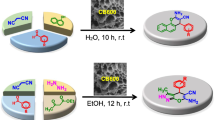
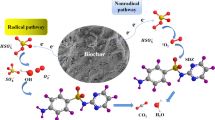
Abstract
In the last few decades, sulfonated carbon materials have garnered significant attention as Brønsted solid acid catalysts. The sulfonation process and catalytic activity of sulfonated biochar can be influenced by the aromaticity and degree of condensation exhibited by biochar. However, the relationships between the aromaticity, sulfonating ability, and resultant catalytic activity are not fully understood. In this study, biochar samples pyrolyzed at 300–650 °C exhibiting different aromaticity and degrees of condensation were sulfonated and employed as sulfonate-bearing solid catalysts for hydrolytically removing tylosin. They exhibited excellent hydrolytic performance and their kinetic constants were positively correlated with the total acidity and negatively correlated with their aromaticity. This study has uncovered the relationship between the structure, properties, sulfonating ability, and subsequent hydrolytic performance of biochar samples. It was observed that the aromaticity of biochar decreased as the pyrolysis temperature increased. Lower pyrolysis temperatures resulted in a reduced degree of condensation, smaller ring size, and an increased number of ring edge sites available for sulfonation, ultimately leading to enhanced catalytic performance. These findings provide valuable insights into the fundamental chemistry behind sulfonation upgrading of biochar, with the aim of developing functional catalysts for mitigating antibiotics in contaminated water.
Graphical Abstract

Highlights
-
Effects of aromatic, condensation and topology of condensed ring in biochar on their sulfonation ability were revealed
-
The total acidity of sulfonated biochar was positively correlated with its hydrolytic ability for tolysin mitigation
-
Low aromaticity, low degree of condensation and abundant edge sites led to high acidity and enhanced catalytic ability
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Solid acid catalysts have the advantages of being highly active, recyclable, environmentally benign, and energy-efficient when compared to mineral acids, such as H2SO4 (Gupta et al. 2014; Nakajima et al. 2012). Solid acid catalysts can be easily recovered through settling or filtration processes and can be reused multiple times in subsequent reactions. This not only reduces the consumption of chemicals but also eliminates the need for waste acid disposal (Nakajima et al. 2012). Sulfonated carbon materials containing -SO3H groups have garnered significant attention in academic research and industry due to their promising properties, including high stability, metal-free composition, and moderate cost. These materials have been extensively studied for various applications, such as biodiesel production, hydrolysis of hemicellulose, biorefinery engineering, and the hydrolytic mitigation of environmental pollutants (Liu et al. 2015; Xie et al. 2020).
Sulfonated carbon-based solid acids can be made from multiple carbonaceous matters such as biomass, cellulose, fiber, polymers and organic waste. On considering waste utilization, resource recovery, and the advantageous properties of abundant supply sources, cost-effectiveness, and easy handling, biochar presents itself as a promising substrate to produce value-added solid acids containing –SO3H groups. Sulfonated biochar materials have already been documented for their application in esterification reactions (Yusuff 2022); the hydrolysis of sugars (Xiong et al. 2018), starch, and cellulose (Yang et al. 2022); and the removal of spiramycin (Xie et al. 2020). The performances of –SO3H-bearing biochar-based solid acids depend on the acidic properties of the catalyst and the operating conditions employed in the hydrolysis reaction. In general, the total acidity and the quantity of –SO3H groups play a pivotal role in determining the hydrolytic kinetics of the sulfonated biochar. A complete removal of 40 mg L−1 of spiramycin was observed within 8 min using sulfonated biochar with a total acidity of 2.0 mmol g−1 and 1.6 mmol g−1 SO3H groups when compared to 73% mitigation using sulfonated biochar (1.0 g L−1) with an acidity of 0.4 mmol g−1 and 0.38 mmol g−1 SO3H groups and microwave irradiation of 200 W (Xie et al. 2020). Enhancing the degree of biochar sulfonation and achieving high total acidity (e.g., ≥ 4.0 mmol g−1) poses a challenge due to the absence of systematic strategies that target diverse biochar substrates. To facilitate the effective development and industrial utilization of these materials, it is crucial to attain a comprehensive understanding of the sulfonation reaction involved in producing sulfonated biochar with elevated acidity. This includes the formation of versatile and abundant acidic surface groups.
Several factors influence the sulfonation of biochar, including the characteristics of the substrate, sulfonation agent, and reaction conditions (Lee et al. 2017; Xiong et al. 2018). Among these factors, the structure and properties of the substrate play a crucial role when considering a specific sulfonation agent and reaction conditions. The degree of aromatic condensation, fusion, and ring size in biochar can impact the total number of available sites and the extent of electrophilic substitution by ClSO3H due to incomplete carbonization. Based on this understanding (Wiedemeier et al. 2015; Yoo et al. 2018), we hypothesized that controlling the aromaticity, degree of condensation, and ring size of the substrate could enhance the total acidity and hydrolytic ability of the resulting sulfonated biochar product.
In a previous study, we successfully employed sulfonated biochar and microwave irradiation for the hydrolytic mitigation of spiramycin (Xie et al. 2020). In this study, we focused on sulfonating biochar samples pyrolyzed at temperatures ranging from 300 to 650 °C, and assessed their efficacies in the hydrolytic removal of tylosin, a representative macrolide antibiotic with a similar structure to spiramycin. The main objectives of our investigation were as follows: (1) to quantitatively differentiate the aromaticity, degree of condensation, and aromatic cluster size of the biochar substrates used for sulfonation, (2) to understand how the aromatic characteristics and model structures influence the sulfonating ability of the biochar samples, and (3) to discover effective strategies for producing exceptional sulfonated biochar catalysts for the hydrolytic mitigation of tylosin.
2 Materials and methods
2.1 Materials
The stems of Crofton weed (Ageratina adenophora), an invasive species with very large harvests, was used as the feedstock of biochar. Its basic chemical composition has been supplied in Additional file 1: Table S1. HPLC grade tylosin (≥ 98.0%) was ordered from Macklin Biochemical Co., Ltd. (Shanghai, China). AR grade chlorosulfonic acid (99.5%) was purchased from Sigma-Aldrich Co., Ltd. (Shanghai, China). HPLC grade acetonitrile (≥ 99.9%) was ordered from Merck Chemicals Co., Ltd. (Shanghai, China). Ultra-pure water with a conductivity of 18.2 MΩ cm−1 was prepared using a Milli-Q purification equipment (Millipore, USA).
2.2 Preparation of biochar
Crofton weeds were dried at 80 °C for 10 h, crushed to 0.15–0.20 mm, and then pyrolyzed in an SGM B3/18 furnace (Luoyang Sigma, China). Prior to heating, the furnace was continuously flushed with 99.9% N2 for 15 min to remove air from the apparatus. The sample (20 g in four crucibles) was heated from room temperature to preset temperatures of 300, 400, 450, 550, 600 and 650 °C at 10 °C min−1 with N2 purged at 25–33 mL min−1, then naturally cooled down to ca. 25 °C. The collected samples were grinded and screened (< 0.25-mm) prior to sulfonation.
2.3 Preparation of sulfonated biochar and characterization of its physico-chemical properties
The biochar samples pyrolyzed at 300–650 °C were hydrothermally treated using ClSO3H to prepare a series of SO3H-bearing solid acids following reported procedures (Xie et al. 2020). The chemical composition, surface properties, and structural properties of the substrate and sulfonated biochar samples were analyzed and recorded. Detailed procedures on sulfonated biochar preparation, sample characterizations, and identification of the hydrolyzed products, were separately provided in Additional file 1: S1, S2 and S3.
2.4 Hydrolysis of tylosin
The hydrolysis experiments were carried out on an ANKS-HX-3 (Qingdao, China) microwave reactor with a power input of 200 W. Tylosin (40 mg L−1) was hydrolyzed by 1 g L−1 of catalyst in 100 mL of solutions. The suspension was stirred at 50 rpm during the hydrolysis reaction. Experimental procedures similar to those previously reported (Xie et al. 2020) were used for the hydrolysis of tylosin and the analysis of the products.
3 Results and discussion
3.1 Characteristics of biochar-based solid acids
The SEM images of the biomass precursor and biochar samples pyrolyzed at 300–650 °C are shown in Additional file 1: Fig. S1. The pore size, surface morphology, and external microstructure can be observed from these images. These samples largely retaining their macroscopic shape and pores were visible in the biochar samples produced at temperatures > 600 °C. These pores may have resulted from the cracking of the volatile fractions upon increasing the pyrolysis temperature. In the FTIR spectrum obtained for pristine biochar (Fig. 1a), typical aromatic C=C stretching vibrations were observed at 1560 cm–1 (Shin et al. 2020), and the in–plane bending of methylene or methyl group vibrations appeared at ~ 1387 cm–1 (Li et al. 2019). The bands observed at 1250 and 1060 cm–1 were due to the C–O stretching of phenol and alkoxy moieties, respectively (Chen et al. 2015; Son et al. 2018). The band observed at 876 cm–1 was assigned to aromatic sp2 C–H bending vibrations (Antor et al. 2022; Semmler et al. 1991). After sulfonation (Fig. 1(b)), the characteristic bands at 1160 and 1030 cm–1 appeared, which were due to O=S=O and S=O stretching vibrations, respectively (Nath et al. 2015). The weak bands observed at 609 cm–1 were assigned to the C–S stretching vibrations (Ouellette et al. 2018). In addition, the bands observed at 1700 and 1585 cm–1 were from the stretching of C=O in –COOH and C–C in phase ring, respectively (You et al. 2013).
The analysis results obtained for these substrates and the sulfonated biochar samples are listed in Table 1 and Additional file 1: Table S2. Prior to sulfonation, the O and H content, and the O/C and H/C ratios gradually decrease from 22.48 to 12.63, 4.09% to 2.06%, 0.26 to 0.13 and 0.77 to 0.34, respectively, while the C content increased from 64.04 to 73.50% with temperature increasing from 300 to 650 °C. The C contents in biochars (Table 1) pyrolyzed at 300 to 650 °C are less than those from wood sawdust, coconut shell and bamboo at 500 °C (Chung et al. 2021; Zhang et al. 2019), since the C contents in herbs are usually less than those in shrubs or trees (Kang et al. 2006). However, the S contents of sulfonated biochars (at or less than 550 °C) are larger than those of sulfonated wood sawdust and coconut shell, indicating more –SO3H groups were introduced during sulfonation for the former than the latter.
In addition, more O-containing moieties (e.g., phenolic hydroxyl, methoxy, and acetoxy groups) remained after pyrolysis at lower temperatures such as at 300 °C (Liu et al. 2015). In addition, the increased H/C ratio indicates the enlarged aromatic features formed during pyrolysis at high temperature. After sulfonation, the S contents increased upon decreasing the pyrolysis temperature, which indicates that more –SO3H groups were loaded onto the solid acids prepared via pyrolysis at low temperature.
The acidity of the sulfonated biochar samples is shown in Fig. 2a and Additional file 1: Table S3. The total acidity ranged from 0.6 to 3.1 mmol g−1 and decreased upon increasing the pyrolysis temperature. The -SO3H groups account for > 90% of the total acidity and the -COOH, Ar–OH, and lactone groups contribute to the remaining acidity. In addition, the -COOH and Ar–OH groups are weakly acidic when compared to the -SO3H groups during the hydrolysis of tylosin. The -SO3H content was negatively correlated with the atomic S/C, O/C, and H/C ratios of the solid acids. This means that increasing the pyrolysis temperature decreases the sulfonating ability of the biochar samples and results in the reduced total acidity of the solid acids. The higher S/C and O/C ratios observed upon pyrolysis at low temperature (e.g., 300 °C) (Fig. 2b–d) indicate that more sulfonate groups (–SO3H) were loaded under these conditions.
3.2 Hydrolytic efficiency and its correlation with the acidic properties of solid acids
The concentration decay curves and the corresponding kinetic fitting results of the solid acid samples toward tylosin are presented in Fig. 3 and Table 2. As observed, the sulfonated biochar samples prepared at decreasing pyrolysis temperatures from 650 to 300 °C display an enhanced efficiency in hydrolyzing tylosin. Consequently, the kinetic constants increase from 0.26 to 1.29 min–1, and the half-lives reduce from 2.65 to 0.53 min. The correlation constants (R2) range from 0.923 to 0.999. These results suggest that the catalytically acidic sites are present in excess relative to tylosin in the solution (Ye et al. 2011).
a Concentration decay curve obtained for tylosin in the presence of the sulfonated biochar samples under microwaving, b the kinetics fitting of the decay curves using a pseudo first-order model, c the recycling and reuse of the CS300 sample over 5 cycles under the same operational conditions used for the hydrolysis of tylosin, and d the total ion chromatogram (TIC) spectra obtained for tylosin and its hydrolyzed products using CS300 after 6 min. Initial concentration of tylosin, 40 mg L−1; catalyst dosage, 1.0 g L−1; microwave irradiation, 200 W; contact time, 10 min; suspension volume, 100 mL
The hydrolytic kinetic constants agree well with the total acidity of the solid acid samples (CS300–650), as shown in Fig. 2a. The CS300 sample was used, recycled, and reused five times, and still catalytically hydrolyzed 100% of tylosin after 6 min (Fig. 3c). The total ion chromatogram (TIC) spectra of tylosin and its hydrolyzed products obtained using CS300 are presented in Fig. 3d and the first-order mass spectra are shown in Additional file 1: Fig. S2. The results indicate that two pseudo-molecular ions at m/z 370.1001 and 432.2759 were produced via the hydrolytic cleavage of glycosidic bonds and the release of three deoxysugars (mycinose, mycarose, and mycaminose) from the 16-membered macrolide ring in tylosin. Accordingly, the degradation pathway for tylosin is shown in Additional file 1: Fig. S3. These results demonstrate that sulfonated biochar is an effective catalyst for the hydrolytic degradation of tylosin.
To understand the individual contributions of each acidic group to the hydrolysis of tylosin, we investigated the correlations between the content of each group and the hydrolytic kinetic constants (Fig. 4a–d). The results revealed positive correlations between the total acidity and the three primary acidic functional groups (–SO3H, –COOH, and Ar–OH) with the hydrolytic kinetic constants. Since -SO3H accounts for ~ 90% of the total acidity (Fig. 2a), it is considered the primary functional group contributing to the hydrolytic mitigation of tylosin, as previously reported for spiramycin (Xie et al. 2020). The content of –SO3H increases as the pyrolysis temperature decreases, indicating that temperature plays a significant role in shaping the structural and surface properties of these substrates. Consequently, it affects the sulfonating ability and final acidic properties of the resulting sulfonated biochar samples. Therefore, it is crucial to conduct a comprehensive investigation into the variations in the structural and surface properties of these substrates, prepared at different pyrolysis temperatures, and their subsequent impacts on the sulfonation reaction.
3.3 Analysis of the aromaticity and its effect on the sulfonation reaction
3.3.1 Effect of aromaticity
Raman and XPS spectroscopies were employed to determine the degree of structural disorder, C sp2/sp3 hybridization, and consequently, the aromaticity of the substrates prepared at 300–650 °C. The Raman spectral analyses and the ratio of the G to D band ratio (ID/IG) vs. the pyrolysis temperature are provided in Additional file 1: Fig. S4, Table S4, and Fig. 5a. The ID/IG ratio increases from 0.48 to 2.40 for the samples pyrolyzed from 300 to 650 °C. The changes in the ID/IG ratio cannot be directly linked to the disorder in the sp2 clusters. An increase in ID/IG ratio was observed upon increasing the pyrolysis temperature from 450 to 1600 °C for saccharose-based biochars (Bernard et al. 2010) and from 500 to 1400 °C for beech wood-based biochar (Guizani et al. 2017). In addition, there are reports on the increase in ID/IG with graphite transforming into reduced graphene oxide (RGO) (Chadha et al. 2021). In this study, it was observed that increasing the pyrolysis temperature led to the development of larger aromatic clusters. Conversely, biochar samples prepared at lower pyrolysis temperatures retained more disordered domains, exhibited defective structures, and showcased a higher abundance of non-graphite features and relatively smaller aromatic cluster sizes. The high-resolution XPS spectra of the C 1 s band for the biochar samples prepared at various temperatures are presented in Additional file 1: Figure S5a–f. The sp2/sp3 ratio deconvoluted from the XPS C1s spectra (Fig. 5b) can be used to infer the aromaticity of the biochar samples. A small sp2/sp3 ratio and large sp3 content obtained at low temperature conditions (e.g., 300 °C) indicate that more transition char and amorphous char remain under such conditions, which may facilitate the sulfonation reaction and result in high total acidity.
a Raman derived area ratio of the G to D bands (ID/IG), b XPS determined sp2/sp3 ratios, and c ultimate analysis determined aromaticity (fa) vs. pyrolysis temperature. d The fa values vs. H/C for the substrates prepared at 300–650 °C compared to those previously reported (Angın et al. 2014; Han et al. 2016; Keiluweit et al. 2010; Park et al. 2018; Yu et al. 2011)
While the connection between the aromatic characteristics and the pyrolysis temperature of the substrates has been established, further investigation is necessary to determine the precise aromaticity values. Quantitatively identifying the differences in the sulfonating ability of these substrates would benefit from determining the exact aromaticity values. The determination of these values was accomplished by employing elemental composition calculations based on the model proposed by Mazumdar (Eqs. 1, 2, 3) (Mazumdar 1999).
where H′/C′ is the corrected atomic H/C ratio (Mazumdar 1999), Mc/d is average molar volume of C atoms, and α is a correction coefficient ranged between 0.115 and 0.125 depending on the type of hydrocarbon analogue. Mc/d was estimated using Eq. 2. H′/C′ in Eq. 2 was determined using Eq. 3 (Wang et al. 2013). Details on building these equations and the constants 5.34, 9.15, and 2.9 have been well documented in previous reports (Mazumdar 1999; Wang et al. 2013).
The results of the aromaticity calculations are presented in Additional file 1: Table S5. These calculations allowed for the assessment of the relationship between the aromaticity of the substrates and both the pyrolysis temperature and the H/C ratio. The corresponding plots are shown in Fig. 5c, d. The aromaticity shows an increasing trend upon increasing the pyrolysis temperature and a negative correlation with the H/C ratio. Increasing the pyrolysis temperature resulted in an enhanced aromaticity, indicating a reduction in carbonaceous and transitional components and a decrease in the presence of randomly arranged aromatic rings (amorphous char) within the sample. Conversely, this led to the development of more graphite domains. These observations were consistent with the changes observed in the ID/IG ratio as depicted in Fig. 5a. The varying aromaticity of the biochar samples was considered a significant factor influencing their sulfonating ability. Lower pyrolysis temperatures corresponded to lower aromaticity, indicating smaller aromatic cluster sizes and a higher availability of reactive edge sites per unit mass. Furthermore, lower pyrolysis temperatures exhibited larger O/C ratios (as shown in Table 1) due to the tendency of carbonaceous residues to retain more oxygen atoms within their structure.
The reduced trend of aromaticity (fa) vs. H/C ratio (Fig. 5d) meant less H-bond sites were reactive at higher pyrolysis temperatures since only the H atoms occurred at the edge sites of the aromatic clusters could participate in the sulfonation reaction, while those in the body parts cannot. To gain a comprehensive understanding of the sulfonating ability of these substrates, further information is required regarding the arrangement of aromatic rings, the degree of condensation and fusion, as well as the cluster size. This detailed knowledge would be instrumental in comprehending the molecular structure and, subsequently, elucidating the variations in sulfonating ability among the substrates.
3.3.2 Effect of the degree of condensation and ring size
Solid-state 13C NMR spectra of the biomass and pyrolyzed products obtained at 300–650 °C were recorded to understand the degree of condensation in the aromatic rings and their arrangements (Fig. 6). Raw biomass presents characteristic chemical shifts (δ) at 0–50, 50–90, and 165–210 ppm, which due to alkyl, O-alkyl and carboxyl C from lignin, cellulose, and hemicellulose, respectively (Fujii et al. 2020). As the pyrolysis temperature was raised from 300 to 400 °C, the presence of O-alkyl C and carboxyl C structures gradually diminished, whereas the signals associated with aromatic structures (δ ~ 128 ppm) intensified significantly. At temperatures exceeding 450 °C, only the aromatic structures were detected. According to Brewer et al. (2009) the degree of aromatic condensation could be quantitatively determined through fitting the 13C-NMR spectra and then revealing the condensed ring size of the aromatic clusters. A direct polarization (DP) NMR method is often employed to quantitatively estimate the composition of the functional carbon groups in ring clusters. According to Mcbeath et al. (2011) the chemical shift obtained from DP MAS was about 1.03–1.04 times that from cross-polarization (CP) MAS and the estimation can be worked out after revision. An aromatic carbon cluster is composed of edge (χedge) and bridge (χbridge) fractions. As a result, the following relationship was derived: χedge + χbridge = 1. When the size of the aromatic cluster increases, χedge decreases. On the contrary, χbridge increases. Aromatic edge carbons mostly originate from aromatic C–H and C–O groups, and their corresponding ratios, χC–H = faC–H/far and χC–O = faC–O/far, can be resolved through fitting the 13C spectral profile, where far is the fraction of aromatic carbons in the total carbon atoms (Brewer et al. 2009; Park et al. 2019). The minimum aromatic edge fraction can be defined as follows:
Additional contributions to aromatic carbon at edges were possible from carbon atoms in alkyl-C and C=O contained-moieties which were connected to aromatic rings. The maximum ratio of edge atoms can be determined according to \({\upchi }_{\mathrm{edge},\,\mathrm{ max}} = {\upchi }_{\mathrm{edge}, \, \mathrm{min}} +{\upchi }_{\mathrm{alkyl}} + {\upchi }_{\mathrm{C}=\mathrm{O}}\). Likewise,\({\upchi }_{\mathrm{alkyl}} ={\mathrm{ f}}_{\mathrm{alkyl}}/{\mathrm{f}}_{\mathrm{ar}}\). Considering the small value of \({\upchi }_{\mathrm{alkyl}}\) and less ratio of C=O in the biochar samples, the range between \({\chi }_{edge, \,min} \mathrm{and }{\upchi }_{\mathrm{edge},\, \mathrm{max}}\) is quite limited (Park et al. 2019). According to a formula generalized by Solum et al., (Solum et al. 1989) the relation between the carbon atom numbers (nc) in clusters and the fractions of edge carbon was defined using Eq. 5.
Thus, \({\upchi }_{\mathrm{edge},\, \mathrm{min}}\), \({\upchi }_{\mathrm{edge}, \,\mathrm{max}}\) (Table 3) and the aromatic carbon and ring numbers in aromatic clusters (Fig. 6) were determined. The carbon numbers of the condensed aromatic clusters were determined to be in the range 13–30 for the biochar samples prepared at 300–650 °C and the ring size (ring number) was estimated to be 3–9.
A decrease in the pyrolysis temperature leads to a reduction in the size of aromatic rings. The arrangement and enlargement of aromatic clusters in these substrates can be conceptualized using hexagonal and lozenge models, as shown in Fig. 6. The results clearly demonstrated the changes in ring size as the pyrolysis temperature was increased from 300 to 650 °C. Pyrolyzing at lower temperatures (e.g., 300 °C) initiates transformations in all biomass components, including cellulose, hemicellulose, and lignin, resulting in minimal condensation and aromatization (Cao et al. 2017). Biochar with a low degree of condensation usually contains more branched residual moieties (e.g., phenolic hydroxyl and methoxy groups) (Liu et al. 2015) and form more edge sites than the highly condensed and aromatized samples, which facilitate the sulfonation reaction to yield excellent solid acid catalysts with high acidity.
3.3.3 Topological analyses of the available sulfonation sites
A general understanding of the dependence of sulfonating ability on the degree of condensation of the biochar samples prepared at 300–650 °C is required. Since sulfonation reactions almost exclusively take place on the edge and defective sites of condensed aromatic rings, it is possible to quantify and compare the total number of reactive sites at these positions through topological analysis. To simplify the structure for this analysis, branched moieties such as phenolic hydroxyl, methoxy, and acetoxy groups connected to the aromatic rings were disregarded. Additionally, only hydrogen atoms were assumed to be present at the edges of the aromatic rings. This simplification allows for the consideration of a polycyclic aromatic hydrocarbon (PAH) model. The defective sites were also ignored because of the small cluster size. All possible aromatic ring arrangements (with sizes of 3–10) are listed in Additional file 1: Table S6. The edge H atoms, total C atoms, Hedge/Ctotal (mmol g−1), and molecular weight of all possible aromatic ring arrangements (Additional file 1: Table S6) were determined using topological analysis. The molar fraction of Hedge/Ctotal for 1 g of solid char (mmol g−1) was calculated using Eq. 6 and presented in Fig. 7a–d, Additional file 1: Fig. S6, and Table S6.
The decrease in Hedge/Ctotal with increasing ring size indicates that the possible reactive sites available for sulfonation decrease with an increase in the degree of condensation and cluster size. Biochar samples produced at low pyrolysis temperatures (e.g., 300 °C) exhibit smaller cluster sizes, which are associated with a higher sulfonating ability. Furthermore, the cluster sizes show a positive correlation with the total acidity of the solid acids. Consequently, lower pyrolysis temperatures are preferred when using a specific biomass, as they result in reduced aromaticity, lower degrees of condensation, and smaller cluster sizes. These factors ultimately contribute to higher acidity in the sulfonated biochar samples. The number of aromatic rings, rather than their specific arrangement, has a significant impact on the availability of edge sites, as indicated by Hedge/Ctotal. Moreover, by adjusting the pyrolysis temperature, it becomes possible to finely tune the total acidity of biochar-based solid acids, thereby influencing the hydrolysis reaction.
a Correlation between the calculated ratio of the edge H atoms to total C atoms [Hedge/Ctotal (mmol g−1)] and the aromatic ring numbers determined using topological analysis, b the total acidity of the solid acids vs. the aromatic ring number of the substrate, and the topological summaries of the c linear-branched and d spherical condensed models with an aromatic ring number of 7
4 Conclusions and outlook
This study investigated the effects of aromatic, condensation and topology arrangement of condensed rings in biochar samples on their sulfonation ability, and then revealed their intrinsic relationship with the hydrolysis properties of tylosin. The findings indicated that a decrease in aromaticity, a lower degree of aromatic condensation, and the presence of ample accessible edge sites facilitate sulfonation reactions, leading to a higher total acidity and a greater abundance of -SO3H groups in the resulting sulfonated biochar. When the sulfonated biochar was utilized as a solid acid catalyst for the hydrolytic mitigation of tylosin, the high total acidity promoted the hydrolytic reactions and exhibited a positive correlation with the kinetic constants. By employing lower pyrolysis temperatures, such as 300 °C, it was possible to achieve reduced aromaticity, less condensation, and increased availability of edge sites. Consequently, fine-tuning the pyrolysis temperature allowed for the attainment of desired biochar characteristics, including heightened acidity and enhanced hydrolytic abilities of the resulting solid acids.
Further research is required to enhance the formation of both amorphous and crystalline domains composed of condensed aromatic rings in biochar substrates and differentiate the subtle structural differences and their effects on the sulfonation reaction. Additionally, determining the overall count of accessible reactive sites on the substrates will contribute to understanding the positional effects of sulfonation due to substituents. The development of high-performance solid acids with sufficient acidity and exceptional hydrolytic activity holds great significance in the mitigation of antibiotic micropollutants from contaminated water. This can be achieved through an environmentally-friendly approach that involves biomass recycling and utilization.
Availability of data and materials
The following files are available free of charge: Additional file 1: Figures S1–S6 and Tables S1–S6 as additional file.
References
Angın D, Şensöz S (2014) Effect of pyrolysis temperature on chemical and surface properties of biochar of rapeseed (Brassica napus l.). Int J Phytoremediat 16:684–693. https://doi.org/10.1080/15226514.2013.856842
Antor NH, Mia S, Hasan MM, Lipi NJ, Jindo K, Sanchez-Monedero MA, Rashid MH (2022) Chemical and biological activation of biochar favors N immobilization in biochar and its release to plant. Pedosphere. https://doi.org/10.1016/j.pedsph.2022.06.050
Bernard S, Beyssac O, Benzerara K, Findling N, Tzvetkov G, Brown GE (2010) XANES, Raman and XRD study of anthracene-based cokes and saccharose-based chars submitted to high-temperature pyrolysis. Carbon 48:2506–2516. https://doi.org/10.1016/j.carbon.2010.03.024
Brewer CE, Schmidt-Rohr K, Satrio JA, Brown RC (2009) Characterization of biochar from fast pyrolysis and gasification systems. Environ Prog Sustain Energy 28:386–396. https://doi.org/10.1002/ep.10378
Cao X, Sun S, Sun R (2017) Application of biochar-based catalysts in biomass upgrading: a review. RSC Adv 7:48793–48805. https://doi.org/10.1039/C7RA09307A
Chadha N, Sharma R, Saini P (2021) A new insight into the structural modulation of graphene oxide upon chemical reduction probed by Raman spectroscopy and X-ray diffraction. Carbon Lett. https://doi.org/10.1007/s42823-021-00234-5
Chen X, Chen B (2015) Macroscopic and spectroscopic investigations of the adsorption of nitroaromatic compounds on graphene oxide, reduced graphene oxide, and graphene nanosheets. Environ Sci Technol 49:6181–6189. https://doi.org/10.1021/es5054946
Chung NH, Dien LQ, Que NT, Thanh NT, Ly GTP (2021) Comparative study on the conversion of Acacia mangium wood sawdust-derived xylose-containing acid hydrolysate to furfural by sulfonated solid catalysts prepared from different lignocellulosic biomass residues. Wood Sci Technol 55:659–679. https://doi.org/10.1007/s00226-021-01284-8
Fujii K, Morioka K, Hayakawa C, Inagaki Y, Hangs RD, Anderson DW, McConkey BG (2020) Litter decomposition and soil organic carbon stabilization in a Kastanozem of Saskatchewan. Can Geoderma Reg 23:e00348. https://doi.org/10.1016/j.geodrs.2020.e00348
Guizani C, Haddad K, Limousy L, Jeguirim M (2017) New insights on the structural evolution of biomass char upon pyrolysis as revealed by the Raman spectroscopy and elemental analysis. Carbon 119:519–521. https://doi.org/10.1016/j.carbon.2017.04.078
Gupta P, Paul S (2014) Solid acids: green alternatives for acid catalysis. Catal Today 236:153–170. https://doi.org/10.1016/j.cattod.2014.04.010
Han L, Qian L, Yan J, Chen M (2016) Contributions of different biomass components to the sorption of 1,2,4-trichlorobenzene under a series of pyrolytic temperatures. Chemosphere 156:262–271. https://doi.org/10.1016/j.chemosphere.2016.04.031
Kang B, Liu S, Zhang G, Chang J, Wen Y, Ma J, Hao W (2006) Carbon accumulation and distribution in Pinus massoniana and Cunninghamia lanceolata mixed forest ecosystem in Daqingshan, Guangxi, China. Acta Ecologica Sinica 26:1320–1327. https://doi.org/10.1016/S1872-2032(06)60024-3
Keiluweit M, Nico PS, Johnson MG, Kleber M (2010) Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ Sci Technol 44:1247–1253. https://doi.org/10.1021/es9031419
Lee J, Kim K-H, Kwon EE (2017) Biochar as a catalyst. Renew Sustain Energy Rev 77:70–79. https://doi.org/10.1016/j.rser.2017.04.002
Li H, Xiong J, Xiao T, Long J, Wang Q, Li K, Liu X, Zhang G, Zhang H (2019) Biochar derived from watermelon rinds as regenerable adsorbent for efficient removal of thallium(I) from wastewater. Process Saf Environ Prot 127:257–266. https://doi.org/10.1016/j.psep.2019.04.031
Liu W, Jiang H, Yu H (2015) Development of biochar-based functional materials: toward a sustainable platform carbon material. Chem Rev 115:12251–12285. https://doi.org/10.1021/acs.chemrev.5b00195
Mazumdar BK (1999) Molecular structure and molar volume of organic compounds and complexes with special reference to coal. Fuel 78:1097–1107. https://doi.org/10.1016/S0016-2361(99)00012-5
McBeath AV, Smernik RJ, Schneider MPW, Schmidt MWI, Plant EL (2011) Determination of the aromaticity and the degree of aromatic condensation of a thermosequence of wood charcoal using NMR. Org Geochem 42:1194–1202. https://doi.org/10.1016/j.orggeochem.2011.08.008
Nakajima K, Hara M (2012) Amorphous carbon with SO3H groups as a solid brønsted acid catalyst. ACS Catal 2:1296–1304. https://doi.org/10.1021/cs300103k
Nath BK, Khan A, Chutia J (2015) Composite plasma polymerized sulfonated polystyrene membrane for PEMFC. Mater Res Bull 70:887–895. https://doi.org/10.1016/j.materresbull.2015.06.028
Ouellette RJ, Rawn JD (2018) 17-Ethers and epoxides. In: Ouellette RJ, Rawn JD (eds) Organic chemistry, 2nd edn. Academic Press, pp 507–536
Park KY, Lee K, Kim D (2018) Characterized hydrochar of algal biomass for producing solid fuel through hydrothermal carbonization. Bioresour Technol 258:119–124. https://doi.org/10.1016/j.biortech.2018.03.003
Park J, Yoo S, Lim KH, Rojas OJ, Hubbe MA, Park S (2019) Impact of oxidative carbonization on structure development of loblolly pine-derived biochar investigated by nuclear magnetic resonance spectroscopy and X-ray photoelectron spectroscopy. Diamond Relat Mater 96:140–147. https://doi.org/10.1016/j.diamond.2019.05.001
Semmler J, Yang PW, Crawford GE (1991) Gas chromatography/Fourier transform infrared studies of gas-phase polynuclear aromatic hydrocarbons. Vib Spectrosc 2:189–203. https://doi.org/10.1016/0924-2031(91)85026-J
Shin H, Tiwari D, Kim D-J (2020) Phosphate adsorption/desorption kinetics and P bioavailability of Mg-biochar from ground coffee waste. J Water Process Eng 37:101484. https://doi.org/10.1016/j.jwpe.2020.101484
Solum MS, Pugmire RJ, Grant DM (1989) Carbon-13 solid-state NMR of Argonne-premium coals. Energy Fuels 3:187–193. https://doi.org/10.1021/ef00014a012
Son E-B, Poo K-M, Chang J-S, Chae K-J (2018) Heavy metal removal from aqueous solutions using engineered magnetic biochars derived from waste marine macro-algal biomass. Sci Total Environ 615:161–168. https://doi.org/10.1016/j.scitotenv.2017.09.171
Wang T, Camps-Arbestain M, Hedley M (2013) Predicting C aromaticity of biochars based on their elemental composition. Org Geochem 62:1–6. https://doi.org/10.1016/j.orggeochem.2013.06.012
Wiedemeier DB, Abiven S, Hockaday WC, Keiluweit M, Kleber M, Masiello CA, McBeath AV, Nico PS, Pyle LA, Schneider MPW, Smernik RJ, Wiesenberg GLB, Schmidt MWI (2015) Aromaticity and degree of aromatic condensation of char. Org Geochem 78:135–143. https://doi.org/10.1016/j.orggeochem.2014.10.002
Xie Q, Yang X, Xu K, Chen Z, Sarkar B, Dou X (2020) Conversion of biochar to sulfonated solid acid catalysts for spiramycin hydrolysis: Insights into the sulfonation process. Environ Res 188:109887. https://doi.org/10.1016/j.envres.2020.109887
Xiong X, Yu IKM, Chen SS, Tsang DCW, Cao L, Song H, Kwon EE, Ok YS, Zhang S, Poon CS (2018) Sulfonated biochar as acid catalyst for sugar hydrolysis and dehydration. Catal Today 314:52–61. https://doi.org/10.1016/j.cattod.2018.02.034
Yang H, Lei S, Xu K, Fang Y, Chen X, Chen Y, Wang X, Chen H (2022) Catalytic pyrolysis of cellulose with sulfonated carbon catalyst to produce levoglucosenone. Fuel Process Technol 234:107323. https://doi.org/10.1016/j.fuproc.2022.107323
Ye Z, Berson RE (2011) Kinetic modeling of cellulose hydrolysis with first order inactivation of adsorbed cellulase. Bioresour Technol 102:11194–11199. https://doi.org/10.1016/j.biortech.2011.09.044
Yoo S, Chung C-C, Kelley SS, Park S (2018) Graphitization behavior of loblolly pine wood investigated by in situ high temperature X-ray diffraction. ACS Sustainable Chem Eng 6:9113–9119. https://doi.org/10.1021/acssuschemeng.8b01446
You T-T, Mao J-Z, Yuan T-Q, Wen J-L, Xu F (2013) Structural elucidation of the lignins from stems and foliage of Arundo donax Linn. J Agric Food Chem 61:5361–5370. https://doi.org/10.1021/jf401277v
Yu JT, Dehkhoda AM, Ellis N (2011) Development of biochar-based catalyst for transesterification of canola oil. Energy Fuels 25:337–344. https://doi.org/10.1021/ef100977d
Yusuff AS (2022) Kinetic and thermodynamic study on the esterification of oleic acid over SO3H-functionalized eucalyptus tree bark biochar catalyst. Sci Rep 12:8653. https://doi.org/10.1038/s41598-022-12539-0
Zhang C, Zhang N, Xiao Z, Li Z, Zhang D (2019) Characterization of biochars derived from different materials and their effects on microbial dechlorination of pentachlorophenol in a consortium. RSC Adv 9:917–923. https://doi.org/10.1039/C8RA09410A
Acknowledgements
Funding from the National Natural Science Foundation of China (No. 51978052 and 42207456) and the State Key Joint Laboratory of Environmental Simulation and Pollution (No. 19K01ESPCR) are gratefully acknowledged. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1A2C2011734). This research was also supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1A6A1A10045235). This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2022M3J4A1091450) and the OJEong Resilience Institute, Korea University.
Funding
This work was supported by the National Natural Science Foundation of China (51978052) and the State Key Joint Laboratory of Environmental Simulation and Pollution (19K01ESPCR).
Author information
Authors and Affiliations
Contributions
QX: data collection, methodology, writing, and visualization; XY: resources, methodology, writing (review and editing), and visualization; BS: formal analysis and writing (review and editing); XD: conceptualization, writing (review and editing), and supervision; PAW: writing (review and editing) and YSO: conceptualization, writing (review and editing), and supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Handling editor: Hailong Wang.
Supplementary Information
Additional file 1: Fig. S1.
SEM images of the biochar substrates pyrolyzed at a 300, b 400, c 450, d 550, e 600, and f 650 °C. Fig. S2. First-order mass spectra obtained for the parent tylosin molecule at a retention time of a 0.932 min and for tylosin hydrolyzed using CS300 at a retention time of b 0.728 and c 2.067 min. Fig. S3. The proposed degradation pathway for tylosin by using CS300. Fig. S4. Raman spectra obtained for the biochar substrates pyrolyzed at 300–650 °C prior to the sulfonation reaction. Fig. S5. High-resolution C 1s spectra obtained for the biochar substrates pyrolyzed at a 300, b 400, c 450, d 550, e 600, and f 650 °C prior to the sulfonation reaction. Fig. S6. The calculated Hedge/Ctotal ratio for the condensed aromatic models with ring numbers in the range of 6 to 10 except 7. The calculated values were obtained from the near-spherical (bottom of each vertical line) to linear (top of each vertical line) samples corresponding to the arrangement models in Table S6. Table S1. The basic chemical composition of the Crofton weed. Table S2. Ultimate analysis of the sulfonated biochar samples obtained from the pyrolyzed samples prepared at 300–650 °C. Table S3. Total acidity and acidic functional groups of the sulfonated biochar samples*. Table S4. Raman spectral analysis of the biochar samples. Table S5. Calculated aromaticity of the biochar samples. Table S6. Possible arrangements of the condensed aromatic rings (3–9) in the biochar samples and topological analysis of the sites available for sulfonation
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xie, Q., Yang, X., Sarkar, B. et al. Conversion of biochar into sulfonate-bearing solid acids used for the hydrolysis of tylosin: the effect of aromaticity and degree of condensation. Biochar 5, 88 (2023). https://doi.org/10.1007/s42773-023-00277-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-023-00277-z