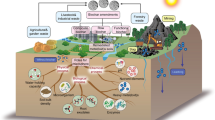
Abstract
Due to anthropogenic activities, heavy metal (HM) pollution in soils has increased, resulting in severe ecological problems and posing a constant threat to human health. Among various remediation methods, bacterial remediation is a relatively clean, efficient, and minimally negative approach. However, bacterial agents face multiple environmental stresses, making them challenging to achieve long-lasting and stable restoration effects. To address this issue, supportive organic substances such as biochar can be added to the soil with bacteria. According to bibliometric studies, integrating biochar and bacteria is extensively researched and widely used for HM-contaminated soil remediation. By integrating biochar and bacteria, heavy metals in the soil can be remediated, and soil conditions can be improved over time. Bacteria can also better promote plant growth or contribute effectively to phytoremediation processes when assisted by biochar. However, the remediation agents integrating biochar and bacteria are still some distance away from large-scale use because of their high cost and possible environmental problems. Therefore, further discussion on the interaction between biochar and bacteria and the integration approach, along with their remediation efficiency and environmental friendliness, is needed to achieve sustainable remediation of HM-contaminated soils by integrating biochar and bacteria. This paper discusses the potential mechanisms of biochar-bacteria-metal interactions, current advancements in biochar-bacteria combinations for HM-contaminated soil treatment, and their application in sustainable remediation, analyzes the interaction between biochar and bacteria and compares the remediation effect of different ways and feedstocks to integrate biochar and bacteria. Finally, future directions of biochar-bacteria combinations are presented, along with evidence and strategies for improving their commercialization and implementation.
Graphical Abstract

Highlights
-
Determining the synergy between biochar and bacteria to identify the optimal ratio for their efficacy as a remediation agent.
-
Biochar-bacteria combinations can remediate metal-contaminated soils by direct or assisted phytoremediation to face different situations.
-
Integration methods and feedstocks are keys to achieving sustainable remediation of HM-contaminated soils by biochar-bacteria combinations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Heavy metals (HMs) are metals with densities greater than 4.5 g/cm3 such as cadmium (Cd), chromium (Cr), lead (Pb), mercury (Hg), cupric (Cu), selenium (Se), arsenic (As), nickel (Ni), and others that tend to accumulate in the environment and are resistant to degradation (Rajendran et al. 2022). Despite numerous painful lessons learned over the last few decades, the world continues to face serious problems with HM pollution in soils (Peng et al. 2022). HM can enter humans through the food chain or drinking water, causing a variety of negative effects (Wang et al. 2022b). Soil, as a complex environmental medium, cannot purify itself of HM pollutants effectively and thus requires remediation through artificial interventions. These interventions are classified as physical, chemical, and bioremediation based on the remediation technology principles (Rajendran et al. 2022). Among the various bioremediation techniques, in situ remediation with bacteria has received more attention due to its low cost, quick results, operability, and eco-friendliness (Dhaliwal et al. 2020). However, the colonization and remediation efficiency of bacteria in HM-contaminated soils and the remediation efficiency of HM-contaminated soils are easily influenced by external conditions such as soil pH, water content, HM stress, nutrient sources, and competition from indigenous microorganisms (Zheng et al. 2022). Therefore, a suitable carrier for bacterial colonization and effective remediation must be found.
Biochar is a carbon-rich material produced by the anoxic pyrolysis of biomass (Wang and Wang 2019). It has a large specific surface area and is porous, allowing it to adsorb and fix HM in soils (Yang et al. 2021). When added to soil, biochar replenishes soil organic carbon (C) content (Ullah et al. 2023), regulates soil pH (Brichi et al. 2023), and increases soil cation exchange capacity (CEC), providing ideal conditions for bacterial survival. Additionally, biochar provides shelter and nutrition to bacteria (Zhao et al. 2020), and even stimulates them to remediate contamination (Guo et al. 2022; Zhou et al. 2022). Therefore, integrating biochar and bacteria is increasingly being used as a more efficient, adaptable, and sustainable remediation agent for HM-contaminated soils (Chen et al. 2021b; Zheng et al. 2022). Bibliometric statistics (Fig. 1a) reveal that research on biochar and bacteria as a complex bacterial agent has recently emerged (around 2010) and is rapidly developing, with an exponential increase in annual publications (Fig. 1b). Among the 724 articles related to bacteria-biochar in the 2022 web of science core database, 15 major topics were identified using the carrot2 system (www.carrot2.org), with the majority related to remediation of contaminated soil, heavy metals, and subtopics such as “Cr(VI) to Cr(III)” and “effects on Cd” (which accounted for 30.4% of the total number of articles posted), as shown in the visualization (Fig. 1c).
Discovering trends in “biochar and bacteria” research using bibliometric methods. a Flowchart of data collection for research articles in biochar-bacteria. b The annual and cumulative number of research articles on biochar-bacteria (Curve: the prediction based on the model equation; Inside: statistics of issuance types). c Articles published on the theme of bacteria-biochar in the Web of Science core database in 2022 are classified into 15 main topics (In brackets: the number of articles under this topic)
The interaction between biochar and bacteria can provide multiple benefits such as promoting plant growth, supporting HM mobilization, improving phytoextraction (Wu et al. 2019), as well as immobilizing or detoxifying HM to alleviate metal stress in crops (Cheng et al. 2020). Furthermore, crop waste can be recycled in a sustainable manner by being converted into biochar and can be used as a slow fertilizer for soil fertility. Although there have been many reviews discussing the benefits of biochar as a negative carrier for bacteria and a summary of the application of bacteria-loaded biochar in environmental remediation (Bolan et al. 2023; Zheng et al. 2022), there has been little attention to the sustainability of biochar-bacteria combinations in practical applications. The extensive use of biochar can lead to huge costs and potential environmental risks (Sabir et al. 2020). There is a need to explore the optimal biochar-to-bacteria ratio, clarify the relationship between biochar and bacteria, and ultimately develop inexpensive, efficient, and sustainable biochar-bacteria combination products. In this review, we investigate recent research on the use of biochar-bacteria combinations for the remediation of HM-contaminated soils, elaborating on their interactions with HM. Additionally, we discuss the mechanisms of direct biochar-bacteria combination-induced immobilization of HM or assisted hyperaccumulators for phytostabilization and phytoextraction. Finally, the influence of raw materials and processes on biochar-bacteria combinations is extensively discussed, which will contribute to the design and commercialization of biochar-bacteria combination remediation agents.
2 Biochar and its potential for remediation
2.1 Biochar properties and production
Biochar, an eco-friendly material, has enormous potential in the remediation of HM-contaminated soils owing to its unique structural properties (Zheng et al. 2022). Key factors that affect its remediation effectiveness include the raw material, application amount, and pH of biochar (Li et al. 2020). As an adsorptive porous material with a large specific surface area and electronegativity, biochar can adsorb metal ions such as Cu(II), Cd(II), Cr(III), and Pb(II), and this adsorption is positively correlated with the pH of the biochar, which is determined by the amount of negative surface charge (Ahmad et al. 2018). Biochar usually displays high CEC values and can release cations for exchange with HM in the soil, which can be trapped in an amorphous structure through complexation with oxygen-containing functional groups or surface deposition (Gholizadeh and Hu 2021). Furthermore, biochar has both oxidizing and reducing properties, acting as electron shuttles, allowing for direct or mediated microbial reduction of Cr(VI) to Cr(III) (Xu et al. 2019b). Biochar surfaces contain a very large number of oxygen-containing functional groups, which play a very important role in the adsorption of HM and can be effective in loading bacteria (Bolan et al. 2023).
The properties and structure of biochar are determined by the feedstock and processes used to produce it. Lignin-based biochar, at the same temperature, has greater C fixation and surface functional groups than cellulose-based biochar but possesses a smaller specific surface area (Wan et al. 2022). Additionally, hemicellulose, such as xylan, is the most efficient biochar for adsorption, surpassing cellulose and lignin biochar in adsorption experiments (Wan et al. 2020). The physicochemical characteristics of biochar are also affected by the temperature, heating rate, residence time, and air conditions during its production (Wang and Wang 2019). For instance, as the pyrolysis temperature increased, the ash content, pH, and specific surface area of rapeseed stem-derived biochar increased, whereas the pore size and number of functional groups decreased (Zhao et al. 2018).
Developing an optimized production process for biochar stands as a sustainable approach to enhance HM remediation via biochar (Qiu et al. 2022). In comparison to virgin biochar obtained through pyrolysis, alkali-activated biochar exhibits a more intricate pore structure and a greater specific surface area. Meanwhile, low-temperature pyrolysis yields biochar with elevated surface functional groups when contrasted with high-temperature pyrolysis. These production processes collectively hold the key to enhancing the biochar’s binding capacity to HMs. Modifying the functional groups on biochar also presents a viable strategy to heighten the efficiency of HM remediation (Wang et al. 2021c).
An in-depth investigation into the elimination mechanisms of various pollutants by biochar utilizing theoretical calculations and molecular simulation techniques can provide insights into the distinct contributions of different functional groups to pollutant binding (Qiu et al. 2022). This understanding, in turn, can inform the design of biochar tailored for optimal usage in the remediation of HM-contaminated soils.
2.2 Biochar–bacteria interaction
When biochar is added to the soil, it interacts with microflora, specifically with bacteria, as illustrated in Fig. 2. Biochar’s effect on bacteria can be categorized into two types: direct and indirect effects (Zhu et al. 2017). The influence of biochar’s abundant functional groups on the surface, which can either support or inhibit bacterial growth and activity, is referred to as direct effects. Furthermore, biochar’s high electrical conductivity can aid in bacterial electron transfer. Indirect effects occur as a result of changes in the soil that bacteria inhabit. Current research has focused on the possible effects of biochar on bacteria (Bolan et al. 2023; Zheng et al. 2022), while the effects of bacteria on biochar have been less studied. Bacteria respond to the stresses imposed on by biochar, which can alter biochar’s chemical and physical properties, leading to its degradation and transformation, as well as accelerating its aging, ultimately impacting bacterial survival. The integration of biochar and bacteria as remediation agents for HM-contaminated soils can only be facilitated if the possible interactions between biochar and bacteria are clarified.
Interactions between biochar and bacteria. a Biochar directly affects bacteria in the following ways: (1) Biochar stimulates or inhibits bacterial growth; (2) Biochar acts as an electron shuttle, receiving and transmitting electrons from bacteria but also transmitting electrons to bacteria; (3) Biochar can act as a mediator for inter-bacterial communication; and (4) Biochar can act as a shelter for bacteria to help them resist stress. b Biochar can help bacteria resist stress by changing the soil environment in which they live indirectly on bacteria. c Bacteria have the potential to alter the physical properties of biochar. The dashed arrows indicate changes in the morphology of biochar under the influence of bacteria. d Bacteria cause changes in the chemical properties of biochar. Cation exchange capacity (CEC); carbon (C); oxygen (O); increase (↑); decrease (↓)
2.2.1 Direct impact of biochar on bacteria
Exogenous microorganisms face challenges when colonizing soils due to competition with indigenous microorganisms and difficulty in adapting to the soil environment and climate (Zheng et al. 2022; Zhu et al. 2017). However, biochar can act as a protective barrier for bacteria, as depicted in Fig. 2a. With its loose and porous structure and large specific surface area, biochar is an ideal environment for bacterial colonization. Furthermore, bacteria residing in biochar can adapt more quickly to environmental stresses, such as high or low temperatures, salt, and HM (Zhang et al. 2023). Aged biochar provides bacteria with a longer colonization time, potentially due to its improved mechanical properties, which reduce the fragmentation of biochar particles and limit the release of unstable components (Wang et al. 2020). For instance, the biochar derived from vegetable wastes effectively adsorbed heavy metals (Pb and Cd) and reduced the Pb and Cd toxicity to soil microbes (Huang et al. 2023).
It is still debatable whether biochar promotes or inhibits bacterial growth (Fig. 2a). On the one hand, biochar is rich in nutrients and can be used to feed bacteria (Zhang et al. 2020a). Furthermore, efficient contact of sp2hybridC with bacteria has been shown to stimulate the growth of certain bacteria (Ouyang et al. 2022), leading to reports that biochar can enhance bacterial growth and metabolism (Guo et al. 2022; Yan et al. 2022; Zhou et al. 2022). On the other hand, biochar may also have a harmful impact on bacteria (inhibiting bacterial cell division and growth rate), with potential toxicity resulting from the presence of hazardous substances (Godlewska et al. 2021), such as polycyclic aromatic hydrocarbons (PAHs), volatile organic compounds (VOCs), HMs, produced during pyrolysis (Godlewska et al. 2021; Zheng et al. 2022). Furthermore, physical properties such as the particle size or morphology of biochar can cause cellular damage to bacteria (Prodana et al. 2019). The total amount of hazardous substances present in biochar is determined by its biomass and production method, but the extent of its toxicity to bacteria is determined by the bioavailability of these hazardous substances (Godlewska et al. 2021). Thus, it is important to consider the toxicity of biochar during fabrication and modify it appropriately to reduce its toxicity (Konczak et al. 2020). Reducing the amount of biochar used also needs to be considered when actually designing remediation agents for biochar and bacteria; low concentrations of toxicity often tend to produce toxic excitatory effects in bacteria, which stimulate multiple bacterial functions, and reduce the risks they may pose.
Moreover, biochar has a significant direct impact on bacteria by promoting extracellular electron transfer (Fig. 2a), which is a newly discovered energy metabolism process of microbes that involves the transfer of electrons from intracellular oxidation to extracellular reduction (Zhao et al. 2021). This process leads to energy production and can enhance many metabolic activities of microorganisms under anaerobic conditions (Zhao et al. 2021). Biochar possesses high electrical conductivity and functional characteristics similar to soil redox-active organic matter (Zheng et al. 2022). As an electron shuttle (Van der Zee and Cervantes 2009), biochar obtained electrons from bacteria and then transferred these electrons to electron acceptors such as iron (Fe) oxides to enhance the reduction of the electron acceptors (Pascual et al. 2020). It can accept electrons from bacteria, and in specific metabolic processes, it can also accept electrons from other bacteria, thus transmitting communication signals between microorganisms and serving as a bridge for bacterial communication (Zhang et al. 2019b, 2021a). Bacterial communication is crucial in the environment (Paquete et al. 2022), and biochar can influence bacterial competition by absorbing or hydrolyzing signaling molecules, altering their communication (Zheng et al. 2022).
Given that the physicochemical attributes of biochar are shaped by both its source materials and production method, these very factors also impact its direct influence on bacteria. Aspects like pore dimensions, functional groups, and pH of biochar play a pivotal role in shaping bacterial colonization (Bolan et al. 2023). Thus, comprehending the interplay between biochar and bacteria is imperative for tailoring biochar materials that facilitate bacterial colonization effectively.
2.2.2 Indirect impact of biochar on bacteria
Biochar’s indirect impact on bacteria is primarily due to changes in living conditions (Fig. 2b). Biochar has the ability to improve soil pH, reduce soil bulk, and increase soil water retention capacity (Zhang et al. 2021b). These improvements in soil physicochemical properties make it easier for bacteria to survive. Biochar improves soil organic C content (Guo et al. 2020), and soil C supplementation improves soil bacterial abundance (Yan et al. 2022). Furthermore, the improvements in soil physicochemical properties increase the fixation of HM and other harmful soil substances, which reduces their biological impact on bacteria (Chen et al. 2021b).
Soil enzymes are important in the decomposition and mineralization of organic matter by microbes. As soil enzyme activity is highly sensitive to environmental factors, it is commonly used as an indicator to assess the impact of various factors on soil bacteria (Liao et al. 2022). Researchers reported that the sucrose and urease in the soil are increasing considerably while increasing the biochar dosages since the biochar application significantly releases organic matter as well as nutrient levels in the soil, and that facilitates suitable conditions for microbial growth and enzyme activity (e.g., redox enzymes) (Tomczyk et al. 2020; Wang et al. 2022a). A meta-analysis revealed that biochar produced at a temperature lower than 500 °C had a significant positive effect on activities of nitrogen (N) and phosphorus (P) related enzymes, and DHA enzymes (Liao et al. 2022).
Biochar integration into soils can increase soil CEC, improving nutrient retention and promoting soil microbial colonization (Wu et al. 2019). Additionally, biochar contains various nutrients that can stimulate bacterial growth and acts as a slow-release fertilizer, resulting in long-term benefits for soil fertility and bacterial growth (Zhang et al. 2020a).
2.2.3 Bacteria accelerate the aging of biochar
Biochar, which comprises mainly recalcitrant C, is degraded very slowly by bacteria, with a reported half-life of about 1000 years (Wang et al. 2020). However, short-term bacterial aging of biochar can be concerning. Bacteria can consume unstable C (such as aliphatic C) and contribute to organic matter dissolution from biochar (Quan et al. 2020). For instance, ryegrass biochar decomposed in soil for 3.2 years showed rapid C decomposition during the first 30 days, followed by a sharp decrease in rate (almost 100-fold) and stabilization after 90 days (Kuzyakov et al. 2009). This decomposition of unstable C and surface oxidation can induce physical changes in biochar (Fig. 2c). For instance, under scanning electron microscopy (SEM), the collapse of rice husk biochar that had aged naturally for 3 years in the Qinghai Tibetan Plateau is noticeable (Rafiq et al. 2020). Similarly, pine bark biochar artificially aged for 10 years displays larger pores than fresh biochar (Zha et al. 2015). Biochar aging can also convert micropores to mesopores and increase biochar pore volume, as demonstrated by the N2 adsorption isotherm (Hua et al. 2020; Wang et al. 2020). This expansion or enlargement of biochar pores causes a change in specific surface area. Several studies have shown that bacterial aging of biochar can increase its specific surface area (Li et al. 2019), providing more binding sites for contaminants and making it more suitable for HM immobilization and microbial colonization (Pei et al. 2021).
During bacterial aging, biochar undergoes changes in its chemical composition (Fig. 2d). Degradation of aliphatic C reduces the C content of biochar while increasing its aromaticity, which accounts for the relative stability of aged biochar (Wang et al. 2020; Zeba et al. 2022). The Fourier Transform Infrared Spectroscopy (FT-IR) and X-ray Photoelectron Spectroscopy (XPS) confirm that bacteria epoxidize part of the aromaticity into oxygen-containing functional groups (e.g., carboxyl, hydroxyl, carbonyl, etc.) on the surface of biochar (Tan et al. 2020). This surface oxidation leads to an increase in the CEC of biochar (Wang et al. 2020), which benefits the fixation of HM ions and adsorption of soil inorganic minerals on the surface of biochar, as well as the increase in ash content (Kapoor et al. 2022).
Overall, bacterial aging of biochar can initially increase soil organic C and subsequently form more stable aged biochar, which increases the inorganic nutrient content of biochar, prolongs bacterial colonization time and improves HM immobilization for long-term soil pollution remediation (Hua et al. 2020; Zhao et al. 2020).
2.3 Ecotoxicological of biochar
To ensure the optimal selection of biochar, it is critical to have knowledge about the biomass source used in its production. Biochar is mainly derived from agricultural waste through a process of calcination carried out under low-oxygen and high-temperature conditions. However, in the case of plant straws already contaminated with pollutants, the resultant biochar may contain significant amounts of HMs, polyhydroxyalkanoates (PHAs), volatile organic compounds (VOCs), or other pollutants even after calcination (Godlewska et al. 2021; Sabir et al. 2020). Therefore, using such biochar as a remediation agent may lead to further soil contamination.
Several studies have indicated that bacteria possess the capability to degrade harmful substances present in biochar (Bolan et al. 2023). As a result, the integration of bacteria and biochar emerges as a safer strategy for remediating soils contaminated with HMs, compared to the use of biochar alone. Nevertheless, in practical application, elevating the dosage ratio of biochar may appear to enhance remediation outcomes (e.g., incorporating a substantial quantity of alkaline biochar to acidic soils for swift pH adjustment). However, this approach could result in increased costs and potential environmental challenges. When integrating biochar and bacteria to remediate HM-contaminated soils, the primary role should be assigned to bacteria. Biochar should be applied sparingly, serving to safeguard bacterial colonization of the soil and potentially enhance certain functions. Thus, it is advisable to minimize the quantity of biochar, employing it primarily as a supportive carrier for the bacteria.
3 Plant-beneficial bacteria and their use in bioremediation
3.1 Plant-beneficial bacteria
In the context of integrating biochar and bacteria to remediate soils contaminated with HMs, our attention pivots toward the bacteria employed to sidestep potential risks linked with excessive biochar usage. As a result, the selection of bacterial species emerges as a critically important consideration. In recent times, there has been widespread utilization of plant-beneficial bacteria (PBB) in research endeavors, either for engaging in the bioremediation of HM-contaminated soils or for supporting phytoremediation through the stimulation of plant growth (Wang et al. 2022b). Microbes have been linked to promoting plant growth, with certain bacteria offering either direct or indirect benefits to plants and are referred to as PBB (Chiaranunt and White 2023). PBB can be categorized into different types such as plant growth-promoting rhizobacteria (PGPR), phyllosphere bacteria, endophytic bacteria, rhizobia, and cyanobacteria, based on their mode of action on plants (Orozco-Mosqueda et al. 2021). PBB can provide nutrients and micronutrients to plants through N fixation, P and potassium (K) solubilization, and siderophore production (Cao et al. 2023). Additionally, they can produce phytohormones (such as indole-3-acetic acid, cytokinin, gibberellin, ethylene, and abscisic acid) that aid in faster growth and help plants cope with stress (Ma et al. 2020b, 2022).
3.2 Role of PBB in metal bioremediation/phytoremediation
Metal-tolerant/resistant PBB can respond to metal stress in a specific way to achieve metal remediation in HM-contaminated soils (Sreedevi et al. 2022; Yin et al. 2019). Common metal-tolerant/resistant PBB species include Bacillus sp., Enterobacter sp., Klebsiella sp., Serratia sp., Agrobacterium sp., Azotobacter sp., Pseudomonas sp., and Alcaligenes sp. (Wang et al. 2022b). Moreover, the interaction between PBB and plants can influence each other’s tolerance to HMs and synergistically promote the efficiency of bioremediation or phytoremediation of HM pollution (Fig. 3) (Ma et al. 2011; Nivetha et al. 2023).
Role of plant-beneficial bacteria in metal bioremediation and phytoremediation. a PBB’s direct action on metals includes the following mechanisms: (1) conversion mechanism; (2) detoxification mechanism; (3) stabilization mechanism; and (4) activation mechanism. PBB can assist plants in dealing with HM stress (Modes 1–3) and promote metal uptake by plants (Mode 4). Meanwhile, PBB can facilitate plant protection and nutrient utilization; produce chemosensitive substances to regulate plant growth and development and protect plants from pathogens; help plants adapt to alleviate stress, and indirectly promote phytoremediation of metal-contaminated soils (Modes 5–7). The solid line represents the metal transfer process, while the dashed line represents the action of bacteria on metals to aid phytoremediation. Extracellular polymer substances (EPS), heavy metals (HMs)
3.2.1 Bioremediation
Bacteria, including metal-tolerant/resistant strains, are ubiquitous in HM-contaminated soils and are frequently used to remediate these contaminants. Diverse bacteria exhibit varying levels of tolerance to different HMs. For instance, the majority of Enterobacter and Klebsiella strains display higher tolerance toward Cd2+ and Pb2+, whereas Agrobacterium and Rhodococcus demonstrate robust resistance to As3+ and As5+, respectively (Henao and Ghneim-Herrera 2021). Additionally, the strategies employed by different bacteria to counteract the effects of HMs vary, and these variations are associated with the functional groups on the bacterial surface and the chemicals they produce (Wang et al. 2022b). Overall, many of these bacteria have been identified as PBB (Henao and Ghneim-Herrera 2021). The oxygen-containing groups and amines found in the polysaccharide mucus layer of PBB have the ability to adsorb metal ions, with an adsorption capacity ranging from 1 to 500 mg/g (Yin et al. 2019). EPS, such as proteins, lipids, and other macromolecules, can also adsorb HMs to protect PBB (Wang et al. 2022b), thereby immobilizing the HMs and reducing their mobility and impact on plants. When HMs penetrate PBB cells, bacteria-secreted enzymes can alter the redox state of the metals and reduce their toxicity (Yin et al. 2019). For instance, some PBB-containing merA genes have been shown to efficiently reduce Hg(II) to Hg(0), with conversion rates of up to 100% (Giovanella et al. 2016; Zhang et al. 2012).
3.2.2 PBB assisted-phytoremediation
PBB (e.g., Pseudomonas sp., Proteus sp., Streptomyces sp., Aspergillus sp., Bacillus sp., Acinetobacter sp., etc.) can directly or indirectly affect the phytoremediation of HMs by releasing various metabolites (e.g., siderophores, organic acids, plant growth regulators, and biosurfactants) (Ma et al. 2011). Phytoremediation of HMs is often hindered by environmental stress (e.g., soil pH, temperature, metal form and availability, essential nutrient scarcity, etc.), which reduces phytoremediation efficiency (Wang et al. 2022b). PBB can reduce the amount of ethylene secreted by aminocyclopropane carboxylic-acid (ACC) deaminase produced by plant roots, improve plant nutrient availability, and promote the production of hormones that stimulate plant growth. Additionally, PBB can also increase plant resistance to pathogenic agents (Rostami and Azhdarpoor 2019). By increasing the amount of plant biomass and inter-root secretions through the above mechanisms, PBB indirectly promote phytoremediation.
PBB directly aid phytoremediation by mobilizing or immobilizing metals (Ma et al. 2011). They can change the solubility, availability, and transport of HMs and nutrients by altering soil pH, releasing chelators, solubilizing phosphate, or inducing redox changes, thereby aiding in HM phytoextraction (Ma et al. 2011; Rostami and Azhdarpoor 2019). For instance, metal-resistant endophytic bacteria isolated from Sedum plumbizincicola were found to enhance the uptake of Cd and zinc (Zn) by 43% and 18% in plants, respectively, while promoting their growth (Ma et al. 2015). Similarly, exogenous arsenic-reducing bacteria have been shown to form biofilms on plant root surfaces, facilitating As uptake by plants (Liu et al. 2023). In addition, PBB can aid with HM detoxification, and endogenous PBB can improve the phytostabilization of HMs through uptake or redox reactions, reducing their toxicity to plants (Wang et al. 2022b).
4 Mechanism underlying biochar–bacteria-metal interactions
Biochar and bacteria have shown promising potential for remediating HMs in soil, while also being environmentally friendly and economically beneficial compared to traditional physical and chemical remediation methods. However, the practical application of using either biochar or bacteria alone has been limited due to their relatively low efficiency in HM remediation (Zheng et al. 2022). By integrating biochar and bacteria, the advantages of both can be combined, amplifying interactions with HMs. In Fig. 4, the mechanism of biochar-bacteria affecting metal is summarized.
4.1 Ion exchange
The ion exchange reactions of metals on biochar and bacteria are classified as cation exchange and anion exchange (Qu et al. 2022a). Biochar is characterized by high CEC and anion exchange capacity (AEC), enabling the exchange of alkali metal ions (K+, Na+, Ca2+, and Mg2+ mainly on the surface of biochar) with the positively charged HMs such as Cr(III), Cd(II), Pb(II) (Qu et al. 2022a; Wu et al. 2021). Meanwhile, negatively charged Cr(VI) can be exchanged with SO42- on the biochar surface (Zhang et al. 2020b). Similarly, bacteria surfaces also contain exchangeable ions for HMs, and immobilized bacteria on biochar have greater ion exchange ability (Huang et al. 2020). For instance, if biochar consists of a high concentration of Mg ions as well as functional groups capable of forming bonds with HM ions to undergo resilient ion exchange. Hence, cation exchange dominates the process of metal adsorption. Biochar produced from different feedstocks will have different ion exchange capacities, with rice straw and chicken manure biochar having higher ion exchange capacity for Cd(II) than sludge biochar (Huang et al. 2020).
4.2 Metal immobilization
Metal cations typically act as central atoms to provide empty orbitals and can complex with electronegative functional groups (e.g., hydroxyl, carboxyl, phosphoryl, and amino groups) present on the surface of biochar and bacteria (Ahmad et al. 2018; Zheng et al. 2022). Upon interaction with bacteria, the aliphatic C portion connecting the aromatic C is broken down, leading to oxidation of the broken portion of the aromatic C and the introduction of more oxygen-containing groups. This process can enhance the complexation of metal ions (Wang et al. 2020).
The adsorption of HM by biochar-bacteria combinations is a combination of passive adsorption by biochar and active adsorption by bacteria (Huang et al. 2020). The higher surface charge of biochar results in electrostatic attraction and Van der Waals forces, which are the primary modes of physical retention of biochar (Hu et al. 2021). Bacteria, on the other hand, can sequester HMs by secreting EPS, which are primarily organic polymers such as polysaccharides, proteins, and lipids that attach to bacterial cell surfaces as capsules or mucus, and have a strong ability to bind HM (Huang et al. 2020; Wang et al. 2022b). Biochar can enhance bacterial survival and promote the production of EPS, which can in turn cover the surface of biochar and transfer HM adsorbed on biochar to the microorganisms via electrostatic gravitational force with accelerated transfer (Zheng et al. 2022).
Bacterial metabolites, such as sulfur ions, phosphate ions, and carbonate ions, can undergo precipitation reactions with metal ions, converting toxic HMs into non-toxic or low-toxic metal precipitates (Ji et al. 2022). Biochar also contributes to HM precipitation by increasing pH (Xu et al. 2018). However, bacterial HM precipitation is influenced by nucleation sites, coexisting ions, pH, and redox potential (Chen et al. 2021a). When exogenously added bacterial agents are used for remediation, indigenous microorganisms can affect the treatment’s effectiveness (Lin et al. 2023). The addition of biochar can improve the bacterial survival environment, provide redox sites, and protect the bacterial agent from indigenous microorganisms, thus enhancing precipitation efficiency (Zhu et al. 2017). Phosphate-solubilizing bacteria (PSB) are commonly used for HM remediation by liberating phosphate from insoluble phosphate complexes and forming stable precipitates with HMs (Xu et al. 2019a). When loaded onto biochar materials, PSB can handle higher Pb(II) concentrations, thus improving treatment efficiency (Qu et al. 2022b).
4.3 Metal detoxification
Valence alteration is frequently regarded as the primary mechanism of the detoxification of variable valence metals (Xia et al. 2021). When bacteria actively take up or adsorb HMs, redox reactions usually occur (Wang et al. 2022b). For instance, Alishewanella sp. WH16-1 can reduce Se(IV) and Cr(VI) to Se(0) and Cr(III) nanoparticles, respectively (Xia et al. 2018), while Pseudomonas sp. B50A can effectively reduce Hg(II) to Hg(0) (Giovanella et al. 2016). The hydroquinone functional groups and persistent free radicals on the surface of biochar can reduce Cr(VI) to Cr(III) and adsorb it on the biochar surface, respectively (Zhang et al. 2019a). Biochar provides a safe haven for bacteria to perform their detoxification function even in a high-HM environment, and the persistent radicals on the surface of biochar can act as electron shuttles to promote the redox of HMs by bacteria (Narayanan and Ma 2022). Furthermore, wheat straw biochar can stimulate the heterotrophic iron-reducing strain Shewanella oneidensis MR-1 to produce Fe(II) and immobilize it on the biochar surface for the removal of Cr(VI) (Liu et al. 2021). Other than the valence changes, the chemical form changes of some heavy metals are of interest. For example, environmental microorganisms are able to convert inorganic As into organic As, which is further converted into volatile substances and reduce the As content in soils.
Altering the bioavailability of HMs is another important mechanism for HM detoxification induced by biochar-bacteria combinations. Cheng et al. (2020) found that combining rice husk biochar with Serratia liquefaciens CL-1 reduced soil Cd and Pb availability by 57% and 27%, respectively, which was twice as effective as using biochar or bacteria alone. Another study by Ma et al. (2020a) showed that coconut shell biochar and Bacillus sp. TZ5 decreased the proportion of acetic acid-extractable (HOAc-extractable) Cd in soils. Inoculating Pseudomonas NT-2 on biochar as shown by Tu et al. (2020) decreased the proportion of soil-exchangeable Cd and carbonate-bound Cu by 12.82% and 26.55%, respectively, reducing the plant availability of HMs.
4.4 Metal mobilization
The mobility of HMs in soils is considered a crucial factor influencing plant extraction efficiency (Ma et al. 2016). Bacteria have the ability to produce various organic acids (such as acetic acid, citric acid, malic acid, and gluconic acid), siderophores, and surfactants that can chelate with HMs, thereby enhancing their biological effectiveness (Wang et al. 2022b; Wu et al. 2019). Additionally, certain bacteria (e.g., Bacillus sp., Pseudomonas sp., Escherichia sp., Methylobacterium sp., Berknolderia sp., etc.) can facilitate the methylation of HMs such as Hg, Pb, and Se, and release them through volatilization (Bali and Sidhu 2021).
Bacterial HM mobilization frequently involves electron transfer, by which biochar can facilitate this transfer by acting as an electron shuttle (Zheng et al. 2022). Biochar produced from rape straw has been shown to improve the mobility of Se and Cd in soils by increasing bacterial Fe reduction (Lyu et al. 2022). In addition, biochar has been shown to protect Serratia sp. SNB6 from Cd while encouraging the growth of the metal hyper-enriched plant Chrysopogon zizanioides L., thereby enhancing the phytoextraction of Cd in soils with elevated Cd concentrations (Wu et al. 2019).
Generally, the adsorption of HMs by biochar can be attributed to a combination of the biochar’s physicochemical properties, the type of feedstock used, the processing techniques applied, and the influence of bacteria on enhancing these physicochemical characteristics. Bacterial involvement in HM interactions might experience decreased efficiency due to HM-induced stress, while the assistance of biochar helps alleviate this stress. The joint action of biochar and bacteria may play a more prominent role in facilitating phytoremediation through HM mobilization and detoxification. However, forthcoming research should prioritize clarifying the long-term durability and survivability of biochar-bacteria complexes in soil environments to establish their sustained impact on HM immobilization.
5 Implementation of biochar and bacteria for sustainable remediation
Through network analysis of keyword co-occurrence using VOSviewer as shown in Fig. 1a, we identified 2318 articles published within the last five years under the theme of biochar and bacteria. The research in this field has primarily focused on modern agriculture, with sustainable remediation (particularly immobilization and transformation) of heavy metals being a current research hotspot (Fig. 5).
Network analysis of keywords co-occurrence of articles with the theme of biochar and bacteria from 2018–2022. The keywords presented are those that appear more than 30 times, including author keywords as well as keywords plus (research topics related to the content of the paper). The size of the circle indicates the frequency of the keywords’ co-occurrence, and the color indicates the oldness of the article containing the keywords, with yellow representing the more recent average publication time and blue representing the older
The success of the integrated biochar and bacterial approach to soil remediation is often dependent on the mechanism of biochar-bacteria action on HMs (Table 1), with bacteria playing a particularly crucial role. For farmland contaminated with HMs, ceasing tillage can lead to significant economic losses, and the severity of HM contamination depends largely on its biological effects on crops. Therefore, adding biochar-bacteria combinations to immobilize HMs in farmland and reduce their biological effectiveness has become an attractive option (Qu et al. 2022b). Biochar-bacteria combinations have been shown to reduce metal accumulation in crop edible parts while improving crop quality (Cheng et al. 2020; Ma et al. 2020a; Sabir et al. 2020). For agricultural land, on the one hand, the concentration of HM is low, and on the other hand, the high cost limits the use of biochar. However, the current research is relatively high-volume for biochar use, which makes it difficult to be promoted on agricultural land. The amount of biochar used should be reduced, and the process of integrating biochar and bacteria should be simplified in order to reduce costs. For mine or site contamination, the focus is more on the biochar-bacteria combinations for the transformation of HMs or supporting phytoremediation for long-term removal. In a potting experiment, the Cr-reducing bacterium Bacillus cereus WHX-1 was immobilized on biochar added to Cr-contaminated soil, resulting in the conversion of 94.22% of Cr(VI) to Cr(III) (Chen et al. 2021b). Combining biochar and PSB can increase microbial abundance in Pb/Cd contaminated soils in mining areas and significantly increase the acid-soluble fraction of Pb/Cd by 5 and 14 times, respectively (Lai et al. 2022). Bacteria-loaded biochar can also enhance the phytoremediation process when carefully designed and thoughtfully applied (Harindintwali et al. 2020). The biochar-PGPR-accumulator system formed by loading biochar with Serratia sp. SNB6 and C. zizanioides L. has been shown to effectively increase soil HOAc-extractable Cd content and metal phytoextraction in Cd-contaminated soil (Wu et al. 2019). In addition to the type of land used for HM-contaminated soils, the physicochemical properties of the soil are also important to consider when using integrated biochar and bacteria for HM-contaminated soils. Biochar-bacteria combinations can be used to remediate acidic and saline soils (Kari et al. 2021; Liang et al. 2023), but it depends on what kind of biochar and what kind of bacteria are combined. Pyrolytic biochar is alkaline and is often used to remediate acidic soils, while hydrothermally prepared biochar is acidic and can be used to remediate saline soils. Regardless of how heavy metals are treated, it is worth noting that the combination of biochar and bacteria can improve the soil environment by adjusting soil pH and capacitance, increasing soil organic C content, nutrient and enzyme activity, and increasing soil organic C content (Gou et al. 2023), making it a sustainable soil remediation agent.
Aside from bacterial selection, the choice of biochar raw material also affects the efficacy and mechanism of HM remediation by biochar-bacteria combinations Huang et al. (2020) conducted a study using various biochar materials loaded with B. cereus RC-1 to immobilize Cd. They found that the predominant mode of Cd immobilization by biochar made from rice straw and chicken manure loaded with bacteria was ion exchange, whereas the mode of Cd immobilization by sewage sludge biochar loaded with Cd fixation by bacteria was mainly complexation, resulting in much lower efficiency compared to the former two groups.
Furthermore, how bacteria and biochar are combined is an important factor to consider in order to improve the effectiveness of the hybrid material and achieve commercial production. The process of immobilizing bacteria onto biochar can be done in a number of ways, including adsorption, electrostatic interactions, and covalent binding to allow bacteria to attach to the surface and within the pores of the biochar, while stabilizers such as alginate can also coat the biochar and bacteria for the production of biochar-bacteria composites (Bolan et al. 2023). The different ways of integrating biochar and bacteria will bring about different remediation effects on HM-contaminated soils. Chen et al. (2021b) demonstrated that the composite material, which combined Bacillus sp. WHX-1 and biochar converted Cr(VI) to Cr(III) at a 12.8% higher rate compared to directly adding equal amounts of bacteria and biochar in Cr-contaminated soils. Refinement of biochar-bacteria combinations can also yield more efficient and targeted materials. For instance, Wang et al. (2021a) showed that modification of biochar-bacteria combinations with Fe3O4 resulted in superior Cd remediation performance in paddy soil compared to unmodified materials. Similarly, Qu et al. (2022b) improved the efficiency of Pb elimination by over 3 times by loading carboxymethyl cellulose and FeS onto biochar-PSB. It is worth mentioning that while complex processes can lead to better restoration results, they also tend to be more costly. It is very important to design cheaper and more efficient biochar-bacteria combinations according to the actual situation.
In Table 1, both biochar and bacteria independently exhibit some capability for remediating HM-contaminated soils. When comparing their individual remediation efficiencies, biochar predominantly assumes a major role in immobilizing metals within the biochar-bacteria context, while bacteria are more instrumental in facilitating metal detoxification and mobilization. Nonetheless, regardless of the metal interaction mechanism, the integration of biochar and bacteria consistently enhances the overall remediation effectiveness. Among the enhancements observed in soil physicochemical properties, biochar primarily elevates soil total organic carbon (TOC) levels and reduces bulk density, whereas bacteria primarily enhance soil enzyme activities. However, the question arises: Is there an optimal ratio between biochar and bacteria? Wang et al. (2021b) used software simulation to determine that a 1.1:1 (w/w) ratio of straw biochar to Bacillus sp. K1 yielded maximum Cd adsorption capacity. Nevertheless, there remains an unexplored area regarding whether a reduction in biochar usage still maintains the efficacy of the biochar-bacteria combination and at what critical threshold this occurs. The reduction of biochar quantity remains imperative due to economic and environmental considerations. Leveraging bacteria as cost-effective remediation agents (Henao and Ghneim-Herrera 2021) while employing minimal amounts of biochar to shield bacteria from soil colonization and potentially enhance their functions, appears to be a promising strategy for the integration of biochar and bacteria in a remediation framework.
6 Conclusion and prospects
The potential mechanisms and broad applications of biochar-bacteria combinations in HM bioremediation/phytoremediation are extensively discussed in this paper. A comparative analysis of the effectiveness of employing biochar and bacteria alone or in combination for remediating HM-contaminated soils reveals distinct primary remediation mechanisms for each approach. However, the interaction between biochar and bacteria enhances the efficacy of both in metal remediation, as biochar improves soil physicochemical characteristics and supports microbial populations by providing nutrients and shelter. The resulting biochar-bacteria-metal interactions create conditions for these composites to be used as remediation agents for soil contaminated with HMs, providing the possibility of long-term remediation. However, practical applications require careful consideration of certain factors:
-
1)
Integrating biochar and bacteria to remediate HM-contaminated soils has been proven effective. However, the dosage and addition methods used are still vague, and many experimental studies involve mixing large doses of biochar and bacteria directly into the soil. Such techniques are clearly not suitable for large-scale replication, and the use of large amounts of biochar may bring unaffordable costs or even secondary contamination. Therefore, it is necessary to investigate the safety of using biochar-bacteria combinations, consideration should also be given to reducing the amount of biochar and designing a more efficient remediation agent.
-
2)
The use of agricultural waste as the raw material for biochar production poses a challenge in controlling the consistency of feedstock components, leading to variations in the properties of different batches of biochar during mass production. This inconsistency can hinder the mass production of biochar-bacteria combined agents.
-
3)
In order to facilitate the practical use of biochar-bacteria combinations in mass production, it is important to consider their shelf life and stability, given the need for long-term transportation and storage. Furthermore, the stability of the combinations should also be a key consideration for future studies. Changing the way of integrating biochar and bacteria, or even making them into remediation agents with stabilizers such as alginate, would be a good decision for their commercialization.
-
4)
To utilize biochar-bacteria combinations in natural environments, their stability in such conditions, including extreme weather, drought, and salinity must also be taken into consideration. At the same time, the effectiveness of remediation in the case of complex pollution needs to be investigated.
Minimizing biochar consumption stands out as a prime strategy to enhance the economic viability and environmental sustainability of biochar-bacteria amalgamations. Future research should place increased emphasis on investigating the intricate interplay between biochar and bacteria. When ascertaining the optimal ratio between biochar and bacteria, the focus ought to shift from the singular goal of maximizing HM remediation to prioritizing the achievement of the utmost returns. Additionally, there is potential for exploring modified biochar variants that exhibit heightened stability and improved capacity to enhance bacterial functionalities.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ahmad Z, Gao B, Mosa A, Yu HW, Yin XQ, Bashir A, Ghoveisi H, Wang SS (2018) Removal of Cu(II), cd(II) and pb(II) ions from aqueous solutions by biochars derived from potassium-rich biomass. J Clean Prod 180:437–449. https://doi.org/10.1016/j.jclepro.2018.01.133
Bali AS, Sidhu GPS (2021) Arsenic acquisition, toxicity and tolerance in plants-from physiology to remediation: a review. Chemosphere 283:22. https://doi.org/10.1016/j.chemosphere.2021.131050
Bolan S, Hou DY, Wang LW, Hale L, Egamberdieva D, Tammeorg P, Li R, Wang B, Xu JP, Wang T, Sun HW, Padhye LP, Wang HL, Siddique KHM, Rinklebe J, Kirkham MB, Bolan N (2023) The potential of biochar as a microbial carrier for agricultural and environmental applications. Sci Total Environ 886:19. https://doi.org/10.1016/j.scitotenv.2023.163968
Brichi L, Fernandes JVM, Silva BM, Vizu JD, Junior JNG, Cherubin MR (2023) Organic residues and their impact on soil health, crop production and sustainable agriculture: a review including bibliographic analysis. Soil Use Manage. https://doi.org/10.1111/sum.12892
Cao MY, Narayanan M, Shi XJ, Chen XP, Li ZL, Ma Y (2023) Optimistic contributions of plant growth-promoting bacteria for sustainable agriculture and climate stress alleviation. Environ Res 217:13. https://doi.org/10.1016/j.envres.2022.114924
Chen X, Zhang D, Larson SL, Ballard JH, Knotek-Smith HM, Nie J, Hu N, Ding DX, Han FXX (2021a) Microbially induced carbonate precipitation techniques for the remediation of heavy metal and trace element-polluted soils and water. Water Air Soil Pollut 232:15. https://doi.org/10.1007/s11270-021-05206-z
Chen YY, Wu HX, Sun P, Liu JX, Qiao SX, Zhang DK, Zhang ZM (2021b) Remediation of chromium-contaminated soil based on Bacillus cereus WHX-1 immobilized on biochar: Cr(VI) transformation and functional microbial enrichment. Front Microbiol 12:10. https://doi.org/10.3389/fmicb.2021.641913
Cheng C, Han H, Wang YP, Wang R, He LY, Sheng XF (2020) Biochar and metal-immobilizing Serratia liquefaciens CL-1 synergistically reduced metal accumulation in wheat grains in a metal-contaminated soil. Sci Total Environ 740:9. https://doi.org/10.1016/j.scitotenv.2020.139972
Chiaranunt P, White JF (2023) Plant beneficial bacteria and their potential applications in vertical farming systems. Plants-Basel 12:27. https://doi.org/10.3390/plants12020400
Dhaliwal SS, Singh J, Taneja PK, Mandal A (2020) Remediation techniques for removal of heavy metals from the soil contaminated through different sources: a review. Environ Sci Pollut R 27:1319–1333. https://doi.org/10.1007/s11356-019-06967-1
Gholizadeh M, Hu X (2021) Removal of heavy metals from soil with biochar composite: a critical review of the mechanism. J Environ Chem Eng 9:25. https://doi.org/10.1016/j.jece.2021.105830
Giovanella P, Cabral L, Bento FM, Gianello C, Camargo FAO (2016) Mercury (II) removal by resistant bacterial isolates and mercuric (II) reductase activity in a new strain of Pseudomonas sp. B50A. New Biotech 33:216–223. https://doi.org/10.1016/j.nbt.2015.05.006
Godlewska P, Ok YS, Oleszczuk P (2021) The dark side of black gold: Ecotoxicological aspects of biochar and biochar-amended soils. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2020.123833
Guo MX, Song WP, Tian J (2020) Biochar-facilitated soil remediation: mechanisms and efficacy variations. Front Environ Sci. https://doi.org/10.3389/fenvs.2020.521512
Guo SS, Liu XM, Wang L, Liu QL, Xia CQ, Tang JC (2022) Ball-milled biochar can act as a preferable biocompatibility material to enhance phenanthrene degradation by stimulating bacterial metabolism. Bioresour Technol 350:10. https://doi.org/10.1016/j.biortech.2022.126901
Gou ZC, Zheng HY, He ZQ, Su YJ, Chen SJ, Chen H, Chen G, Ma NL, Sun Y (2023) The combined action of biochar and nitrogen-fixing bacteria on microbial and enzymatic activities of soil N cycling. Environ Pollut 317:10. https://doi.org/10.1016/j.envpol.2022.120790
Harindintwali JD, Zhou JL, Yang WH, Gu QY, Yu XB (2020) Biochar-bacteria-plant partnerships: eco-solutions for tackling heavy metal pollution. Ecotox Environ Safe 204:14. https://doi.org/10.1016/j.ecoenv.2020.111020
Henao SG, Ghneim-Herrera T (2021) Heavy metals in soils and the remediation potential of bacteria associated with the plant microbiome. Front Env Sci 9:17. https://doi.org/10.3389/fenvs.2021.604216
Hu FN, Xu CY, Ma RT, Tu K, Yang JY, Zhao SW, Yang MY, Zhang FB (2021) Biochar application driven change in soil internal forces improves aggregate stability: based on a two-year field study. Geoderma 403:10. https://doi.org/10.1016/j.geoderma.2021.115276
Hua Y, Zheng XB, Xue LH, Han LF, He SY, Mishra T, Feng YF, Yang LZ, Xing BS (2020) Microbial aging of hydrochar as a way to increase cadmium ion adsorption capacity: process and mechanism. Bioresour Technol 300:9. https://doi.org/10.1016/j.biortech.2019.122708
Huang F, Li K, Wu RR, Yan YJ, Xiao RB (2020) Insight into the Cd2+ biosorption by viable Bacillus cereus RC-1 immobilized on different biochars: roles of bacterial cell and biochar matrix. J Clean Prod 272:13. https://doi.org/10.1016/j.jclepro.2020.122743
Huang K, Zhang J, Tang GM, Bao D, Wang TY, Kong DP (2023) Impacts and mechanisms of biochar on soil microorganisms. Plant Soil Environ 10. https://doi.org/10.17221/348/2022-pse
Ji XW, Wan J, Wang XD, Peng C, Wang GH, Liang WY, Zhang W (2022) Mixed bacteria-loaded biochar for the immobilization of arsenic, lead, and cadmium in a polluted soil system: Effects and mechanisms. Sci Total Environ 811:10. https://doi.org/10.1016/j.scitotenv.2021.152112
Kapoor A, Sharma R, Kumar A, Sepehya S (2022) Biochar as a means to improve soil fertility and crop productivity: a review. J Plant Nutr 45:2380–2388. https://doi.org/10.1080/01904167.2022.2027980
Kari A, Nagymate Z, Romsics C, Vajna B, Toth E, Lazanyi-Kovacs R, Rizo B, Kutasi J, Bernhardt B, Farkas E, Marialigeti K (2021) Evaluating the combined effect of biochar and PGPR inoculants on the bacterial community in acidic sandy soil. Appl Soil Ecol 160:10. https://doi.org/10.1016/j.apsoil.2020.103856
Konczak M, Pan B, Ok YS, Oleszczuk P (2020) Carbon dioxide as a carrier gas and mixed feedstock pyrolysis decreased toxicity of sewage sludge biochar. Sci Total Environ 723:10. https://doi.org/10.1016/j.scitotenv.2020.137796
Kuzyakov Y, Subbotina I, Chen HQ, Bogomolova I, Xu XL (2009) Black carbon decomposition and incorporation into soil microbial biomass estimated by C-14 labeling. Soil Biol Biochem 41:210–219. https://doi.org/10.1016/j.soilbio.2008.10.016
Lai WW, Wu YY, Zhang CN, Dilinuer Y, Pasang L, Lu YQ, Wang YH, Chen HM, Li Z (2022) Combination of biochar and phosphorus solubilizing bacteria to improve the stable form of toxic metal minerals and microbial abundance in lead/cadmium-contaminated soil. Agronomy-Basel 12:17. https://doi.org/10.3390/agronomy12051003
Li HX, Lu XQ, Xu Y, Liu HT (2019) How close is artificial biochar aging to natural biochar aging in fields? A meta-analysis. Geoderma 352:96–103. https://doi.org/10.1016/j.geoderma.2019.06.006
Li XP, Wang CB, Zhang JG, Liu JP, Liu B, Chen GY (2020) Preparation and application of magnetic biochar in water treatment: a critical review. Sci Total Environ 711:14. https://doi.org/10.1016/j.scitotenv.2019.134847
Liang S, Wang SN, Zhou LL, Sun S, Zhang J, Zhuang LL (2023) Combination of biochar and functional bacteria drives the ecological improvement of saline-alkali soil. Plants-Basel 12:13. https://doi.org/10.3390/plants12020284
Liao XL, Kang H, Haidar G, Wang WF, Malghani S (2022) The impact of biochar on the activities of soil nutrients acquisition enzymes is potentially controlled by the pyrolysis temperature: a meta-analysis. Geoderma 411:13. https://doi.org/10.1016/j.geoderma.2021.115692
Lin H, Zhou MY, Li B, Dong YB (2023) Mechanisms, application advances and future perspectives of microbial-induced heavy metal precipitation: a review. Int Biodeterior Biodegrad 178:15. https://doi.org/10.1016/j.ibiod.2022.105544
Liu LC, Liu GF, Zhou JT, Jin RF (2021) Interaction between hexavalent chromium and biologically formed iron mineral-biochar composites: kinetics, products and mechanisms. J Hazard Mater 405:12. https://doi.org/10.1016/j.jhazmat.2020.124246
Liu YB, Zhang BY, Han YH, Yao Y, Guo P (2023) Involvement of exogenous arsenic-reducing bacteria in root surface biofilmformation promoted phytoextraction of arsenic. Sci Total Environ 858:13. https://doi.org/10.1016/j.scitotenv.2022.160158
Lyu C, Li L, Liu XW, Zhao ZQ (2022) Rape straw application facilitates Se and Cd mobilization in Cd-contaminated seleniferous soils by enhancing microbial iron reduction. Environ Pollut 310:12. https://doi.org/10.1016/j.envpol.2022.119818
Ma Y, Prasad MNV, Rajkumar M, Freitas H (2011) Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol Adv 29:248–258. https://doi.org/10.1016/j.biotechadv.2010.12.001
Ma Y, Oliveira RS, Nai FJ, Rajkumar M, Luo YM, Rocha I, Freitas H (2015) The hyperaccumulator Sedum plumbizincicola harbors metal-resistant endophytic bacteria that improve its phytoextraction capacity in multi-metal contaminated soil. J Environ Manage 156:62–69. https://doi.org/10.1016/j.jenvman.2015.03.024
Ma Y, Oliveira RS, Freitas H, Zhang C (2016) Biochemical and molecular mechanisms of plant-microbe-metal interactions: relevance for phytoremediation. Front Plant Sci 7:19. https://doi.org/10.3389/fpls.2016.00918
Ma H, Wei MY, Wang ZR, Hou SY, Li XD, Xu H (2020a) Bioremediation of cadmium polluted soil using a novel cadmium immobilizing plant growth promotion strain Bacillus sp. TZ5 loaded on biochar. J Hazard Mater 388:9. https://doi.org/10.1016/j.jhazmat.2020.122065
Ma Y, Dias MC, Freitas H (2020b) Drought and salinity stress responses and microbe-induced tolerance in plants. Front Plant Sci 11:18. https://doi.org/10.3389/fpls.2020.591911
Ma Y, Freitas H, Dias MC (2022) Strategies and prospects for biostimulants to alleviate abiotic stress in plants. Front Plant Sci 13:15. https://doi.org/10.3389/fpls.2022.1024243
Narayanan M, Ma Y (2022) Influences of biochar on bioremediation/phytoremediation potential of metal-contaminated soils. Front Microbiol 13:11. https://doi.org/10.3389/fmicb.2022.929730
Nivetha N, Srivarshine B, Sowmya B, Rajendiran M, Saravanan P, Rajeshkannan R, Rajasimman M, Pham THT, Shanmugam V, Dragoi EN (2023) A comprehensive review on bio-stimulation and bio-enhancement towards remediation of heavy metals degeneration. Chemosphere 312:31. https://doi.org/10.1016/j.chemosphere.2022.137099
Orozco-Mosqueda MD, Flores A, Rojas-Sanchez B, Urtis-Flores CA, Morales-Cedeno LR, Valencia-Marin MF, Chavez-Avila S, Rojas-Solis D, Santoyo G (2021) Plant growth-promoting bacteria as bioinoculants: attributes and challenges for sustainable crop improvement. Agronomy-Basel 11:15. https://doi.org/10.3390/agronomy11061167
Ouyang P, Liang CZ, Liu FS, Chen Q, Yan ZQ, Ran JY, Mou SY, Yuan Y, Wu X, Yang S-T (2022) Stimulating effects of reduced graphene oxide on the growth and nitrogen fixation activity of nitrogen-fixing bacterium Azotobacter chroococcum. Chemosphere 294:133702. https://doi.org/10.1016/j.chemosphere.2022.133702
Paquete CM, Rosenbaum MA, Baneras L, Rotaru A-E, Puig S (2022) Let’s chat: Communication between electroactive microorganisms. Bioresour Technol. https://doi.org/10.1016/j.biortech.2022.126705
Pascual MB, Sanchez-Monedero MA, Cayuela ML, Li S, Haderlein SB, Ruser R, Kappler A (2020) Biochar as electron donor for reduction of N2O by Paracoccus denitrificans. Fems Microbiol Ecol 96:11. https://doi.org/10.1093/femsec/fiaa133
Pei JM, Li JQ, Mia S, Singh B, Wu JH, Dijkstra FA (2021) Biochar aging increased microbial carbon use efficiency but decreased biomass turnover time. Geoderma 382:3. https://doi.org/10.1016/j.geoderma.2020.114710
Peng JY, Zhang S, Han YY, Bate B, Ke H, Chen YM (2022) Soil heavy metal pollution of industrial legacies in China and health risk assessment. Sci Total Environ 816:12. https://doi.org/10.1016/j.scitotenv.2021.151632
Prodana M, Silva C, Gravato C, Verheijen FGA, Keizer JJ, Soares A, Loureiro S, Bastos AC (2019) Influence of biochar particle size on biota responses. Ecotox Environ Safe 174:120–128. https://doi.org/10.1016/j.ecoenv.2019.02.044
Qiu MQ, Liu LJ, Ling Q, Cai YW, Yu SJ, Wang SQ, Fu D, Hu BW, Wang XK (2022) Biochar for the removal of contaminants from soil and water: a review. Biochar 4:25. https://doi.org/10.1007/s42773-022-00146-1
Qu JH, Shi JJ, Wang YH, Tong H, Zhu YJ, Xu LS, Wang YF, Zhang B, Tao Y, Dai X, Zhang H, Zhang Y (2022a) Applications of functionalized magnetic biochar in environmental remediation: a review. J Hazard Mater 434:17. https://doi.org/10.1016/j.jhazmat.2022.128841
Qu JH, Wei SQ, Liu Y, Zhang XM, Jiang Z, Tao Y, Zhang GS, Zhang B, Wang L, Zhang Y (2022b) Effective lead passivation in soil by bone char/CMC-stabilized FeS composite loading with phosphate-solubilizing bacteria. J Hazard Mater 423:11. https://doi.org/10.1016/j.jhazmat.2021.127043
Quan GX, Fan QY, Zimmerman AR, Sun JX, Cui LQ, Wang HL, Gao B, Yan JL (2020) Effects of laboratory biotic aging on the characteristics of biochar and its water-soluble organic products. J Hazard Mater 382:9. https://doi.org/10.1016/j.jhazmat.2019.121071
Rafiq MK, Bai YF, Aziz R, Rafiq MT, Masek O, Bachmann RT, Joseph S, Shahbaz M, Qayyum A, Shang ZH, Danaee M, Long RJ (2020) Biochar amendment improves alpine meadows growth and soil health in tibetan plateau over a three year period. Sci Total Environ 717:11. https://doi.org/10.1016/j.scitotenv.2019.135296
Rajendran S, Priya TAK, Khoo KS, Hoang TKA, Ng HS, Munawaroh HSH, Karaman C, Orooji Y, Show PL (2022) A critical review on various remediation approaches for heavy metal contaminants removal from contaminated soils. Chemosphere 287:14. https://doi.org/10.1016/j.chemosphere.2021.132369
Rostami S, Azhdarpoor A (2019) The application of plant growth regulators to improve phytoremediation of contaminated soils: a review. Chemosphere 220:818–827. https://doi.org/10.1016/j.chemosphere.2018.12.203
Sabir A, Naveed M, Bashir MA, Hussain A, Mustafa A, Zahir ZA, Kamran M, Ditta A, Nunez-Delgado A, Saeed Q, Qadeer A (2020) Cadmium mediated phytotoxic impacts in Brassica napus: managing growth, physiological and oxidative disturbances through combined use of biochar and Enterobacter sp. MN17. J Environ Manage 265:10. https://doi.org/10.1016/j.jenvman.2020.110522
Simiele M, Sferra G, Lebrun M, Renzone G, Bourgerie S, Scippa GS, Morabito D, Scaloni A, Trupiano D (2021) In-depth study to decipher mechanisms underlying Arabidopsis thaliana tolerance to metal(loid) soil contamination in association with biochar and/or bacteria. Environ Exp Bot 182:14. https://doi.org/10.1016/j.envexpbot.2020.104335
Sreedevi PR, Suresh K, Jiang GM (2022) Bacterial bioremediation of heavy metals in wastewater: a review of processes and applications. J Water Process Eng 48:15. https://doi.org/10.1016/j.jwpe.2022.102884
Tan LS, Sun CC, Wang Y, Wang TT, Wu GL, He HH, Zheng JY (2020) Changes in biochar properties in typical loess soil under a 5-year field experiment. J Soils Sediment 20:340–351. https://doi.org/10.1007/s11368-019-02398-0
Tomczyk A, Sokolowska Z, Boguta P (2020) Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev Environ Sci Bio-Technol 19:191–215. https://doi.org/10.1007/s11157-020-09523-3
Tu C, Wei J, Guan F, Liu Y, Sun YH, Luo YM (2020) Biochar and bacteria inoculated biochar enhanced cd and cu immobilization and enzymatic activity in a polluted soil. Environ Int 137:9. https://doi.org/10.1016/j.envint.2020.105576
Ullah S, Ali I, Yang M, Zhao Q, Iqbal A, Wu XY, Ahmad S, Muhammad I, Khan A, Adnan M, Yuan PL, Jiang LG (2023) Partial substitution of urea with biochar induced improvements in soil enzymes activity, ammonia-nitrite oxidizers, and nitrogen uptake in the double-cropping rice system. Microorganisms 11:20. https://doi.org/10.3390/microorganisms11020527
Van der Zee FR, Cervantes FJ (2009) Impact and application of electron shuttles on the redox (bio)transformation of contaminants: a review. Biotechnol Adv 27:256–277. https://doi.org/10.1016/j.biotechadv.2009.01.004
Wan J, Liu L, Ayub KS, Zhang W, Shen GX, Hu SQ, Qian XY (2020) Characterization and adsorption performance of biochars derived from three key biomass constituents. Fuel 269:7. https://doi.org/10.1016/j.fuel.2020.117142
Wan J, Liu L, Wang GH, Sang L, Liang WY, Zhang W, Peng C, Fu RB (2022) Unveiling the mechanisms of carbon conversion and loss in biochars derived from characteristic lignocellulosic biomass. J Environ Chem Eng 10:8. https://doi.org/10.1016/j.jece.2022.108403
Wang JL, Wang SZ (2019) Preparation, modification and environmental application of biochar: a review. J Clean Prod 227:1002–1022. https://doi.org/10.1016/j.jclepro.2019.04.282
Wang LW, O’Connor D, Rinklebe J, Ok YS, Tsang DCW, Shen ZT, Hou DY (2020) Biochar aging: mechanisms, physicochemical changes, assessment, and implications for field applications. Environ Sci Technol 54:14797–14814. https://doi.org/10.1021/acs.est.0c04033
Wang L, Chen HR, Wu JZ, Huang LB, Brookes PC, Rodrigues JLM, Xu JM, Liu XM (2021a) Effects of magnetic biochar-microbe composite on cd remediation and microbial responses in paddy soil. J Hazard Mater 414:9. https://doi.org/10.1016/j.jhazmat.2021.125494
Wang L, Li ZT, Wang Y, Brookes PC, Wang F, Zhang QC, Xu JM, Liu XM (2021b) Performance and mechanisms for remediation of cd(II) and as(III) co-contamination by magnetic biochar-microbe biochemical composite: competition and synergy effects. Sci Total Environ 750:9. https://doi.org/10.1016/j.scitotenv.2020.141672
Wang Y, Zheng K, Zhan W, Huang L, Liu Y, Li T, Yang Z, Liao Q, Chen R, Zhang C, Wang Z (2021c) Highly effective stabilization of cd and Cu in two different soils and improvement of soil properties by multiple-modified biochar. Ecotoxicol Environ Saf 207:111294. https://doi.org/10.1016/j.ecoenv.2020.111294
Wang HH, Zhang R, Zhao YY, Shi HZ, Liu GS (2022a) Effect of biochar on rhizosphere soil microbial diversity and metabolism in tobacco-growing soil. Ecologies 3:539–556. https://doi.org/10.3390/ecologies3040040
Wang Y, Narayanan M, Shi XJ, Chen XP, Li ZL, Natarajan D, Ma Y (2022b) Plant growth-promoting bacteria in metal-contaminated soil: current perspectives on remediation mechanisms. Front Microbiol 13:17. https://doi.org/10.3389/fmicb.2022.966226
Wu B, Wang ZR, Zhao YX, Gu YM, Wang Y, Yu J, Xu H (2019) The performance of biochar-microbe multiple biochemical material on bioremediation and soil micro-ecology in the cadmium aged soil. Sci Total Environ 686:719–728. https://doi.org/10.1016/j.scitotenv.2019.06.041
Wu JW, Wang T, Wang JW, Zhang YS, Pan WP (2021) A novel modified method for the efficient removal of pb and cd from wastewater by biochar: enhanced the ion exchange and precipitation capacity. Sci Total Environ 754:10. https://doi.org/10.1016/j.scitotenv.2020.142150
Xia X, Wu SJ, Li NH, Wang D, Zheng SX, Wang GJ (2018) Novel bacterial selenite reductase CsrF responsible for Se(IV) and cr(VI) reduction that produces nanoparticles in Alishewanella sp WH16-1. J Hazard Mater 342:499–509. https://doi.org/10.1016/j.jhazmat.2017.08.051
Xia X, Wu SJ, Zhou ZJ, Wang GJ (2021) Microbial cd(II) and cr(VI) resistance mechanisms and application in bioremediation. J Hazard Mater 401:13. https://doi.org/10.1016/j.jhazmat.2020.143685
Xu YL, Seshadri B, Sarkar B, Wang HL, Rumpel C, Sparks D, Farrell M, Hall T, Yang XD, Bolan N (2018) Biochar modulates heavy metal toxicity and improves microbial carbon use efficiency in soil. Sci Total Environ 621:148–159. https://doi.org/10.1016/j.scitotenv.2017.11.214
Xu JC, Huang LM, Chen CY, Wang J, Long XX (2019a) Effective lead immobilization by phosphate rock solubilization mediated by phosphate rock amendment and phosphate solubilizing bacteria. Chemosphere 237:9. https://doi.org/10.1016/j.chemosphere.2019.124540
Xu XY, Huang H, Zhang Y, Xu ZB, Cao XD (2019b) Biochar as both electron donor and electron shuttle for the reduction transformation of cr(VI) during its sorption. Environ Pollut 244:423–430. https://doi.org/10.1016/j.envpol.2018.10.068
Yan S, Ren TB, Mahari WAW, Feng HL, Xu CS, Yun F, Waiho K, Wei YW, Lam SS, Liu GS (2022) Soil carbon supplementation: improvement of root-surrounding soil bacterial communities, sugar and starch content in tobacco (N. tabacum). Sci Total Environ 802:11. https://doi.org/10.1016/j.scitotenv.2021.149835
Yang Q, Wang YJ, Zhong H (2021) Remediation of mercury-contaminated soils and sediments using biochar: a critical review. Biochar 3:23–35. https://doi.org/10.1007/s42773-021-00087-1
Yin K, Wang QN, Lv M, Chen LX (2019) Microorganism remediation strategies towards heavy metals. Chem Eng J 360:1553–1563. https://doi.org/10.1016/j.cej.2018.10.226
Zeba N, Berry TD, Panke-Buisse K, Whitman T (2022) Effects of physical, chemical, and biological ageing on the mineralization of pine wood biochar by a Streptomyces isolate. PLoS ONE 17:18. https://doi.org/10.1371/journal.pone.0265663
Zha RD, Coles N, Wu JP (2015) Carbon mineralization following additions of fresh and aged biochar to an infertile soil. CATENA 125:183–189. https://doi.org/10.1016/j.catena.2014.10.026
Zhang WW, Chen LX, Liu DY (2012) Characterization of a marine-isolated mercury-resistant Pseudomonas putida strain SP1 and its potential application in marine mercury reduction. Appl Microbiol Biotechnol 93:1305–1314. https://doi.org/10.1007/s00253-011-3454-5
Zhang KK, Sun P, Zhang YR (2019a) Decontamination of Cr(VI) facilitated formation of persistent free radicals on rice husk derived biochar. Front Env Sci Eng 13:9. https://doi.org/10.1007/s11783-019-1106-7
Zhang XQ, Xia J, Pu JY, Cai C, Tyson GW, Yuan ZG, Hu SH (2019b) Biochar-mediated anaerobic oxidation of methane. Environ Sci Technol 53:6660–6668. https://doi.org/10.1021/acs.est.9b01345
Zhang GX, He LX, Guo XF, Han ZW, Ji L, He QS, Han LF, Sun K (2020a) Mechanism of biochar as a biostimulation strategy to remove polycyclic aromatic hydrocarbons from heavily contaminated soil in a coking plant. Geoderma 375:12. https://doi.org/10.1016/j.geoderma.2020.114497
Zhang H, Xiao R, Li RH, Ali A, Chen AL, Zhang ZQ (2020b) Enhanced aqueous cr(VI) removal using chitosan-modified magnetic biochars derived from bamboo residues. Chemosphere 261:12. https://doi.org/10.1016/j.chemosphere.2020.127694
Zhang MY, Zhang L, Riaz M, Xia H, Jiang CC (2021a) Biochar amendment improved fruit quality and soil properties and microbial communities at different depths in citrus production. J Clean Prod 292:12. https://doi.org/10.1016/j.jclepro.2021.126062
Zhang YF, Wang JM, Feng Y (2021b) The effects of biochar addition on soil physicochemical properties: a review. CATENA 202:19. https://doi.org/10.1016/j.catena.2021.105284
Zhang MY, Xia H, Riaz M, Liu B, Zeinab El D, Jiang CC (2023) Various beneficial microorganisms colonizing on the surface of biochar primarily originated from the storage environment rather than soil environment. Appl Soil Ecol 182:9. https://doi.org/10.1016/j.apsoil.2022.104700
Zhao B, O’Connor D, Zhang JL, Peng TY, Shen ZT, Tsang DCW, Hou DY (2018) Effect of pyrolysis temperature, heating rate, and residence time on rapeseed stem derived biochar. J Clean Prod 174:977–987. https://doi.org/10.1016/j.jclepro.2017.11.013
Zhao L, Xiao D, Liu Y, Xu H, Nan H, Li D, Kan Y, Cao X (2020) Biochar as simultaneous shelter, adsorbent, pH buffer, and substrate of Pseudomonas citronellolis to promote biodegradation of high concentrations of phenol in wastewater. Water Res. https://doi.org/10.1016/j.watres.2020.115494
Zhao JT, Li F, Cao YX, Zhang XB, Chen T, Song H, Wang ZW (2021) Microbial extracellular electron transfer and strategies for engineering electroactive microorganisms. Biotechnol Adv 53:19. https://doi.org/10.1016/j.biotechadv.2020.107682
Zheng XM, Xu WH, Dong J, Yang T, Shangguan ZC, Qu J, Li X, Tan XF (2022) The effects of biochar and its applications in the microbial remediation of contaminated soil: a review. J Hazard Mater 438:18. https://doi.org/10.1016/j.jhazmat.2022.129557
Zhou X, Zhang X, Ma C, Wu F, Jin X, Dini-Andreote F, Wei Z (2022) Biochar amendment reduces cadmium uptake by stimulating cadmium-resistant PGPR in tomato rhizosphere. Chemosphere. https://doi.org/10.1016/j.chemosphere.2022.136138
Zhu XM, Chen BL, Zhu LZ, Xing BS (2017) Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: a review. Environ Pollut 227:98–115. https://doi.org/10.1016/j.envPol.2017.04.032
Acknowledgements
Not applicable.
Funding
This work is carried out at the College of Resources and Environment, Southwest University, supported by the Fundamental Research Funds for the Central Universities (No. SWU 020010).
Author information
Authors and Affiliations
Contributions
PO: Investigation, analysis, data collection, methodology, and writing the draft manuscript. MN: Investigation, review and editing. XS, XC, ZL, and YL revised the manuscript. YM: Conceptualization, funding acquisition, project administration and supervision, writing the draft manuscript, review and editing. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Handling editor: Wenfu Chen.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ouyang, P., Narayanan, M., Shi, X. et al. Integrating biochar and bacteria for sustainable remediation of metal-contaminated soils. Biochar 5, 63 (2023). https://doi.org/10.1007/s42773-023-00265-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-023-00265-3