Abstract
Modification serves as an excellent approach to enhancing the adsorption performance of biochar for tetracycline. Selective modification further allows the attainment of biochar materials that are not only more efficient but also cost-effective. However, the key structural factors influencing the adsorption of tetracycline by biochar remain unclear at present, hindering the effective guidance for modification strategies. This study established the relationship between carbonization degree and adsorption capacity, constructed a standardized microscopic model for biochar adsorption of tetracycline, and explored potential reaction mechanisms. The results indicated that with increases in the degree of carbonization, the tetracycline adsorption capacity of biochar increased from 16.08 mg L−1 to 98.35 mg L−1. The adsorption energy exhibited a strong correlation with the aromatic condensation of biochar at p ≤ 0.01, with a linear relationship (r2 ≥ 0.94). For low carbonization degrees, the adsorption of tetracycline by biochar was primarily driven by chemical bonds (69.21%) and complemented with electrostatic interactions, weak van der Waals forces or π-π interactions. For high carbonization degrees, the synergistic effects of hydrogen bonding, van der Waals forces, and π-π interactions determined the adsorption of tetracycline on biochar (91.1%). Additionally, larger carbon clusters resulted in stronger and more stable adsorption interactions. Furthermore, carboxyl-functionalized highly carbonized biochar displayed the highest reaction energy of − 1.8370 eV for adsorption of tetracycline through electrostatic interactions. This study suggests that a high degree of aromatic condensation in the carbon structure of biochar is crucial for the efficient adsorption of tetracycline.
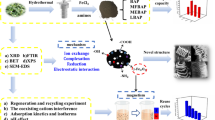
Graphical Abstract

Highlights
-
Low-carbonized biochar primarily adsorbs tetracycline through chemical bonds.
-
High degree of aromatic condensation facilitates biochar adsorption of tetracycline.
-
-COOH provides the highest binding affinity for tetracycline adsorption by high-carbonized biochar.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Tetracycline (TTC) is the most widely utilized antibiotic in China and the second most frequently employed antimicrobial globally (Balakrishnan et al. 2023), (Mohammadi et al. 2023). In recent years, tetracycline has been detected in various environments, including water, soil, and even plants (Zhang et al. 2021a), (Zhu et al. 2021), (Chang et al. 2023), (Lenart-Boroń et al. 2022). This exacerbates concerns regarding public health, as the indiscriminate use of antibiotics is inducing modifications in bacteria and their genetic material, thereby fostering mutations and increased resistance (Leichtweis et al. 2022). Strategies for tetracycline removal include membrane separation (Liu et al. 2019), adsorption, (Minale et al. 2020; Zhang et al. 2023a) advanced oxidation technologies, (Hou et al. 2022), (Zhang et al. 2023b) etc. Adsorption is widely favored due to its low energy consumption, ease of operation, and environmental friendliness (Liang et al. 2022). Nevertheless, conventional adsorbents such as carbon nanotubes (Li et al. 2023), graphene (Zhao et al. 2023), and activated carbon16 (Vinayagam et al. 2023) are constrained by intricate synthesis and relatively elevated costs. Biochar (BC) is a high-value adsorbent generated via biomass pyrolysis under oxygen-limited conditions, and it exhibits a novel negative carbon emission level (Xu et al. 2020; Yi et al. 2020). The biomass for biochar production originates from solid wastes generated at various sources, including agriculture, aquaculture, and industry, and it is abundant and cost-effective (Liang et al. 2021). Additionally, the porous structure and abundant functional groups of biochar provide exceptional adsorption capacities, suggesting its potential as a promising adsorbent (Yang et al. 2021b).
Pristine biochar demonstrates limited capacity for tetracycline adsorption, while modifications significantly enhanced the absorption of tetracycline. Modifications influence the structural characteristics of biochar, such as the surface area, porosity, aromaticity, surface functional groups, and mineral content (Qiu et al. 2021). For instance, acid‒base modifications alter the surface characteristics of biochar, expanding specific regions and pore structures, thereby exerting a significant influence on physical adsorption of pollutants (Cheng et al. 2021).Organic modifications involve the conjugation of biochar with organic compounds containing a multitude of functional groups, thereby increasing the number of adsorption sites (Qiu et al. 2021). Iron modification facilitates the formation of the carbon structure in biochar, concurrently increasing the specific surface area and functional group abundance (Karunaratne et al. 2022). Therefore, modifications of diverse structures in biochar have varying impacts on tetracycline adsorption. Mei et al. (Mei et al. 2021) utilized Fe–N doping to modify biochar at 700 °C. By manipulating the surface area, functional groups, and graphite carbon structure, the maximum adsorption capacity for tetracycline exhibited a 5.4-fold enhancement relative to pristine biochar. Yu et al. (2020) modified grapefruit peel extract and enriched the surface functional groups of grapefruit peel-based biochar prepared at 600 °C. The maximum capacity for tetracycline adsorption was raised to 2.3 times that of the original material. Mu et al. (Mu et al. 2021). formed mesopores in tea biochar by utilizing KOH at 700 °C and concurrently enriched the surface functional groups. However, the maximum tetracycline adsorption capacity of the modified biochar was only 1.827 times that of the unmodified material. Therefore, identifying the key structures that regulate the tetracycline adsorption capacity of biochar and selectively modifying the biochar structure for a specific purpose contribute to the development of more cost-effective and efficient biochar adsorption materials.
However, there has been no prior identification of a correlation between biochar structure and tetracycline adsorption in previous studies. This limitation arises from the inherent heterogeneity in biochar structures, which is influenced by both the feedstock and carbonization degrees (Keiluweit et al. 2010). Therefore, it is important to develop standardized construction of biochar. Xiao and Chen proposed that biochar has a quaternary structure, and despite substantial discrepancies in the composition and characteristics of biochars, their fundamental architectures remain analogous (Xiao and Chen 2017). This provides the possibility of constructing a microscopic structural model for tetracycline adsorption by biochar. Research indicates that the mechanisms for tetracycline adsorption onto biochar involve chemical reactions and intermolecular forces, including electrostatic interactions, hydrogen bonding, π-π interactions, and van der Waals forces (Ngigi et al. 2019; Yue et al. 2019; Zhang et al. 2021b). However, no studies have identified the specific interaction sites, so there is no mechanistic rationale for these interactions.
At a pyrolysis temperature of 300 °C, the biomass exhibits a low pore volume and low crystallinity, with subtle variations indicative of biochar with a low degree of carbonization (Leng et al. 2021). With increases in the pyrolysis temperature, amorphous carbon is transformed into crystalline carbon through condensation, leading to increases in the specific surface area and porosity, elevated ash content, and the formation of a “molten ring two-dimensional structure” characterized by abundant pores (Ahmad Farid and Andou 2022). At 700 °C, the disordered layered structure of biochar transitions to graphite (Chen et al. 2017). Biochar maintained at 700 °C exhibited a graphitic microcrystalline structure. Due to the widespread adoption of straw biochar for investigating the structural properties of biochar, this study focused on the adsorption of tetracycline by biochar prepared within the pyrolysis range of 300 to 700 °C (Zhang et al. 2023c), (Fu et al. 2021), (Liao et al. 2023). The primary focuses were (1) identifying the biochar structures influencing tetracycline adsorption and determining the key influencing factors; (2) establishing the relationships among the key factors influencing biochar adsorption and constructing a standardized model for biochar and (3) investigating the micromechanisms of tetracycline adsorption by biochar and unveiling the adsorption sites. It is anticipated that this study will offer valuable insights advancing the development of more efficient and cost-effective biochar materials.
2 Materials and methods
2.1 Chemicals
More information can be seen in Additional file 1: Text S1.
2.2 Preparation and characterization of rice straw biochars
Rice straw biochar was produced in a vacuum tube furnace (GSL-1600, China) by slow pyrolysis method (Hoslett et al. 2021; Fan et al. 2023; Abumelha et al. 2023; He et al. 2019). Briefly, the collected rice straw was washed three times and dried in a hot air oven at 80 °C until reaching a constant weight. Then, the dried biomass was ground and sieved through a 120-mesh sieve. Next, the powdered biomass was placed in a tube furnace and heated at a rate of 5 °C min−1 and carbonized for 2 h under continuous nitrogen flow to avoid oxidation. The heat treatment temperatures were 300 °C, 400 °C, 500 °C, 600 °C, and 700 °C; the products obtained with these different heating treatment temperatures were labeled BC300, BC400, BC500, BC600, and BC700, respectively.
The surface morphologies of the biochar were examined with scanning electron microscopy (SEM; Hitachi S-4800, Japan). The Brunauer–Emmett–Teller specific surface areas and total pore volumes of the biochar were evaluated with N2 adsorption at 77 K and a surface area analyzer (Quantachrome, Autosorb-iQ-Station 3, USA). The elemental contents of different biochars were determined with an elemental analyzer (Elementar: Vario EL cube, Germany). The ash contents were determined by heating the biochars at 800 °C for 4 h in a muffle furnace with ambient atmosphere (Thermo BF51866KC-1, Germany). The percentage of oxygen was determined by subtraction. Solid-state 13C CP/MAS NMR spectroscopy was performed with a Bruker Avance 400 MHz NMR spectrometer (Karlsruhe, Germany) operating at 100 MHz.
Quantification of functional groups was performed with the Boehm titration method. Further details can be found in Additional file 1: Text S2.
2.3 Batch adsorption experiment
The kinetics and isotherms for tetracycline adsorption on the biochars were investigated. The adsorption experiments were carried out in 50 mL brown plastic centrifuge tubes containing 25 mL of tetracycline aqueous solution and 0.01 g biochar. The pH was adjusted to 7 with 0.01 M hydrochloric acid or sodium hydroxide, since this is the optimal pH for tetracycline adsorption by biochar (Zhang et al., 2023c), (Hoslett et al., 2021a). At elevated or reduced pH conditions, electrostatic repulsion diminishes the adsorption performance of biochar (Fan et al., 2023). The pHpzc of biochar and the pH variations before and after tetracycline adsorption are presented in Additional file 1: Table S1. Before adsorption by biochar, tetracycline existed in two states, namely, TCH2+ and TCH− (pKa2 = 7.68, pKa3 = 9.68) (Abumelha et al. 2023). Thus, pH 7 falls within the optimal range to minimize interference from electrostatic repulsion. An initial concentration of 10–120 mg L−1 tetracycline was prepared with ultrapure water for the adsorption isotherm experiments. All experiments involved continuous shaking at 200 rpm at a constant temperature of 25 °C for 72 h. Two milliliters of the supernatant was removed and filtered through a 0.22 μm filter membrane (Tianjin Jinteng, China) for further analyses. Tetracycline concentrations of 40 and 80 mg L−1were used for the kinetics experiments, with aliquots taken at specific time intervals. All batch experiments were conducted in triplicate. The concentrations of tetracycline in the samples were quantified with high-performance liquid chromatography (Agilent, 1260 Infinity II, USA) with a tunable UV (TUV) detector set to a wavelength of 350 nm. Competitive adsorption experiments and detection details are described in Additional file 1: Text S3.
2.4 Fitting models
Four adsorption kinetic models, namely, the pseudofirst-order (PFO), pseudosecond-order (PSO), Elovich, and liquid film diffusion (LFD) models, were used to fit the kinetic data. The details of the model equations are presented in Additional file 1: Text S4. The isotherms for tetracycline adsorption on the biochar showed the best fit with the Dubinin-Ashtahkov model (DAM) (He et al. 2019; Jang and Kan 2019; Ma et al. 2021) . The DA model has been used with carbonaceous materials in many cases (Bai et al. 2021; Shen et al. 2015; Yang et al. 2016). The EDA (kJ mol−1) is the characteristic adsorption energy, which includes all forces for direct interactions between the molecule and the surface (Crittenden et al. 1999). More details are shown in Additional file 1: Text S5.
2.5 Density functional theory computational simulations
The 13C NMR spectra of the biochar produced at 300–700 °C were analyzed with MestReNova. The aromaticity (Far) was obtained by integrating the peaks found in the 13C NMR spectra at 95 to 165 ppm. The degree of aromatic condensation (χb) was described by the sizes of the aromatic structures, and it was calculated from the ratio of the fraction of aromatic bridgehead carbons relative to all aromatic carbons (Yang et al. 2021a; McBeath et al. 2011).
where Fb is the fraction of aromatic bridgehead carbons relative to total carbons, obtained by subtracting the integration results for aromatic C−O groups (145–165 ppm). The mean weighted square error (MWSE) was calculated to examine the goodness of fit with the model. More details can be found in Additional file 1: Text S6. Fits and calculations were determined with SPSS 23 and Origin v8.0 software.
Density functional theory (DFT) calculations were performed with Gaussian16 and the Vienna ab initio simulation package. The adsorption energies were calculated with projector augmented wave potentials and the generalized gradient approximation (GGA) with the Perdew-Burke-Ernzerhof (PBE) functional (Kresse and Furthmüller 1996; Kresse and Joubert 1999; Perdew et al. 1996). The van der Waals interactions were described by the DFT-D2 method in this work (Grimme et al. 2010). The binding energies (Eab) were calculated with Eq. 2. For geometry optimization and frequency calculations, 6–311 + G(d) was used as the basis set. The PBE0 functional was adopted with the D3 (BJ) empirical dispersion correction. The solvent effect of water was examined with the self-consistent reaction field (SCRF) method based on the SMD model.
where E(slab + organics) and E(slab) are the total energy of the substrate system that adsorbs or does not adsorb organics, respectively. E(organics) is the total energy of the organics.
3 Results and discussion
3.1 Characterization of the biochar and adsorption of TTC
The capacities for TTC adsorption on biochar increased with increasing treatment temperatures, as shown with two different concentrations (Additional file 1: Figure S1). Among the biochar samples examined, BC700 exhibited the most efficient adsorption and removed up to 54.5% and 49.17% of the TTC from the two water systems, respectively. This is consistent with the TTC adsorption capacities reported in prior studies (Additional file 1: Table S2), suggesting that the trend is generalizable across various feedstocks. The specific surface area (SSA) of BC700 was significantly higher than that of the other biochar examined (Additional file 1: Table S3), implying the presence of more adsorption sites in BC700. This was corroborated by the total pore volume. However, the volume of the micropores in BC700 was lower than that in BC600, possibly due to pore collapse.
resulting from charring deactivation and carbon melting during high-temperature combustion (Lian and Xing 2017). Additionally, the increased ash content led to a reduction in the microporous surface area (Leng et al. 2021). Micropores facilitate the adsorption of tetracycline; however, they restrict its diffusion in liquid, especially within nanometer-sized pores with diameters less than 3 nm (Wang et al. 2022) An increase in the mesopore volume of BC700 was observed, with an average pore diameter of only 4.391 nm. This may compensate for the deficiency in adsorption sites due to the reduction in micropore volume.
3.2 Possible chemical bond in adsorption
The taxonomic categories of the functional groups present in the five types of biochar, along with their respective proportions are illustrated in Fig. 1a. At a pyrolysis temperature of 700 °C, a marked reduction in functional groups, particularly acidic functional groups, was observed (Fig. 1b). This phenomenon was attributed to the thermal decomposition reactions that occurred during high-temperature pyrolysis of the biochar. The FTIR data in Additional file 1: Figure S3 and Table S4 show a broad peak in the range of 2800 to 3100 cm−1 for O–H stretching vibrations of BC300. The C = O stretching vibrations gave a peak at 1730 ± 20 cm−1 and the O–H bending vibration observed at 960 ± 20 cm−1 indicated the presence of carboxylic functional groups. With increases in the adsorption time, the intensity of the carboxylic acid peak gradually decreased, indicating a reaction between the carboxyl groups and TTC. The nucleophilic character of the carboxyl group in TTC enabled it to attack the carbonyl groups (C=O) in functional groups such as carboxyls, carbonyls, and esters on the biochar surface, resulting in the formation of ester linkages (−COO−). This was supported by previous studies on the reactivity of carbonyl functional groups with organic compounds (Zhou et al. 2019). Figure 1c shows that the content of acid functional groups in BC700 was lower than that in the other biochar, suggesting that acidic functional groups were not the primary sites for adsorption of TTC. However, BC700 exhibited the highest content of alkaline functional groups. The peak at 3300–3600 cm−1 was attributed to N–H stretching vibrations, indicating the presence of amines. The peaks at 688 ± 20 cm−1 and 1580 ± 20 cm−1 indicated the presence of primary amines, which could react with the carbonyl groups of TTC in condensation reactions to form amide bonds. The peak at 1630–1690 cm−1 for BC700 confirmed the presence of amide groups (−CONH−). However, these results were not observed with copper sulfate tests, possibly due to their relatively low content.
3.3 Adsorption behavior
Adsorption kinetics were used to assess the adsorption of TTC with various concentrations on biochar prepared with different pyrolysis temperatures (Additional file 1: Figure S4–S6) (Hoslett et al. 2021). At a relatively low concentration of 40 mg L−1, all adsorption processes exhibited characteristics consistent with the Elovich model, which is an empirical model derived from the assumption of chemical adsorption. The rate-limiting step resulted from the energy barrier present on the surface where adsorption occurred (Choudhary et al. 2020). The adsorption rate constants ranged from 0.26 to 2.74 mg·g−1·min−1, indicating slow adsorption via chemical reactions (Additional file 1: Table S5). It is noteworthy that the desorption rate for BC300 exceeded the adsorption rate, which could account for its relatively low adsorption capacity. Nevertheless, it is theoretically expected that BC300, owing to its high concentration of acidic functional groups, would form stable chemical bonds to enhance adsorption. This apparent discrepancy suggested that the adsorption capacity resulting from acidic functional groups was limited, underscoring the operation of other processes. Furthermore, it was evident that the remaining biochar was better described by the intraparticle diffusion model, as indicated by the relatively low ki3 value, which suggested that insufficient adsorption sites were one of the factors limiting the adsorption process; additionally, a plot of the intraparticle diffusion model did not pass through the origin, indicating the operation of other rate-determining steps (Liu et al. 2021).
The Dubinin-Ashtahkov model is based on the assumption of a single site; that is, adsorption sites have different adsorption capacities, which are related to the temperature. Accordingly, thermodynamic experiments were conducted at three temperatures and yielded good fits (0.97–0.99), which attested to the strong correlation between the experimental and theoretical data (Fig. 2 and Table 1). The EDA was used to determine the adsorption affinity and was found to be independent of Q0. These results indicated that the capacity for tetracycline adsorption by biochar gradually increased with an increase in heat treatment temperature, suggesting that biomass carbonization was a contributing factor to tetracycline adsorption by biochar. During carbonization, the biomass underwent continuous deoxygenation and dehydrogenation, resulting in the formation of carbon flakes. Furthermore, as the temperature increased, these carbon flakes coalesced, resulting in the creation of more bulk carbon.
3.4 Carbon structure and correlation
To develop a more comprehensive understanding of the structure of the biochar, the carbon distribution was examined and is presented in Fig. 3b; it was classified into five primary regions based on the 13C NMR spectra shown in Additional file 1: Figure S7. The carbon structures contained methoxy (50–60 ppm), carboxyl and carbonyl carbons (160–210 ppm), and the side chains contained alkyl (0–50 ppm) and O-alkyl (60–110 ppm) groups. It is noteworthy that the composition of the biochar evolved considerably with the heating temperature. For instance, at the lower heating temperature of BC300, the proportion of aliphatic carbon (such as alkyl and methoxy carbon) was the highest, mainly due to incomplete pyrolysis of the lignin. However, as the carbonization temperature was increased, more aliphatic carbon atoms were transformed into sp2-hybridized carbon atoms (such as furan and aromatic carbon atoms, 110–160 ppm), thereby increasing the level of polyaromaticity (Li et al. 2020). Therefore, the aromaticity (Far) and aromatic condensation (χb) indices also increased and were valuable indicators providing information about the carbon structure of the biochar. Aromatic condensation refers to the combination of aromatic compounds to form larger molecules, which is often accompanied by the loss of small molecules such as water or hydrogen chloride. As χb increases, the biochar exhibits a higher carbon mass and an increased proportion of aromatic structures. These aromatic structures increase the SSA and porosity of the biochar, thereby enhancing its adsorption capacity and chemical stability (Wang et al. 2018). The values of Far and χb for the biochar are presented in Table 2.
Consistent with the aforementioned results, the polarity index [(O + N)/C] and H/C atomic ratio exhibited decreasing trends, shifting from 0.7789 to 0.2726 and 0.8997 to 0.3410, respectively, as the heating temperature was increased (Table 2). In addition, the hydrophilic index [O/C] decreased significantly, from 0.7543 to 0.2540, indicating the loss of polar functional groups. However, as the polarity decreased, both the SSA and the pore volume increased, providing more binding sites for tetracycline. This suggests that factors other than polar functional groups had a greater impact on the adsorption of tetracycline onto the biochar.
Therefore, thirteen different parameters were used to evaluate the correlations between each factor and the adsorption capacity with Pearson correlation analyses (Fig. 3a). The heatmap revealed that qe was highly correlated with EDA at the 0.001 level, with a correlation coefficient (p) between 0.8 and 1. This was explained by the fact that EDA includes all forces that contribute to the adsorption affinity, and it plays a crucial role in determining the adsorption capacity. Furthermore, qe demonstrated a strong correlation with χb at the 0.01 level (p = 0.8–1), suggesting that the level of aromatic condensation in the biochar played a significant role in influencing the adsorption capacity. This is not surprising since a higher χb corresponds to higher condensation of the biochar, resulting in larger SSA and pore volumes, as confirmed by the strong correlation between SSA and χb. Moreover, a significant correlation was observed between qe and the basic functional groups at 0.05. This was attributed to the higher reactivity of the two amino groups in TTC compared to the two carboxyl groups, leading to stronger reactions with basic functional groups rather than acid functional groups. Additionally, qe was negatively correlated with O/C and (O + N)/C, which was consistent with previous findings.
The impact of χb on EDA was determined by plotting a linear correlation graph, as shown in Fig. 4. The results demonstrated a significant correlation between χb and EDA at all three temperatures (R2 = 0.94–0.98). As the degree of aromatic condensation in the biochar increased, there was a corresponding increase in its affinity for tetracycline. This suggested that biochar with higher levels of χb may be a more effective adsorbent for tetracycline removal (Peng et al. 2016). Concurrently, competitive adsorption experiments demonstrated that as the degree of aromatic condensation increased, the proportion of chemical reactions gradually decreased (refer to Additional file 1: Figure S8 and Figure S3c). This was attributed to a decrease in the number of acidic functional groups at the chemical reaction sites, resulting from the increased treatment temperature.
3.5 Establishment of microscale models and adsorption mechanisms
Hückel’s rule is a fundamental principle that describes the aromaticity of molecular systems. Specifically, it states that a planar cyclic molecular system exhibits aromaticity when the number of π electrons in the system is equal to 4n + 2 (where n is a nonnegative integer). Based on Hückel’s rule of aromaticity, χb can be used to calculate the theoretical sizes of aromatic ring clusters. Additionally, DFT can be employed to establish different microunit carbon ring structures for biochar formed with various treatment temperatures, as shown in Additional file 1: Figure S9 (Yang et al. 2016). All constructed models were optimized to achieve the most stable configurations. Furthermore, different adsorption configurations between the biochar and TTC were studied, and three positions were selected, as shown in Additional file 1: Figure S10. The three positions described in this study were ultimately selected based on multiple experiments and represent different binding characteristics. The binding energies of the resulting complexes were calculated and are presented in Table 3. The positive BC300 adsorption energies indicate that the reaction with tetracycline had thermodynamic barriers, which may impede the progress of the reaction and require a greater energy input. The observed effect was ascribed to the relatively low degree of carbonization exhibited by BC300, which could lead to a reduction in the extent of carbon framework condensation and a consequent decrease in the surface charge density (Yang et al. 2021b).
The adsorption energies of BC300 were all relatively low positive values, indicating that adsorption may be governed by weak van der Waals forces (0.4–4 kJ mol−1) or π-π interactions (1.5–3 kJ mol−1) (Aliakbar Tehrani and Kim 2016). The carbon rings in the biochar have π electron densities, which enable π-π interactions with the four arene rings in tetracycline (Zeng et al. 2019), (Chen et al. 2007). Studies have shown that for most compounds containing arene rings, the distance at which π-π interactions occur is commonly less than 3.5 Å (Fiori et al. 2017). Within this range, the interactions between π electron clouds reach a maximum value, and stable π-π bonds are formed. Except for BC300, Position 1 was selected as the optimal binding model for investigating the adsorption of tetracycline on the biochar; all other biochar exhibited exothermic adsorption of tetracycline at this position, suggesting that the binding reaction was thermodynamically feasible and facile. The maximum binding energy of BC700 ( −0.946 eV) indicated that in addition to the van der Waals forces and π-π interactions that are known to operate in such systems, electrostatic interactions or hydrogen bonds (25–40 kJ mol−1) may also be contributing factors (Deng et al. 2023).
However, the binding configuration observed at position 1 revealed that the tetracycline binding site on the biochar contained many oxygen-containing functional groups with negative charges similar to those of the biochar (Additional file 1: Figure S11a), making electrostatic interactions highly unlikely. Therefore, it is suggested that hydrogen bonding and the combined effects of van der Waals and π-π interactions may be responsible for the observed binding behavior. The oxygen functional groups in tetracycline are capable of serving as hydrogen bond donors, thereby forming hydrogen bonds with the π electron clouds of the carbon structures present in the biochar (Yang et al. 2011). It was observed that the shortest bond lengths between tetracycline and the five types of biochar ranged between 2.66 and 2.84 Å, which were consistent with the known bond lengths for intermolecular forces (Das et al. 2014). Furthermore, the size of the carbon clusters present in the biochar, which was the only variable in the simulated adsorption experiments, was strongly correlated with the strength of the π electron cloud, indicating that larger carbon clusters resulted in stronger and more stable adsorption interactions. The optimal adsorption configuration and minimum bond distance for the tetracycline on biochar are shown in Fig. 5.
The microscopic interactions between the biochar functional groups and tetracycline were investigated with the syntheses of various functionalized biochar. X-ray photoelectron spectroscopy (XPS) was employed to characterize the biochar surfaces, revealing that hydroxyl (−OH), carboxyl (−COOH−), pyridine-N (−PDN), pyrrole-N (−PLN), and graphitic-N (−GN) were the primary functional groups present on the biochar (Fig. 1c). These functional groups are common in biochar (Xiao et al. 2018). Therefore, based on the BC700 simulation model, the functionalized biochar was composed of five distinct functional groups, namely, BC-OH, BC-COOH, BC-GN, BC-PDN, and BC-PLN, as illustrated in Additional file 1: Figure S12. The molecular electrostatic potential map revealed that the carbon framework of the biochar was rich in π electrons, thus displaying strongly electrophilic character (Additional file 1: Figure S12) (Ai et al. 2019). It was observed that, with the exception of BC-GN, all of the functionalized biochars exhibit negative charges (Xiao et al. 2020). Moreover, all of the functionalized biochar exhibited a higher binding energy with tetracycline (−1.0866 to −1.8370 eV) than BC700, indicating the possibility of chemical adsorption (Table 4). This may be attributable to the high negative charge carried by the functionalized biochar, which could interact electrostatically with the nucleophilic nitrogen (amine functional group and acyl amide functional group) in tetracycline. The calculated frontier orbitals revealed that the Eg1 values of all biochar specimens were lower than Eg2, thereby signifying that the electrons present on the surface of the biochar were stimulated and transmitted to the tetracycline molecules (Additional file 1: Table S6) (Hou et al. 2021). Moreover, the charge density images provided a lucid depiction of the binding locations between the functionalized biochar and tetracycline (Fig. 6) (Guo et al. 2016). As demonstrated in Fig. 6c, the incorporation of graphite nitride decreased the electrostatic potential and energy, thereby generating overlap between the nucleophilic regions of tetracycline and the nondoped electron-rich zone of the biochar (Dai et al. 2020). However, the higher adsorption energy (−1.0866 > −0.946 eV) suggested that adsorption may have involved electrostatic interactions. Moreover, the intermolecular forces also increased the binding energy. BC-COOH exhibited the highest overlap with tetracycline and showed the greatest adsorption binding energy (−1.8370 eV), possibly due to the formation of hydrogen bonds between the carboxyl groups of the biochar and the nitrogen atoms of the amine functional groups in tetracycline, thereby enhancing the intermolecular interactions (Ai et al. 2019). Likewise, BC-OH bound in a similar manner (Peiris et al. 2017), (Zhu et al. 2019). Binding between the BC-PDN and BC-PLN with tetracycline occurred through the formation of hydrogen bonds with the acyl amide functional groups (Zhang et al. 2021b).
3.6 Potential adsorption mechanisms and binding sites
The possible micromechanisms and binding sites are summarized in Fig. 7. BC300 mainly adsorbed tetracycline (69.21%) through chemical bonding. This is attributed to the high acidity functional group content (4 mmol g−1) of low-carbonized biochar. The nucleophilic nature of the carboxyl groups in TTC allows them to attack carbonyl groups (C=O) in functional groups on the biochar surface, forming ester bonds (−COO−). By comparison, due to low surface charge density, intermolecular forces dominated by van der Waals or π-π interactions play a limited role. With increasing carbonization, the functional groups in biochar decrease, and the carbon structure becomes more ordered. High-carbonized biochar is mainly controlled by intermolecular forces (91.1%), with strong π electron clouds on large cluster carbon structures forming π-π interactions with the conjugated benzene rings of tetracycline. Additionally, oxygen functional groups in tetracycline can act as hydrogen bond donors, forming hydrogen bonds with the π electron clouds in the carbon structure of biochar. Van der Waals forces also occur in the adsorption system. The high affinity of functionalized biochar for tetracycline is attributed to the strengthening of electrostatic interactions. The electron-rich regions of biochar can engage in electrostatic interactions with the nucleophilic regions (amine and amide functional groups) in the nitrogen area of tetracycline. BC-COOH exhibits the highest degree of overlap with tetracycline and has the maximum adsorption binding energy (−1.8370 eV). This may be due to the formation of hydrogen bonds between the carboxyl groups of biochar and the nitrogen atoms of the amine functional groups in tetracycline, enhancing intermolecular interactions.
3.7 Comparative study
Regardless of the modification method employed, the efficient adsorption of tetracycline is the criterion for assessing the performance quality of biochar. Additional file 1: Table S7 provides a comprehensive comparison of the adsorption efficiency and mechanisms of modified biochar on tetracycline over the past two years, encompassing various feedstock types. It is noteworthy that 86.7% (13 out of 15) of the studies opted for elevated temperatures (≥ 500 °C) as the preparation temperature. This indirectly corroborates the favorable adsorption of tetracycline by biochar under conditions of high aromatic condensation. The impact of modification on the structural regulation of biochar for the adsorption of tetracycline can be broadly categorized into three types: increased functional groups, enhanced graphitization, and the doping of metals. Simultaneously, an increase in SSA and pore volume was observed across all studies, thereby augmenting the adsorption sites. The modification of functional groups alone has limited impact on enhancing the adsorption efficiency of tetracycline. The La-activated orange peel biochar (La-BC) prepared by Chen et al. (Chen et al. 2023) exhibited an impressive specific surface area (SSA) of 2888.33m2 g−1, accompanied by an augmentation in both the quantity and diversity of functional groups. However, its adsorption capacity for tetracycline is only 143.2 mg g−1, indicating a low enhancement efficiency attributed to the surface functional groups and adsorption sites. However, with the concurrent increase in graphitization and functional groups, the adsorption efficiency of biochar for tetracycline can be significantly enhanced. Wang et al. (Wang et al. 2023) synthesized microalgae-derived biochar (CVAC) with nitrogen doping modification, concurrently enhancing the presence of oxygen-containing functional groups and graphitization level. Compared to La-BC, CVAC exhibits a significantly lower specific surface area (SSA) of only 491.16 m2 g−1. Nevertheless, it achieves a remarkable adsorption capacity of 381.584 mg g−1 for tetracycline. Therefore, enhancing the aromaticity of biochar is crucial for improving its adsorption capability for tetracycline. Additionally, the impact of metal doping on the performance of biochar varies. Zhang et al. employed Iron-zinc modified biochar, resulting in a significant enhancement of SSA (747.76 m2 g−1); however, the adsorption capacity was only 94.63 mg g−1. This is attributed to the potential occupancy of certain microspatial domains by iron and zinc ions, resulting in a limited increase in adsorption sites. In contrast, Luo et al. (Luo et al. 2022) incorporated Fe3O4 nanoparticles into biochar, effectively reducing pore size and introducing additional functional groups. Despite a SSA of only 75.34 m2 g−1, the adsorption capacity for tetracycline reached 184.5 mg g−1.
4 Conclusions
In this study, key factors and microscopic adsorption sites of biochar with varying carbonization degrees (300–700 °C) in the adsorption of tetracycline were explored. The results indicated that biochar pyrolyzed at higher temperatures exhibited superior adsorption performance for tetracycline, confirming the aromatic condensation of biochar as a key factor influencing adsorption. Thus, different biochar-tetracycline adsorption configurations were established under various carbonization temperatures, which circumvents the limitations imposed by variations in raw material types and pyrolysis temperatures, contributing to the standardization of biochar research. Although low-carbonized biochar predominantly adsorbs tetracycline through chemical bonding, the available adsorption sites are limited. On the other hand, high-carbonized biochar, with larger carbon clusters offering numerous adsorption sites, predominantly adsorbs tetracycline through intermolecular forces. Overall, this study reveals the most valuable directions for the modification of biochar to enhance tetracycline adsorption, offering insights for the development of more cost-effective and efficient biochar adsorption materials.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abumelha HM, Alzahrani SO, Alrefaee SH, Al-bonayan AM, Alkhatib F, Saad FA, El-Metwaly NM (2023) Evaluation of tetracycline removal by magnetic metal organic framework from aqueous solutions: adsorption isotherm, kinetics, thermodynamics, and Box-Behnken design optimization. J Saudi Chem Soc 27(5). https://doi.org/10.1016/j.jscs.2023.101706
Ahmad Farid MA, Andou Y (2022) A route towards graphene from lignocellulosic biomass: Technicality, challenges, and their prospective applications. J Cleaner Prod 380. https://doi.org/10.1016/j.jclepro.2022.135090
Ai Y, Liu Y, Huo Y, Zhao C, Sun L, Han B, Cao X, Wang X (2019) Insights into the adsorption mechanism and dynamic behavior of tetracycline antibiotics on reduced graphene oxide (RGO) and graphene oxide (GO) materials. Environ Sci Nano 6(11):3336–3348
Aliakbar Tehrani Z, Kim KS (2016) Functional molecules and materials by π-Interaction based quantum theoretical design. Int J Quantum Chem 116(8):622–633
Bai S, Jin C, Zhu S, Ma F, Wang L, Wen Q (2021) Coating magnetite alters the mechanisms and site energy for sulfonamide antibiotic sorption on biochar. J Hazard Mater 409:125024
Balakrishnan A, Chinthala M, Polagani RK, Vo DN (2023) Removal of tetracycline from wastewater using g-C(3)N(4) based photocatalysts: A review. Environ Res 216(Pt 3):114660
Bayly CI, Cieplak P, Cornell W, Kollman PA (1993) A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: the RESP model. J Phys Chem 97(40):10269–10280
Chang D, Mao Y, Qiu W, Wu Y, Cai B (2023) The source and distribution of tetracycline antibiotics in China: a review. Toxics 11(3). https://doi.org/10.3390/toxics11030214
Chen W, Duan L, Zhu D (2007) Adsorption of polar and nonpolar organic chemicals to carbon nanotubes. Environ Sci Technol 41(24):8295–8300
Chen Y, Zhang X, Chen W, Yang H, Chen H (2017) The structure evolution of biochar from biomass pyrolysis and its correlation with gas pollutant adsorption performance. Bioresour Technol 246:101–109
Chen Z, Lin B, Huang Y, Liu Y, Wu Y, Qu R, Tang C (2023) Pyrolysis temperature affects the physiochemical characteristics of lanthanum-modified biochar derived from orange peels: Insights into the mechanisms of tetracycline adsorption by spectroscopic analysis and theoretical calculations. Sci Total Environ 862:160860
Cheng N, Wang B, Wu P, Lee X, Xing Y, Chen M, Gao B (2021) Adsorption of emerging contaminants from water and wastewater by modified biochar: a review. Environ Pollut 273:116448
Choudhary M, Kumar R, Neogi S (2020) Activated biochar derived from Opuntia ficus-indica for the efficient adsorption of malachite green dye, Cu(+2) and Ni(+2) from water. J Hazard Mater 392:122441
Crittenden JC, Sanongraj S, Bulloch JL, Hand DW, Rogers TN, Speth TF, Ulmer M (1999) Correlation of aqueous-phase adsorption isotherms. Environ Sci Technol 33(17):2926–2933
Dai Y, Li J, Shan D (2020) Adsorption of tetracycline in aqueous solution by biochar derived from waste Auricularia auricula dregs. Chemosphere 238:124432
Das P, Jain CK, Dey SK, Saha R, Chowdhury AD, Roychoudhury S, Kumar S, Majumder HK, Das S (2014) Synthesis, crystal structure, DNA interaction and in vitro anticancer activity of a Cu(ii) complex of purpurin: dual poison for human DNA topoisomerase I and II. RSC Adv 4(103):59344–59357
Deng J-H, Luo J, Mao Y-L, Lai S, Gong Y-N, Zhong D-C, Lu T-B (2020) π-π stacking interactions: non-negligible forces for stabilizing porous supramolecular frameworks. Sci Adv 6(2):eaax9976
Fan Y, Su J, Wang Z, Liu S, Li X, Hou C (2023) Improvement of the specific surface area of biochar by calcium-precipitated nanoparticles synthesized by microbial induction as a template skeleton: removal mechanism of tetracycline in water. J Environ Manage 348:119279
Fiori ATM, Nakahata DH, Cuin A, Lustri WR, Corbi PP (2017) Synthesis, crystallographic studies, high resolution mass spectrometric analyses and antibacterial assays of silver(I) complexes with sulfisoxazole and sulfadimethoxine. Polyhedron 121:172–179
Fu Y, Jia M, Wang F, Wang Z, Mei Z, Bian Y, Jiang X, Virta M, Tiedje JM (2021) Strategy for mitigating antibiotic resistance by biochar and hyperaccumulators in cadmium and oxytetracycline co-contaminated soil. Environ Sci Technol. https://doi.org/10.1021/acs.est.1c03434
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132(15):154104
Guo X, Zhang X, Zhao S, Huang Q, Xue J (2016) High adsorption capacity of heavy metals on two-dimensional MXenes: an ab initio study with molecular dynamics simulation. Phys Chem Chem Phys 18(1):228–233
He Y, Liu C, Tang X-Y, Xian Q-S, Zhang J-Q, Guan Z (2019) Biochar impacts on sorption-desorption of oxytetracycline and florfenicol in an alkaline farmland soil as affected by field ageing. Sci Total Environ 671:928–936
Hoslett J, Ghazal H, Katsou E, Jouhara H (2021) The removal of tetracycline from water using biochar produced from agricultural discarded material. Sci Total Environ 751:141755
Hou J, Zhang S, Zhang X, Wang K, Zhang Q, Shi Y (2021) Insights into ferulic acid detoxification mechanism by using a novel adsorbent, AEPA250: the microinteraction of ferulic acid with AEPA250 and Saccharomyces cerevisiae. J Hazard Mater 415:125685
Hou C, Jiang X, Chen D, Zhang X, Liu X, Mu Y, Shen J (2022) Ag-TiO(2)/biofilm/nitrate interface enhanced visible light-assisted biodegradation of tetracycline: the key role of nitrate as the electron accepter. Water Res 215:118212
Jang HM, Kan E (2019) A novel hay-derived biochar for removal of tetracyclines in water. Bioresour Technol 274:162–172
Janiak C (2000) A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands †. J Chem Soc, Dalton Trans 21:3885–3896
Karunaratne TN, Nayanathara RMO, Navarathna CM, Rodrigo PM, Thirumalai RVKG, Pittman CU, Kim Y, Mlsna T, Zhang J, Zhang X (2022) Pyrolytic synthesis of graphene-encapsulated zero-valent iron nanoparticles supported on biochar for heavy metal removal. Biochar 4(1). https://doi.org/10.1007/s42773-022-00196-5
Keiluweit M, Nico PS, Johnson MG, Kleber M (2010) Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ Sci Technol 44(4):1247–1253
Kresse G, Furthmüller J (1996) Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput Mater Sci 6(1):15–50
Kresse G, Joubert D (1999) From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B 59(3):1758–1775
Leichtweis J, Vieira Y, Welter N, Silvestri S, Dotto GL, Carissimi E (2022) A review of the occurrence, disposal, determination, toxicity and remediation technologies of the tetracycline antibiotic. Process Saf Environ Prot 160:25–40
Lenart-Boroń A, Prajsnar J, Guzik M, Boroń P, Grad B, Żelazny M (2022) Antibiotics in groundwater and river water of Białka—a pristine mountain river. Appl Sci 12(34). https://doi.org/10.3390/app122412743
Leng L, Xiong Q, Yang L, Li H, Zhou Y, Zhang W, Jiang S, Li H, Huang H (2021) An overview on engineering the surface area and porosity of biochar. Sci Total Environ 763:144204
Li Y, Wang F, Miao Y, Mai Y, Li H, Chen X, Chen J (2020) A lignin-biochar with high oxygen-containing groups for adsorbing lead ion prepared by simultaneous oxidization and carbonization. Bioresour Technol 307:123165
Li J, Tian T, Jia Y, Xu N, Yang S, Zhang C, Gao S, Shen W, Wang Z (2023) Adsorption performance and optimization by response surface methodology on tetracycline using Fe-doped ZIF-8-loaded multi-walled carbon nanotubes. Environ Sci Pollut Res Int 30(2):4123–4136
Lian F, Xing B (2017) Black carbon (biochar) in water/soil environments: molecular structure, sorption, stability, and potential risk. Environ Sci Technol 51(23):13517–13532
Liang L, Xi F, Tan W, Meng X, Hu B, Wang X (2021) Review of organic and inorganic pollutants removal by biochar and biochar-based composites. Biochar 3(3):255–281
Liang H, Zhu C, Ji S, Kannan P, Chen F (2022) Magnetic Fe2O3/biochar composite prepared in a molten salt medium for antibiotic removal in water. Biochar 4(1). https://doi.org/10.1007/s42773-021-00130-1
Liao X, Chen C, Liang Z, Zhao Z, Cui F (2023) Selective adsorption of antibiotics on manganese oxide-loaded biochar and mechanism based on quantitative structure-property relationship model. Bioresour Technol 367:128262
Liu J, Liu D, Liu S, Li Z, Wei X, Lin S, Guo M (2019) Preparation and characterization of sulfated cellulose nanocrystalline and its composite membrane for removal of tetracycline hydrochloride in water. Energy Environ Mater 3(2):209–215
Liu Q, Li D, Cheng H, Cheng J, Du K, Hu Y, Chen Y (2021) High mesoporosity phosphorus-containing biochar fabricated from Camellia oleifera shells: impressive tetracycline adsorption performance and promotion of pyrophosphate-like surface functional groups (C-O-P bond). Bioresour Technol 329:124922
Luo S, Qin J, Wu Y, Feng F (2022) Tetracycline adsorption on magnetic sludge biochar: size effect of the Fe(3)O(4) nanoparticles. R Soc Open Sci 9(1):210805
Ma Y, Li M, Li P, Yang L, Wu L, Gao F, Qi X, Zhang Z (2021) Hydrothermal synthesis of magnetic sludge biochar for tetracycline and ciprofloxacin adsorptive removal. Bioresour Technol 319:124199
McBeath AV, Smernik RJ, Schneider MPW, Schmidt MWI, Plant EL (2011) Determination of the aromaticity and the degree of aromatic condensation of a thermosequence of wood charcoal using NMR. Org Geochem 42(10):1194–1202
Mei Y, Xu J, Zhang Y, Li B, Fan S, Xu H (2021) Effect of Fe-N modification on the properties of biochars and their adsorption behavior on tetracycline removal from aqueous solution. Bioresour Technol 325:124732. https://doi.org/10.1016/j.biortech.2021.124732
Minale M, Gu Z, Guadie A, Kabtamu DM, Li Y, Wang X (2020) Application of graphene-based materials for removal of tetracyclines using adsorption and photocatalytic-degradation: a review. J Environ Manage 276:111310
Mohammadi M, Sabbaghi S, Binazadeh M, Ghaedi S, Rajabi H (2023) Type-1 alpha-Fe(2)O(3)/TiO(2) photocatalytic degradation of tetracycline from wastewater using CCD-based RSM optimization. Chemosphere 336:139311
Mu Y, He W, Ma H (2021) Enhanced adsorption of tetracycline by the modified tea-based biochar with the developed mesoporous and surface alkalinity. Bioresour Technol 342:126001
Muzyka R, Misztal E, Hrabak J, Banks SW, Sajdak M (2023) Various biomass pyrolysis conditions influence the porosity and pore size distribution of biochar. Energy 263. https://doi.org/10.1016/j.energy.2022.126128
Ngigi AN, Ok YS, Thiele-Bruhn S (2019) Biochar-mediated sorption of antibiotics in pig manure. J Hazard Mater 364:663–670
Peiris C, Gunatilake SR, Mlsna TE, Mohan D, Vithanage M (2017) Biochar based removal of antibiotic sulfonamides and tetracyclines in aquatic environments: a critical review. Bioresour Technol 246:150–159
Peng B, Chen L, Que C, Yang K, Deng F, Deng X, Shi G, Xu G, Wu M (2016) Adsorption of antibiotics on graphene and biochar in aqueous solutions induced by pi-pi interactions. Sci Rep 6:31920
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77(18):3865–3868
Qiu M, Hu B, Chen Z, Yang H, Zhuang L, Wang X (2021) Challenges of organic pollutant photocatalysis by biochar-based catalysts. Biochar 3(2):117–123
Shen X, Guo X, Zhang M, Tao S, Wang X (2015) Sorption mechanisms of organic compounds by carbonaceous materials: site energy distribution consideration. Environ Sci Technol 49(8):4894–4902
Tang J, Zhang S, Zhang X, Chen J, He X, Zhang Q (2020) Effects of pyrolysis temperature on soil-plant-microbe responses to Solidago canadensis L.-derived biochar in coastal saline-alkali soil. Sci. Total Environ. 731:138938
Vinayagam R, Quadras M, Varadavenkatesan T, Debraj D, Goveas LC, Samanth A, Balakrishnan D, Selvaraj R (2023) Magnetic activated carbon synthesized using rubber fig tree leaves for adsorptive removal of tetracycline from aqueous solutions. Environ Res 216(Pt 3):114775
Wang H, Wang X, Cui Y, Xue Z, Ba Y (2018) Slow pyrolysis polygeneration of bamboo (Phyllostachys pubescens): product yield prediction and biochar formation mechanism. Bioresour Technol 263:444–449
Wang Y, Xu L, Wei F, Ding T, Zhang M, Zhu R (2022) Insights into the adsorption mechanism of tetracycline on hierarchically porous carbon and the effect of nanoporous geometry. Chem Eng J 437. https://doi.org/10.1016/j.cej.2022.135454
Wang S, Yuan C, Zafar FF, Wei M, Marrakchi F, Cao B, Fu Y (2023) Facile synthesis of chlorella-derived autogenous N-doped porous biochar for adsorption on tetracycline. Environ Pollut 330:121717
Xiao X, Chen B (2017) A direct observation of the fine aromatic clusters and molecular structures of biochars. Environ Sci Technol 51(10):5473–5482
Xiao X, Chen Z, Chen B (2016) H/C atomic ratio as a smart linkage between pyrolytic temperatures, aromatic clusters and sorption properties of biochars derived from diverse precursory materials. Sci Rep 6:22644
Xiao X, Chen B, Chen Z, Zhu L, Schnoor JL (2018) Insight into multiple and multilevel structures of biochars and their potential environmental applications: a critical review. Environ Sci Technol 52(9):5027–5047
Xiao Y, Lyu H, Tang J, Wang K, Sun H (2020) Effects of ball milling on the photochemistry of biochar: enrofloxacin degradation and possible mechanisms. Chem Eng J 384:123311
Xu Q, Zhou Q, Pan M, Dai L (2020) Interaction between chlortetracycline and calcium-rich biochar: enhanced removal by adsorption coupled with flocculation. Chem Eng J 382:122705. https://doi.org/10.1016/j.cej.2019.122705
Yang W, Zheng F, Lu Y, Xue X, Li N (2011) Adsorption interaction of tetracyclines with porous synthetic resins. Ind Eng Chem Res 50(24):13892–13898
Yang K, Yang J, Jiang Y, Wu W, Lin D (2016) Correlations and adsorption mechanisms of aromatic compounds on a high heat temperature treated bamboo biochar. Environ Pollut 210:57–64
Yang J, Pignatello JJ, Yang K, Wu W, Lu G, Zhang L, Yang C, Dang Z (2021a) Adsorption of organic compounds by biomass chars: Direct role of aromatic condensation (ring cluster size) revealed by experimental and theoretical studies. Environ Sci Technol 55(3):1594–1603
Yang Q, Wang Y, Zhong H (2021b) Remediation of mercury-contaminated soils and sediments using biochar: a critical review. Biochar 3(1):23–35
Yi Y, Huang Z, Lu B, Xian J, Tsang EP, Cheng W, Fang J, Fang Z (2020) Magnetic biochar for environmental remediation: a review. Biores Technol 298:122468
Yu H, Gu L, Chen L, Wen H, Zhang D, Tao H (2020) Activation of grapefruit derived biochar by its peel extracts and its performance for tetracycline removal. Bioresour Technol 316:123971
Yue Y, Shen C, Ge Y (2019) Biochar accelerates the removal of tetracyclines and their intermediates by altering soil properties. J Hazard Mater 380:120821
Zeng Z, Ye S, Wu H, Xiao R, Zeng G, Liang J, Zhang C, Yu J, Fang Y, Song B (2019) Research on the sustainable efficacy of g-MoS2 decorated biochar nanocomposites for removing tetracycline hydrochloride from antibiotic-polluted aqueous solution. Sci Total Environ 648:206–217
Zhang P, Li Y, Cao Y, Han L (2019) Characteristics of tetracycline adsorption by cow manure biochar prepared at different pyrolysis temperatures. Bioresour Technol 285:121348
Zhang M, Cai Z, Zhang G, Zhang Y, Xue N, Zhang D, Pan X (2021a) Effectively reducing antibiotic contamination and resistance in fishery by efficient gastrointestine-blood delivering dietary millispheres. J Hazard Mater 409:125012
Zhang Z, Li Y, Ding L, Yu J, Zhou Q, Kong Y, Ma J (2021b) Novel sodium bicarbonate activation of cassava ethanol sludge derived biochar for removing tetracycline from aqueous solution: Performance assessment and mechanism insight. Bioresour Technol 330:124949
Zhang X, Zhen D, Liu F, Chen R, Peng Q, Wang Z (2023a) An achieved strategy for magnetic biochar for removal of tetracyclines and fluoroquinolones: adsorption and mechanism studies. Bioresour Technol 369:128440
Zhang H, Yu Y, Li Y, Lin L, Zhang C, Zhang W, Wang L, Niu L (2023b) A novel BC/g-C(3)N(4) porous hydrogel carrier used in intimately coupled photocatalysis and biodegradation system for efficient removal of tetracycline hydrochloride in water. Chemosphere 317:137888
Zhang S, Hou J, Zhang X, Cheng L, Hu W, Zhang Q (2023c) Biochar-assisted degradation of oxytetracycline by Achromobacter denitrificans and underlying mechanisms. Biores Technol 387:129673
Zhao N, Ma Q, Zhang B, Liu D, Wei Y, Li M, Yu T, Li H, Shen Y, Yuan P (2023) New insight into removal of tetracycline by a two-dimensional graphene-like carbon adsorbent prepared using one-step pyrolysis of spent bleaching earth: adsorption behaviors, mechanisms and cost analysis. Sep Purif Technol 327. https://doi.org/10.1016/j.seppur.2023.124950
Zhou Y, He Y, He Y, Liu X, Xu B, Yu J, Dai C, Huang A, Pang Y, Luo L (2019) Analyses of tetracycline adsorption on alkali-acid modified magnetic biochar: site energy distribution consideration. Sci Total Environ 650(Pt 2):2260–2266
Zhu S, Liu Y, Huo Y, Chen Y, Qu Z, Yu Y, Wang Z, Fan W, Peng J, Wang Z (2019) Addition of MnO2 in synthesis of nano-rod erdite promoted tetracycline adsorption. Sci Rep 9(1):16906
Zhu D, Delgado-Baquerizo M, Su JQ, Ding J, Li H, Gillings MR, Penuelas J, Zhu YG (2021) Deciphering potential roles of earthworms in mitigation of antibiotic resistance in the soils from diverse ecosystems. Environ Sci Technol 55(11):7445–7455
Acknowledgements
Not applicable
Funding
This project was funded by the Natural Science Foundation of China (52300166), National Natural Science Foundation of China (No. 52270125), the National Key Research and Development Program of China (2018YFC1901005), the Shanghai Science and Technology Committee (No. 21ZR1421300 and 22DZ1209600), and the Shanghai Sailing Program (23YF1446200).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by XZ, JH, SZ, TC, SL, WH, QZ. The first draft of the manuscript was written by Xiaotong Zhang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Handling editor: Baoshan Xing
Supplementary Information
Additional file 1.
Text S1. Chemicals and Reagents. Text S2. Titration Experiment of Functional Groups. Text S3. Competitive adsorption experiments and detection methods of tetracycline. Text S4. Fitting of dynamical equations. Text S5. Model equations of Dubinin-Ashtahkov model. Text S6. MWSE calculation. Table S1. pH values before and after adsorption at 7, pHpzc of biochars. Table S2. The tetracycline adsorption capacity of BC for comparison with this study. Table S3. Ash content, elemental compositions and pore propertiesof biochar. Table S4. FTIR bands and functional groups of biochar. Table S5. Kinetic parameters of tetracycline adsorption on biochar. Table S6. The Frontier Orbital (HOMO and LUMO) Energy of BC700 and Functionalized Biochars, Their Energy Gaps with Tetracycline Orbitals, and the Corresponding π-Electron Donors. Table S7. Comparative study of tetracycline adsorption by biochar. Figure S1. Adsorption capacity of tetracycline by biochar under different hitting treatment temperatures and concentrations. Figure S2. SEM images of biochar. Figure S3. FTIR spectra of before- and after-TTC adsorption at different time. Figure S4. Adsorption kinetics of TTC onBC300 (a), BC400 (b), BC500 (c), BC600 (d) and BC700 (e) by fitting the model of pseudo-first order (PFO), pseudo-second order (PSO), and Elovich. Figure S5. Adsorption kinetics of TTC on BC300 (a), BC400 (b), BC500 (c), BC600 (d) and BC700 (e) by fitting the model of Liquid film diffusion under initial concentrations of 40 mg·L-1 and 80 mg·L-1. Figure S6. Adsorption kinetics of TTC on BC300 (a), BC400 (b), BC500 (c), BC600 (d) and BC700 (e) by fitting the model of Intra-particle diffusion under initial concentrations of 40 mg·L-1and 80 mg·L-1. Figure S7. 13C NMR spectra of biochar. Figure S8. Bisolute competitive experiments at different TTC initial concentration. Error bar represents standard error of at least three replicates. Figure S9. Dft simulated models of tetracycline and Biochars. (a) tetracycline; (b) BC300; (c) BC400; (d) BC500; (e) BC600; (f) BC700. Figure S10. Adsorption configuration of tetracycline adsorbed on Biochars. (A, F, K: BC300; B, G, L: BC400; C, H, M: BC500; D, I, N: BC600; E, J, O: BC700; position 1: A, B, C, D, E; position 2: F, G, H, I, J; position 3: K, L, M, N, O). Figure S11. Electrostatic potential (ESP) distribution of TTC and functionalized Biochars. (a) TTC; (b) BC-OH; (c) BC-COOH; (d) BC-GN; (e) BC-PDN; (f) BC-PLN. Figure S12. Dft simulated models of functionalized Biochars. (a) BC-OH; (b) BC-COOH; (c) BC-GN; (d) BC-PDN; (e) BC-PLN. Figure S13. The frontier orbitals (HOMO and LUMO) of TTC and functionalized Biochars. (a) TTC; (b) BC-OH; (c) BC-COOH; (d) BC-GN; (e) BC-PDN; (f) BC-PLN.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, X., Hou, J., Zhang, S. et al. Standardization and micromechanistic study of tetracycline adsorption by biochar. Biochar 6, 12 (2024). https://doi.org/10.1007/s42773-023-00299-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-023-00299-7