correlated to the DOC content (P < 0.05).
According to Fig. 5B, the content of available As, soil pH, and FA content were positively correlated with the relative abundances of plylum Firmicutes, Proteobacteria, Chloroflexi, and Desulfobacterota, respectively. Moreover, amorphous Fe oxide and free Fe oxide concentrations were also positively correlated with the relative abundances of plylum Proteobacteria.
3.7 Functional prediction of soil bacteria communities
The functional prediction of soil bacterial community based on the FAPROTAX database can reflect the elemental biogeochemical cycling. The results of functional predictions for different treatment groups are shown in Fig. 6. The relative abundances of some community functions such as dissimilatory arsenate reduction, aromatic compound degradation, and arsenite oxidation detoxification were the highest for the RZVI/BC treatment. This result can indirectly prove that arsenic immobilization by ZVI/BC was affected by the arsenic redox associated microbes in the OM-reduced soil. This could also indicate that the reductive transformation of arsenate in available As was affected by not only ZVI but also the As(V)-reducing bacteria in the OM-reduced soil. However, in the pristine soil, the relative abundances of these three community functions were relatively low, implying that the high proportion of arsenite from the available As in the soil treated by ZVI/BC can be ascribed to the reduction effect by ZVI.
Functional predictability of soil bacterial community based on the FAPROTAX database. CK means control group, and BC means the soil treated by biochar (BC), and ZVI/BC means the soil treated by biochar-supported zero valent iron (ZVI/BC). RCK, RBC, and RZVI/BC represent the respective soil with organic matter being removed
4 Discussion
4.1 Transformation of As
According to Sect. 3.1, biochar addition into soil increased the As availability, which was consistent with the results of previous studies (Wang et al. 2017; Fan et al. 2020). This can be mainly attributed to the soil pH increase induced by biochar, enhancing the electrostatic repulsion, thus facilitating As desorption from soil particles (Fan et al. 2020). Although the formation of dissolved complexes of dissolved organic matter (DOM) with As and the competition effect from DOM molecules for adsorption sites could also improve the lability and availability of As in the soil (Aftabtalab et al. 2022), DOM may not be the key factor influencing available As in the soil according to the results of correlation analysis (R = 0.068) (Additional file 1: Table S2) in the present study. ZVI/BC addition decreased the As availability in comparison to the respective control soil. These can mainly be ascribed to the increased concentration of amorphous iron oxide (Fig. 2C), which facilitated the immobilization of As via adsorption and co-precipitation (Manning et al. 2002). It was reported that ferrihydrite, magnetite, lepidocrocite, and goethite were formed as the oxidative products of ZVI in the realistic soil, and these iron oxides have strong affinity to arsenite or arsenate (Hui et al. 2022; Mitzia et al. 2023), immobilizing As(V) and As(III). The result of Wenzel sequential extraction experiment indicated that ZVI/BC addition could facilitate the transformation of the labile As species in the two soils to the more stable As species. However, the application of individual biochar can increase the release of As in the two soils (Fig. 1B). This result was also in line with the previous study (Fan et al. 2020). ZVI/BC addition increased the As(III) proportion in the available As, which could be ascribed to the reductive transformation of As(V) to As(III) by ZVI (Fan et al. 2020). However, the generated As(III) can also be immobilized by the amorphous iron oxide or the secondary iron minerals in the soils, though As(III) was more toxic than As(V) (Chen et al. 2023).
4.2 Effects of soil physicochemical properties on As availability
A previous study indicated that the amorphous Fe oxide can play an important role in immobilizing As in the soil (Liu et al. 2015). Therefore, ZVI/BC addition can facilitate the immobilization of As, probably resulting from the increased amorphous Fe oxide in the soil. Further, after adding ZVI/BC, the amorphous Fe oxide contents in the pristine soil was significantly higher than that in the OM-reduced soil (P < 0.05), which supported the result of available As in the soil (Fig. 1A). Higher soil pH made OH− compete with anionic As for adsorption sites, which was not conducive to immobilization of As in the soil. In this study, Fe(II) concentration was negatively correlated to available As (Fig. 5A), indicating that Fe(II) generation was a necessary process for As immobilization in soil by ZVI/BC. Moreover, HA content was negatively correlated to available As, indicating that HA could be in favor of As immobilization (Fang et al. 2022). The result indicated that the oxidation, destruction, and decomposition of soil organic matter by the oxidizing agent (i.e., hydrogen peroxide) resulted in the destruction of the organic-mineral complexes in the soil and the increase of FA carbon content with small molecular weight and strong mobility (Fig. 3C). Previous studies have shown that the presence of humic substance decreases the adsorption capacity of Fe(OH)3 towards arsenate, and the competition effect of FA was stronger than that of HA (Saldaña-Robles et al. 2017; Marzi et al. 2022). In addition, the positive correlation between FA carbon content and available As indicated that FA could compete with arsenate or arsenite for the adsorption site in soil. Overall, the effect of SOM removal mainly influenced the soil pH and FA carbon content in soil to alter As availability in soil. Previous studies have also indicated that these two parameters have important influences on the immobilization of As on Fe oxides in the soil (Du et al. 2014; Yang et al. 2022a).
4.3 Effects of microorganism on As transformation
It was reported that Pseudomonas could reduce arsenate to arsenite, which also supported the result of Sect. 3.1 (Liao et al. 2011). Bacillus and Arthorbacter were functionally classified as toxic element-resistant bacteria due to substrate stimulation (i.e., more available arsenic). Both biochar and ZVI/BC addition stimulated the growth of Ammoniphilus that was reported as iron-reducing bacteria (Luo et al. 2021). Similarly, the relative abundance of Acinetobacter was also the highest, which may be resulted from Fe oxides from the oxidation of ZVI as an electron acceptor, facilitating the proliferation of Acinetobacter as iron-reducing bacteria (Yang et al. 2022b). On another hand, Pseudomonas, Acinetobacter, and Bacillus can be also arsenite-oxidizing bacteria that can transform arsenite to arsenate by using either O2 as a terminal electron acceptor (TEA) under the oxic condition or NO3−, NO2−, and SeO32− as an TEA under the strict anoxic condition. Therefore, these arsenite-oxidizing bacteria also participated in As transformation in the current condition. A previous study also indicated that low dosage addition of ZVI/BC can stimulate the growth of sulfate-reducing bacteria in an anaerobic matrix (Liu et al. 2021). OH− ion and FA molecules can increase the quantity of negative charge on the surface of soil colloids, facilitating desorption and release of As (Meng et al. 2022a). This indicated that more OH− and more FA did not favor As immobilization in the soil. Previous studies had indicated that Ammoniphilus and Bacillus can participate in the reduction of Fe(III) and As(V) in the soil (Wang et al. 2023). Therefore, the more As(III) in the available As in the OM-reduced soil can be generated by the microbial reduction of As(V) after ZVI/BC addition, while the more As(III) in the available As in the pristine soil mainly derive from the reduction of ZVI.
Overall, the removal of SOM compromised the immobilization of arsenic by ZVI/BC. This is mainly due to the significant increase in soil pH and fulvic carbon content after the removal of SOM. In addition, the removal of SOM may lead to the fragmentation of iron oxides-organic matter complexes, exposing more iron oxides, thus stimulating the proliferation of the bacteria affiliated with Firmicutes and Proteobacteria. Pseudomonas belonged to Proteobacteria can reduce As(V) to As(III), possibly improving the migration ability of As, and Bacillus can reduce Fe(III) minerals, probably enhancing the release of As bound to iron minerals though they can also oxidize As(III) to As(V). Therefore, the removal of SOM was not conducive to the immobilization of arsenic in soils by ZVI/BC. Moreover, the anaerobic microbial reduction of Fe-As minerals due to the stimulation of ZVI/BC to Fe(III)- and As(V)-reducing bacteria could play an important role in the increased proportion of As(III) in the available fraction (Cai et al. 2021), especially in the OM-reduced soil.
4.4 Mechanism of As immobilization and transformation
Although high fulvic acid contents are not in favor of the immobilization of As, ZVI/BC can significantly reduce the available As concentration in the soils compared to the respective control soil. It was indicated that ZVI that was added into soils can be transformed to γ-Fe2O3 and Fe3O4, which occurred in the form of Fe oxides-organic matter aggregates (Wang et al. 2021). In order to further elucidate the mechanism of As immobilization by ZVI/BC in the SOM-reduced soil, the XPS spectra of O and As of the reacted products after adsorption of As by ferrihydrite in the presence of humic acid were analyzed. The results of O 1 s peak showed that the proportion of Fe-O-As bond increased from 26.5% (Fe-O-H) to 36.1% after adsorption (Additional file 1: Fig. S1C), which indicated that ferrihydrite can also immobilize a part of As in the presence of humic acid. The adsorbed As mainly existed in the form of arsenate (Additional file 1: Fig. S2D). Previous studies have indicated that formation of ternary complexes (i.e., As-Fe(III)-OM complexes) is the main interaction mechanism of As, Fe, and OM under oxidized or reducing conditions (Mikutta and Kretzschmar 2011; Hoffmann et al. 2013). Further, the high-resolution TEM mapping images showed that the distribution tendencies of C, O, Fe, and As were coincident (Fig. 7). Collectively, the formation of ternary complexes i.e., As-Fe(III)-OM complexes could be the mainly mechanism of As immobilization by ZVI/BC in the SOM-reduced soil.
Overall, in the pristine soil, ZVI/BC mainly increased the content of amorphous Fe oxide in soil to immobilize As. The elevated proportion of arsenite was mainly due to the reduction of ZVI. However, in the OM-reduced soil, immobilization of As by ZVI/BC was inhibited by the increase of soil pH and the high content of FA caused by the oxidative reduction of SOM. The migration of As was enhanced by Fe(III)-reducing bacteria and As(V)-reducing bacteria stimulated by more iron oxides exposure after reducing SOM. Therefore, in the OM-reduced soil arsenate reduction was affected by not only ZVI but also the Fe/As biogeochemical cycle associated microbes.
5 Conclusion
This study investigated the influence of SOM depletion on the remediation of As-contaminated paddy soil by ZVI/BC. Biochar addition significantly increased available As concentrations in the two soils. However, available As concentrations in pristine and OM-reduced soils treated by ZVI/BC were reduced by 64.2% and 5.1%, respectively, ascribing to the increase of amorphous iron oxide. ZVI/BC supplementation facilitated the transformation of labile As to a more stable faction, thus benefiting As immobilization. The removal of SOM compromised the remediation efficiency of As by ZVI/BC, attributing to the increased soil pH and FA carbon concentration. The removal of SOM also stimulated the proliferation of the bacteria such as Bacillus and Ammoniphilus. The addition of ZVI/BC improved the proportion of As(III) in available As in two soils. The removal of SOM increased the contribution of arsenic/iron-reducing bacteria to As(V) reduction in soils; however, As(V) reduction process may be mainly controlled by ZVI in the pristine soil.
Availability of data and materials
Not applicable.
References
Aftabtalab A, Rinklebe J, Shaheen SM, Niazi NK, Moreno-Jiménez E, Schaller J, Knorr K-H (2022) Review on the interactions of arsenic, iron (oxy)(hydr)oxides, and dissolved organic matter in soils, sediments, and groundwater in a ternary system. Chemosphere 286:131790
Cai X, ThomasArrigo LK, Fang X, Bouchet S, Cui Y, Kretzschmar R (2021) Impact of organic matter on microbially-mediated reduction and mobilization of arsenic and iron in Arsenic(V)-bearing ferrihydrite. Environ Sci Technol 55:1319–1328
Cai D, Kong S, Shao Y, Liu J, Liu R, Wei X, Bai B, Werner D, Gao X, Li C (2022) Mobilization of arsenic from As-containing iron minerals under irrigation: Effects of exogenous substances, redox condition, and intermittent flow. J Hazard Mater 440:129736
Chen Y, Liu Y, Li Y, Wu Y, Chen Y, Zeng G, Zhang J, Li H (2017) Influence of biochar on heavy metals and microbial community during composting of river sediment with agricultural wastes. Biores Technol 243:347–355
Chen P, Zhang H-M, Yao B-M, Chen S-C, Sun G-X, Zhu Y-G (2020) Bioavailable arsenic and amorphous iron oxides provide reliable predictions for arsenic transfer in soil-wheat system. J Hazard Mater 383:121160
Chen X, Xu X, Wei Y, Wang X, Cao X (2023) Constructing the active surface soil layer with ZVI-biochar amendment for simultaneous immobilization of As and Zn in both contaminated soil and groundwater: continuous versus intermittent infiltration mode. J Hazard Mater 445:130518
Du J, Jing C, Duan J, Zhang Y, Hu S (2014) Removal of arsenate with hydrous ferric oxide coprecipitation: effect of humic acid. J Environ Sci 26:240–247
Fan J, Chen X, Xu Z, Xu X, Zhao L, Qiu H, Cao X (2020) One-pot synthesis of nZVI-embedded biochar for remediation of two mining arsenic-contaminated soils: arsenic immobilization associated with iron transformation. J Hazard Mater 398:122901
Fang W, Yang D, Williams PN, Yang Y (2022) Distinct response of arsenic speciation and bioavailability to different exogenous organic matter in paddy soil. Chemosphere 309:136653
Feng F, Jiang Y, Jia Y, Lian X, Shang C, Zhao M (2023) Exogenous-organic-matter-driven mobilization of groundwater arsenic. Environmental Science and Ecotechnology 15:100243
Hoffmann M, Mikutta C, Kretzschmar R (2013) Arsenite binding to natural organic matter: spectroscopic evidence for ligand exchange and ternary complex formation. Environ Sci Technol 47:12165–12173
Hu L, Zhang P, Xu X, Ren J, Zhao L, Qiu H, Cao X (2023) Immobilization of arsenic in different contaminated soils by zero-valent iron-embedded biochar: effect of soil characteristics and treatment conditions. Sci Total Environ 868:161597
Hui C, Liu B, Du L, Xu L, Zhao Y, Shen D, Long Y (2022) Transformation of sulfidized nanoscale zero-valent iron particles and its effects on microbial communities in soil ecosystems. Environ Pollut 306:119363
Kaur J, Anand V, Srivastava S, Bist V, Naseem M, Singh P, Gupta V, Singh PC, Saxena S, Bisht S, Srivastava PK, Srivastava S (2023) Mitigation of arsenic toxicity in rice by the co-inoculation of arsenate reducer yeast with multifunctional arsenite oxidizing bacteria. Environ Pollut 320:120975
Kou B, Hui K, Miao F, He Y, Qu C, Yuan Y, Tan W (2022) Differential responses of the properties of soil humic acid and fulvic acid to nitrogen addition in the North China Plain. Environ Res 214:113980
Li H, Liu H, Zeng Q, Xu M, Li Y, Wang W, Zhong Y (2020a) Isolation and appraisal of a non-fermentative bacterium, Delftia tsuruhatensis, as denitrifying phosphate-accumulating organism and optimal growth conditions. J Water Process Eng 36:101296
Li L, Jia R, Qu Z, Li T, Shen W, Qu D (2020b) Coupling between nitrogen-fixing and iron(III)-reducing bacteria as revealed by the metabolically active bacterial community in flooded paddy soils amended with glucose. Sci Total Environ 716:137056
Liao VH-C, Chu Y-J, Su Y-C, Hsiao S-Y, Wei C-C, Liu C-W, Liao C-M, Shen W-C, Chang F-J (2011) Arsenite-oxidizing and arsenate-reducing bacteria associated with arsenic-rich groundwater in Taiwan. J Contam Hydrol 123:20–29
Liu G, Fernandez A, Cai Y (2011) Complexation of arsenite with humic acid in the presence of ferric iron. Environ Sci Technol 45:3210–3216
Liu C, Yu H-Y, Liu C, Li F, Xu X, Wang Q (2015) Arsenic availability in rice from a mining area: Is amorphous iron oxide-bound arsenic a source or sink? Environ Pollut 199:95–101
Liu Q, Sheng Y, Wang W, Liu X (2021) Efficacy and microbial responses of biochar-nanoscale zero-valent during in-situ remediation of Cd-contaminated sediment. J Clean Prod 287:125076
Liu M, Zhu J, Yang X, Fu Q, Hu H, Huang Q (2022) Mineralization of organic matter during the immobilization of heavy metals in polluted soil treated with minerals. Chemosphere 301:134794
Liu Q, Huang Y, Zhou Y, Chen Z, Luo J, Yan X (2023a) Impacts of wet-dry alternations on cadmium and zinc immobilisation in soil remediated with iron oxides. J Environ Manag 326:116660
Liu X, Rong R, Dai M, Bian H, Peng C (2023b) Preparation of red mud-based zero-valent iron materials by biomass pyrolysis reduction: reduction mechanism and application study. Sci Total Environ 864:160907
Lukwambe B, Nicholaus R, Zhao L, Yang W, Zhu J, Zheng Z (2020) Microbial community and interspecies interaction during grazing of ark shell bivalve (Scapharca subcrenata) in a full-scale bioremediation system of mariculture effluents. Mar Environ Res 158:104956
Luo D, Meng X, Zheng N, Li Y, Yao H, Chapman SJ (2021) The anaerobic oxidation of methane in paddy soil by ferric iron and nitrate, and the microbial communities involved. Sci Total Environ 788:147773
Manning BA, Hunt ML, Amrhein C, Yarmoff JA (2002) Arsenic(III) and Arsenic(V) reactions with zerovalent iron corrosion products. Environ Sci Technol 36:5455–5461
Marzi M, Towfighi H, Shahbazi K, Farahbakhsh M, Kazemian H (2022) Study of arsenic adsorption in calcareous soils: competitive effect of phosphate, citrate, oxalate, humic acid and fulvic acid. J Environ Manage 318:115532
Medaiyese AO, Wu J, Unc A (2023) Utility of wood ash, paper sludge and biochar for the mitigation of greenhouse gases emissions from acid boreal soils. J Environ Manage 330:117202
Meng F, Huang Q, Cai Y, Xiao L, Wang T, Li X, Wu W, Yuan G (2022a) A comparative assessment of humic acid and biochar altering cadmium and arsenic fractions in a paddy soil. J Soils Sediments. https://doi.org/10.1007/s11368-022-03385-8
Meng H, Yan Z, Li X (2022b) Effects of exogenous organic acids and flooding on root exudates, rhizosphere bacterial community structure, and iron plaque formation in Kandelia obovata seedlings. Sci Total Environ 830:154695
Mikutta C, Kretzschmar R (2011) Spectroscopic evidence for ternary complex formation between arsenate and ferric iron complexes of humic substances. Environ Sci Technol 45:9550–9557
Mitzia A, Vítková M, Ratié G, Chotěborský R, Vantelon D, Neaman A, Komárek M (2023) Revealing the long-term behaviour of nZVI and biochar in metal(loid)-contaminated soil: focus on Fe transformations. Environ Sci Nano 10:2861–2879
Molamahmood HV, Qin J, Zhu Y, Deng M, Long M (2020) The role of soil organic matters and minerals on hydrogen peroxide decomposition in the soil. Chemosphere 249:126146
Nascimento ÍVD, Fregolente LG, Pereira APDA, Nascimento CDVD, Mota JCA, Ferreira OP, Sousa HHDF, Silva DGGD, Simões LR, Souza Filho AG, Costa MCG (2023) Biochar as a carbonaceous material to enhance soil quality in drylands ecosystems: A review. Environ Res 233:116489
Peng X-X, Gai S, Cheng K, Yang F (2022) Roles of humic substances redox activity on environmental remediation. J Hazard Mater 435:129070
Qiu H, Liu J, Chen X, Hu Y, Su Y, Ge T, Li D, Wu J (2022) Rice straw carbon mineralization is affected by the timing of exogenous glucose addition in flooded paddy soil. Appl Soil Ecol 173:104374
Saldaña-Robles A, Saldaña-Robles N, Saldaña-Robles AL, Damian-Ascencio C, Rangel-Hernández VH, Guerra-Sanchez R (2017) Arsenic removal from aqueous solutions and the impact of humic and fulvic acids. J Clean Prod 159:425–431
Song P, Xu H, Sun S, Xiong W, Yang Z (2022) Remediation of arsenic-spiked soil by biochar-loaded nanoscale zero-valent iron: performance, mechanism, and microbial response. J Clean Prod 380:134985
Stolze L, Zhang D, Guo H, Rolle M (2019) Model-based interpretation of groundwater arsenic mobility during in situ reductive transformation of ferrihydrite. Environ Sci Technol 53:6845–6854
Tran THH, Kim SH, Lee H, Jo HY, Chung J, Lee S (2023) Variable effects of soil organic matter on arsenic behavior in the vadose zone under different bulk densities. J Hazard Mater 447:130826
Wang N, Xue X-M, Juhasz AL, Chang Z-Z, Li H-B (2017) Biochar increases arsenic release from an anaerobic paddy soil due to enhanced microbial reduction of iron and arsenic. Environ Pollut 220:514–522
Wang Y, Liu Y, Su G, Yang K, Lin D (2021) Transformation and implication of nanoparticulate zero valent iron in soils. J Hazard Mater 412:125207
Wang Y, Joseph S, Chen C, Qi X, Mitchell DRG, Si H, Shang J (2023) Goethite-enriched biochar mitigates soil emissions of CO2 during arsenic passivation: effect and mechanisms. Chem Eng J 476:146542
Wen E, Yang X, Chen H, Shaheen SM, Sarkar B, Xu S, Song H, Liang Y, Rinklebe J, Hou D, Li Y, Wu F, Pohořelý M, Wong JWC, Wang H (2021) Iron-modified biochar and water management regime-induced changes in plant growth, enzyme activities, and phytoavailability of arsenic, cadmium and lead in a paddy soil. J Hazard Mater 407:124344
Xue Q, Ran Y, Tan Y, Peacock CL, Du H (2019) Arsenite and arsenate binding to ferrihydrite organo-mineral coprecipitate: Implications for arsenic mobility and fate in natural environments. Chemosphere 224:103–110
Yang Y, Rao X, Fu Q, Zhang X, Gao J, Wan X, Zhu J, Huang G, Hu H (2022a) The inhibiting effects of organic acids on arsenic immobilization by ferrihydrite: gallic acid as an example. Chemosphere 299:134286
Yang Z-M, Guo R-B, Dong X-H (2022b) Promoting biomethane production from propionate with Fe2O3@carbon nanotubes composites. Sci Total Environ 818:151762
Yang X, Dai Z, Ge C, Yu H, Bolan N, Tsang DCW, Song H, Hou D, Shaheen SM, Wang H, Rinklebe J (2023) Multiple-functionalized biochar affects rice yield and quality via regulating arsenic and lead redistribution and bacterial community structure in soils under different hydrological conditions. J Hazard Mater 443:130308
Yuan Y, Liu Q, Zheng H, Li M, Liu Y, Wang X, Peng Y, Luo X, Li F, Li X, Xing B (2023) Biochar as a sustainable tool for improving the health of salt-affected soils. Soil & Environmental Health 1:100033
Zhang J, Yang X, Shi J, Zhao M, Yin W, Wang X, Wang S, Zhang C (2022) Carbon matrix of biochar from biomass modeling components facilitates electron transfer from zero-valent iron to Cr(VI). Environ Sci Pollut Res 29:24309–24321
Zhang J, Jiang Y, Ding C, Wang S, Zhao C, Yin W, Wang B, Yang R, Wang X (2023a) Remediation of lead and cadmium co-contaminated mining soil by phosphate-functionalized biochar: performance, mechanism, and microbial response. Chemosphere 334:138938
Zhang J, Qian Y, Wang S, Yin W, Wang B, Yang R, Wang X (2023b) Effect and mechanism of biochar as a support on immobilization of different heavy metals by iron oxides in a multi-contaminated soil. J Environ Chem Eng 11:109895
Zhang J, Yang X, Wang S, Li T, Li W, Wang B, Yang R, Wang X, Rinklebe J (2023c) Immobilization of zinc and cadmium by biochar-based sulfidated nanoscale zero-valent iron in a co-contaminated soil: performance, mechanism, and microbial response. Sci Total Environ 902:165968
Zhou S-J, Du Y-J, Sun H-Y, Yuan H, Feng Y-S, Xia W-Y (2022) Evaluation of the effectiveness of ex-situ stabilization for arsenic and antimony contaminated soil: Short-term and long-term leaching characteristics. Sci Total Environ 848:157646
Acknowledgements
This work was supported by the National Natural Science Foundation of China [Grant numbers 42277040; 41977117; 41977085], the National Key Research and Development Program of China [Grant number 2021YFD1700800], Qing-Lan Project of Yangzhou University [2020], and High-level Talent Support Plan of Yangzhou University [2019].
Funding
National Natural Science Foundation of China [Grant numbers 42277040; 41977117; 41977085], National Key Research and Development Program of China [Grant number 2021YFD1700800], Qing-Lan Project of Yangzhou University [2020], and High-level Talent Support Plan of Yangzhou University [2019].
Author information
Authors and Affiliations
Contributions
SW wrote the manuscript. WL, CD, JZ, and NZ finished the experiment. YCL, BG, BW, and XW reviewed and provided valuable comments to the manuscript. SW has made substantial contributions to manuscript writing and revision. All authors have reviewed the manuscript and given their permission to be named.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling editor: Xiangzhou Yuan
Supplementary Information
Additional file 1: Table S1.
The Wenzel sequential extraction procedure of the As-contaminated soil. Table S2. The specific surface areas, total pore volumes, and pore sizes of the obtained biochar (BC) and biochar-supported zero-valent iron (ZVI/BC). Table S3. The results of Pearson correlation analysis for the tested parameters in soils (n=18). Fig. S1. The XRD spectrum of the obtained biochar (BC) and biochar-supported zero-valent iron (ZVI/BC). Fig. S2. The SEM images of the obtained biochar (BC) (A) and biochar-supported zero-valent iron (ZVI/BC) (B). Fig. S3. The XPS spectrum of O 1s and As 3d before (A and B) and after (C and D) adsorption of arsenate by ferrihydrite in the presence of humic acid.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, S., Li, W., Ding, C. et al. Biochar-supported zero-valent iron enhanced arsenic immobilization in a paddy soil: the role of soil organic matter. Biochar 6, 26 (2024). https://doi.org/10.1007/s42773-024-00318-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-024-00318-1