Abstract
Functional constipation (FC) can seriously affect the physical and mental health of children. The goal of this study is to assess the efficacy and safety of Bifidobacterium animalis subsp. lactis XLTG11 in treating FC in children through a randomized, double-blinded, placebo-controlled approach. Eligible children were randomized into either the intervention group (IG, n = 65, receiving conventional treatment with probiotics) or the control group (CG, n = 66, receiving conventional treatment without probiotics). The primary outcome measure was fecal frequency. Fecal gut microbiota analysis and PICRUSt (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States) were used to predict gene family abundances based on 16S information. Over the course of treatment, the weekly frequency of feces within each group increased significantly (F = 41.97, p < 0.001). The frequency of feces (times/week (t/w)) in the IG was significantly higher than that in the CG (3.69 ± 2.62 t/w vs.3.18 ± 1.43 t/w, 4.03 ± 2.54 t/w vs. 2.89 ± 1.39 t/w and 3.74 ± 2.36 t/w vs. 2.94 ± 1.18 t/w and 3.45 ± 1.98 vs. 3.17 ± 1.41 t/w for the 1st, 2nd, 3rd, and 4th week after intervention, respectively) (F = 7.60, p = 0.0067). After the intervention, dominate species shifted to Bifidobacterium longum, Bifidobacterium breve, and Escherichia coli in the IG. Additionally, genes related to short-chain fatty acid (SCF) metabolism were upregulated, while methane metabolism was downregulated. Administration of XLTG11 at a dose of 1 × 1010 CFU/day to children increased fecal frequency, induced beneficial changes in gut microbiota, and regulated SCFs and methane metabolism–related genes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The incidence of infant constipation is influenced by many factors, and the reported incidence rate varies in China, ranging from 0.7 to 29.6%. It constitutes 10 to 25% of the cases in the Gastroenterology Department, with functional constipation (FC) accounting for 90 to 95% of children’s constipation [1, 2]. FC refers to constipation that is not caused by identifiable diseases or drug factors, posing a significant impact on the physical and mental health of children, especially infants. Long-term constipation can affect their nutritional status, growth, and development [3], imposing greater economic and mental pressure on parents [4].
FC can be categorized into three types: slow transit constipation, outlet obstruction constipation, and mixed constipation. The pathogenesis of FC mainly includes insufficient intake of food fibers and water, reduced intestinal smooth muscle tension and intestinal peristalsis, mechanically obstructed intestinal peristalsis, dysfunction of defecation muscles, and disturbance of the intestinal microbiome [5, 6].
The intestinal microbiome plays an important role in the physiology and pathophysiology of constipation. It interacts with the immune system, enteric nervous system, and central nervous system, and modifies intestinal secretion and hormonal milieu. Specific microbial metabolites, such as short-chain fatty acids (SCFAs) and tryptophan catabolites, play central roles in microbiota-mediated intestinal functions [6, 7]. Recent randomized, controlled trials (RCTs) have demonstrated the anti-constipation effects of certain probiotic strains, particularly in children [8,9,10,11]. Probiotic supplementation is thus suggested as a complementary treatment for constipation.
However, the efficiency of probiotics is highly dependent on both strain specificity and disease specificity [12]. Different probiotic strains vary in efficacy due to different mechanisms-of-action against pathogens, manufacturing processes, and product quality control. Clinical guidelines and meta-analyses should recognize the importance of reporting outcomes by specific strain(s) of probiotics and the type of disease. Currently, hundreds of different probiotic products are available in the market. These products differ in excipients, amount and strains of microorganisms, and activity [13,14,15,16].
Bifidobacterium animalis subsp. lactis XLTG11 is a specific strain isolated from the intestines of healthy infants in China with independent intellectual property rights. The strain has been assigned a preservation number of CGMCC No. 18738 by the China General Microbiological Culture Collection Center (CGMCC). Worldwide, other similar probiotic strains [17,18,19] of Bifidobacterium animalis subsp. lactis have been shown to contribute to relieving gut dysmotility–related constipation through several mechanisms. Therefore, Bifidobacterium animalis subsp. lactis XLTG11 is also expected to have a positive effect on normalizing gut motility and maintaining gut health.
To our knowledge, no study has investigated whether XLTG11 can achieve good colonization and become a dominant microorganism to fulfill its role in gastrointestinal regulation function in children with FC. Therefore, the purpose of this research is to study the adjunctive clinical efficacy of the XLTG11 strain on FC in children.
Materials and methods
Subjects and ethical approval
This is a multi-center, parallel randomized, controlled, double-blinded clinical intervention. Children of both sexes and aged 0–6 years who were outpatients with FC were recruited. The study was conducted at Chengdu Women’s and Children’s Central Hospital and five sub-centers, namely Chongzhou Maternal and Child Health Care Hospital, Dayi Maternal and Child Health Care Hospital, Xindu Maternal and Child Health Care Hospital, Jinniu Maternal and Child Health Care Hospital, and Qingbaijiang Maternal and Child Health Care Hospital, spanning from November 2021 to September 2022.
The enrollment and research plan were reviewed and approved by the institutional ethics committee of the Shanghai Nutrition Society of China (ethics number of Shanghai Nutrition Society: Lun Shen [2021] No. 24). Written informed consent was obtained from each child’s parents/guardians. This study complied with the code of ethics of the World Medical Association (Declaration of Helsinki) and received approval and registration in the Chinese Clinical Trial Registration Center with the registration number ChiCTR2100053695.
Inclusion, exclusion, and withdrawal criteria
Diagnostic criteria [20] for FC
Must include 2 or more of the following symptoms at least once per week for a minimum of 1 month, with insufficient criteria for a diagnosis of irritable bowel syndrome:
-
1)
Two or fewer defecations in the toilet per week in a child of a developmental age of at least 4 years.
-
2)
At least 1 episode of fecal incontinence per week.
-
3)
History of retentive posturing or excessive volitional stool retention.
-
4)
History of painful or hard bowel movements.
-
5)
Presence of a large fecal mass in the rectum.
-
6)
History of large diameter stools that can obstruct the toilet.
After appropriate evaluation, the symptoms cannot be fully explained by another medical condition.
Inclusion criteria:
-
1)
Conforming to Rome IV diagnostic criteria for FC in children.
-
2)
Birth weight 2500–4000 g.
-
3)
Age of enrollment: 0–6 years old.
-
4)
Parents voluntarily delaying significant changes in infant feeding patterns.
-
5)
Parents willing and able to fill in diaries and questionnaires.
-
6)
Signed written informed consent collected from parents/guardians of enrolled children.
-
7)
Regular and effective visits can be made during the trial.
Exclusion criteria:
-
1)
Congenital intestinal malformation leading to outlet obstruction and difficult defecation.
-
2)
Nervous system dysplasia and severe organic diseases, severe malnutrition, immune deficiency diseases, pancreatic dysfunction.
-
3)
Same probiotics taken within 1 month before the diagnosis of this illness.
-
4)
Children expected to receive antibiotic treatment during the trial.
-
5)
Children diagnosed with allergic diarrhea.
Withdrawal criteria:
-
1)
Erroneous inclusion and misdiagnosis.
-
2)
Children without any clinical records for evaluation.
-
3)
Children taking drugs prohibited by the study, including hormones, immunosuppressive drugs, and other probiotics, during the treatment.
-
4)
Poor compliance (the number of sachets consumed was less than 80% of the expected amount).
-
5)
Situation where the probiotic cannot be taken from the digestive tract during the treatment.
-
6)
Children treated with antibiotics during the treatment.
Grouping and intervention
A biostatistician, who was not directly involved in the execution of the study, used the RAND function in Excel to generate random numbers. Children who met the inclusion criteria were coded by the random numbers and assigned into the two groups based on the sequence of the random numbers. Each group was randomly assigned with 75 children.
Socio-demographic data were collected at baseline. Clinical evaluation of each child at the time of enrollment followed the Rome IV criteria [20]. Recruited children were managed per the Rome IV criteria, which included education efforts to guide families in recognizing withholding behaviors and implementing behavioral interventions such as regular toileting, maintaining diaries to monitor bowel movements, and utilizing reward systems for successful evacuations. Lifestyle counseling and dietary recommendations were developed based on the Chinese Dietary Guidelines 2022 and expert consensus and guidelines for FC in children [1, 21, 22]. The use of other laxatives, antibiotics, probiotics, fiber, fermented dairy products, and yogurt was prohibited during the study period. Glycerin suppositories were only permitted in the absence of defecation for more than 3 days.
Children in the intervention group (IG) received the oral probiotic in the form of a single sachet (SunFlower Group, Production No.: SC10632021400614). The probiotic could be taken directly or added to warm water below 45 ℃, milk, rice paste, or other liquid foods. One sachet (containing XLTG11 strain 1 × 1010 CFU/sachet) was taken daily for 28 consecutive days starting on the first day of the clinical treatment.
Children in the control group (CG) were treated with the placebo sachet containing only maltodextrin. The probiotic and placebo had similar appearance, taste, and smell and were provided in identical sachets with identical labeling expect for the subject specific randomization number.
If the children vomited within 30 min after taking the sachet, an additional dose was given (up to one extra dose in 4 h). Dosing and re-dosing were recorded in a case report form (CRF) by the treating physicians. The children’s parents and/or guardians, clinicians, laboratory personnel, data manager, and statistician remained blinded to group assignments until the end of data analysis.
Data collection
After enrollment, study staff performed assessments, recorded data on CRF, and collected laboratory samples according to the protocol. Clinical medications were then assigned. During the trial period, parents took daily pictures of their child’s feces and sent them to the researcher for objective records. A fecal sample was collected daily to verify the fecal type by Bristol Stool Scale and to evaluate the treatment efficacy. The fecal consistency score, or fecal score, was evaluated based on the Bristol Stool Scale in the same order (i.e., Bristol stool type 7 had a fecal score of 7 while type 1 had a fecal score of 1). The clinicians used the CRF to record the incidence of abdominal cramps, nausea, vomiting, fever, constipation, and low appetite in children during the treatment. The mean value of weekly fecal score was defined as the sum of weekly fecal score divided by the fecal frequency in a given week.
Fecal microbiome analysis
A total of 158 fecal samples from the children were collected for gut microbiome analyses, including 82 samples from 41 children in the IG before and after the intervention and 76 samples from 38 children in the CG. Total genome DNA from the samples was extracted by the CTAB/SDS method using a QIAamp Fast DNA fecal Mini Kit (Qiagen, Valencia, California, USA) according to the manufacturer’s instructions. The isolated genomic DNA for the bacterial 16S rRNA gene V3-V4 region was amplified using the TransGen AP221-02 Kit (TransGen, Beijing, China), with the 16S V34: 341F-806R PCR primers. The Uparse software (Uparse v7.0.1001) [23] and Quantitative Insights Into Microbial Ecology (QIIME) software [24] were utilized for 16S rRNA sequence analysis. Sequences with ≥ 97% similarity were assigned to the same operational taxonomic units (OTUs). We further selected a representative sequence for each OTU and annotated its taxonomic information based on the RDP classifier [25]. The QIIME (Version 1.9.1) calculated both alpha- (within sample) and beta- (between sample) diversity. Shannon, Simpson, Chao1, and ACE indices were used as indicators of the alpha diversity. Principal coordinate analysis (PCoA), based on Bray–Curtis distance, was used to analyze the β-diversity. The differential abundance of taxa between groups was analyzed using Kruskal–Wallis test. Differential enrichment of gut microbiota was analyzed by linear discriminant analysis effect size (LEfSe). Furthermore, LDA scores (> 4.0) derived from the LEfSe analysis at genus and species levels identified several bacterial genera and species that differed in the two groups.
To explore the functional profiles of the gut microbiota, Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) was performed based on 16S information from the Greengenes database [26]. The functional genes predictive analysis was performed by the NovoMagic website (https://magic.novogene.com/customer/main#/homeNew).
Outcome measures
The primary outcome measure was the fecal frequency. Secondary outcome measures included the sum of weekly Bristol fecal score and the mean of weekly Bristol fecal score throughout the entire FC treatment episode. Moreover, adverse effects and fecal gut microbiota analysis before and after intervention were considered. Concurrently, common symptoms such as abdominal cramps, nausea, vomiting, fever, and loss of appetite were monitored throughout the entire intervention period.
Statistical analysis
SAS version 9.2 for Windows (SAS Institute Inc., Cary, NC, USA) was used for all analyses. The quantitative data were expressed in two ways: mean ± standard deviation (SD), and median (P25, P75). t-test was used to compare normally distributed data. Wilcoxon rank sum test was used for data without a normal distribution. χ2 test was used to compare the difference of the treatment efficacy between the two groups for countable data. The frequency of feces, sum of weekly Bristol fecal score, and mean of weekly Bristol fecal score between the two groups before and after the intervention were compared using repeated measures analysis of variance (ANOVA). A p-value less than 0.05 was considered statistically significant.
Sample size
In the present study, we considered a clinical significance to be present if the difference in weekly fecal frequency between the CG and the IG after the intervention was assumed to be more than 2 times. With a power of β = 0.8 and a significance level of α = 0.05 (bilateral), the calculated sample size for each group was approximately 60 subjects. Accounting for a 25% dropout rate, we selected a total sample size of 150 subjects with 75 subjects in each group.
Result
Basic clinical and demographic data
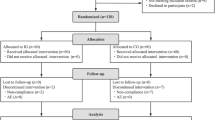
A total of 150 eligible children were enrolled in the study, with approximately 12.7% (19/150) dropped out during the study period. Among these, five children were withdrawn by their parents at the initial stage, four children were treated with antibiotics during the intervention, three children took other prohibited probiotics, and seven children had poor compliance. Thus, primary and secondary outcome measures were obtained from 131 infants (65 in IG and 66 in CG, respectively) (Fig. 1).
Figure l is a flowchart illustrating participant involvement. There was no significant difference in demographics, total and mean Bristol fecal score, and weekly fecal frequency before the intervention between the two groups (p > 0.05, Table 1).
Effect of probiotic intervention on weekly fecal frequency
The weekly frequency of feces within each group increased significantly (F = 41.97, p < 0.001) with the extension of treatment time. The frequency of feces (t/w) in the IG was significantly higher than that in the CG (3.69 ± 2.62 t/w vs.3.18 ± 1.43 t/w, 4.03 ± 2.54 t/w vs. 2.89 ± 1.39 t/w and 3.74 ± 2.36 t/w vs. 2.94 ± 1.18 t/w and 3.45 ± 1.98 vs. 3.17 ± 1.41 t/w for the 1st, 2nd, 3rd, and 4th week after the intervention, respectively) (F = 7.60, p = 0.0067). However, there was no significant interaction between treatment time and intervention method (F = 2.11, p = 0.0798) (Supplementary Table 1, Fig. 2).
Effect of probiotic intervention on weekly fecal consistency
The repeated measures ANOVA indicated a significant increase in the sum of weekly Bristol fecal score for children in both groups with the extension of treatment time (F = 64.9, p < 0.001). However, children in the IG had a significantly higher sum of weekly Bristol fecal score than children in the CG (F = 13.94, p = 0.0003). There was also a significant interaction between treatment time and intervention method (F = 3.90, p = 0.0045) (Supplementary Table 2, Fig. 3).
Effect of probiotic intervention on the mean of weekly Bristol fecal score
The repeated measures ANOVA showed a significant increase in the mean of weekly Bristol fecal score for children in both groups with the extension of treatment time (F = 197.17, p < 0.001). Children in the IG had significantly higher mean of weekly Bristol fecal score than children in the CG (F = 6.93, p = 0.00096). Additionally, a significant interaction between treatment time and intervention method was observed (F = 2.73, p = 0.0338) (Supplementary Table 3, Fig. 4).
Effect of probiotic intervention on gut microbiota
A total of 158 fecal samples, both before and after the intervention, were collected from the IG and CG groups for the assessment of gut microbiota composition using high-throughput sequencing of the 16S rRNA gene. As shown in Fig. 5, there was no significant difference in the three alpha diversity indices between the two groups before the intervention (all p-values >0.05). However, after the intervention, the diversity estimates (Shannon and Simpson indices) in the IG were significantly higher than those before the intervention (all p<0.05). Additionally, the richness estimate indices (calculated in observed species) were significantly higher in the IG after the intervention compared to children in the CG (p-value <0.05).
The PCoA plot based on Bray-Curtis distance showed that axis 1 (PC1) explained 10.81% of the variability, and axis 2 (PC2) explained 6.71% of the variability of before the intervention. The PCoA plot demonstrated that the samples from children in both the IG and the CG were closely situated spatially. However, after the intervention, there was a notable spatial separation between the samples from the two groups (Fig. 6).
The gut microbiota composition is presented in Fig. 7. Before intervention, Firmicutes, Actinobacteria, and Proteobacteria dominated at the phylum level, while Enterococcus, Bifidobacterium, Escherichia-Shigella, and Streptococcus were prominent at the genus level. At the species level, Enterococcus faecium, Escherichia coli, and Bifidobacterium longum were predominant. After the intervention, the dominate species shifted to Bifidobacterium longum, Bifidobacterium breve, and Escherichia coli in the IG, while in the CG, it remained with Enterococcus faecium, Bifidobacterium longum, Bifidobacterium breve, and Escherichia coli (Fig. 7A, B, C).
Taxa abundance at phylum (A), genus (B), and species (C) levels. Only the top 10 most abundant phyla, genera, and species were shown. D The MetaStat Complex Heat map showing the differential abundance at species level between the two groups with statistical significance. Con.A, control group after intervention; XLT.C.A, intervention group after intervention; Con.P, control group before intervention; XLT.C.P, intervention group before intervention
Furthermore, the MetaStat method confirmed that the abundance of Lactobacillus plantarum, Rothia mucilaginosa, and Enterococcus faecium in the CG was significantly higher than that in the IG (p<0.05) (Fig. 8D).
LEfSe analysis identified the most deferentially abundant taxa between the intervention and control groups. Cladogram: taxonomic representation of statistically and biologically consistent differences among intestinal microbiota of different groups. Differences were represented by the color of the most abundant taxa (green indicated a taxon with significantly higher relative abundance in the intervention group, red indicated a taxon significantly more abundant in the control group, and yellow indicated no significant difference). LAD SCORE: histogram of linear discriminant analysis (LDA) scores for deferentially abundant taxon. Cladogram was calculated by LefSe and displayed according to effect size. A, B LDA score and Cladogram before intervention; C, D LDA score and Cladogram after intervention; Con.P, control group before intervention; XLT.C.P, intervention group before intervention; Con.A, control group after intervention; XLT.C.A, intervention group after intervention
To compare the differences in gut microbiota community composition between the IG and CG, LEfSe analysis was performed. LEfSe analysis identified 19 taxa that exhibited differential abundance between the two groups before the intervention. Following the intervention, 12 taxa showed differential abundance (Fig. 8B, D). In comparison to the CG, XLTG11 treatment increased abundance of two families (Erysipelatoclostridiaceae and Lachnospiraceae), three orders (Erysipelotrichales, Lachnospirales, and Oscillospirales), and one class (Clostridia).
According to LDA scores, notable high abundance was observed in the Bifidobacterium breve species and Erysipelotrichaceae_UCG_003 genus in children from the IG, while the children in the CG showed enrichment with the Akkermansia muciniphila and Enterococcus faecium species, and the Akkermansia and Enterococcus genera after the intervention (Fig. 8A, B).
XLTG11 treatment changed the functional gene composition of gut microbiota
To explore the effects of XLTG11 intervention on physiological functions, PICRUSt was used to analyze and predict the composition of the functional genes in the metabolic pathways. The result showed a significant difference in functional genes within the gut microbiota before and after XLTG11 treatment, suggesting a potential impact on the metabolic pathways of the gut microbiota. Notably, the proportion of 50 sub-functional genes in the gut microbiota exhibited evident changes after XLTG11 treatment (Fig. 9). Before XLTG11 treatment, functional genes involved in peptidases, chromosome, amino acid–related enzymes, ribosome biogenesis, arginine and proline metabolism, methane metabolism, and DNA replication proteins were upregulated. Conversely, after XLTG11 treatment, different functional genes such as pyruvate metabolism, bacterial motility proteins, butanoate metabolism, propanoate metabolism, citrate cycle, lipid biosynthesis proteins, tyrosine metabolism, and fatty acid metabolism were upregulated. These findings indicated a significant influence of XLTG11 treatment on the composition of functional genes within the gut microbiota.
Incidence of probiotic intervention related adverse reactions during treatment
No incidence of abdominal colic, nausea, vomiting, fever, low appetite, and other symptoms related to probiotic intervention was reported in both groups.
Discussion
Bifidobacterium animalis subsp. lactis XLTG11 is a specific strain isolated from the intestines of healthy infants. It demonstrates good gastrointestinal passage abilities and strong antibacterial effect against common human pathogens, such as Escherichia coli, Salmonella, and Staphylococcus aureus. Animal studies and in vitro research have found that XLTG11 can alleviate DSS-induced colitis and ameliorate antibiotic-related diarrhea by inhibiting the activation of the TLR4/MYD88/NF-κB signaling pathway, regulating inflammatory cytokines, improving intestinal barrier function, and modulating gut microbiota composition and intestinal immunity [27,28,29].
As an auxiliary treatment, the use of XLTG11 independently demonstrates effective improvement in FC in children, starting from the first week of treatment. This finding aligns with a recent study in China that used Bifidobacterium lactis Bb-12 strain for treating FC in infants [30]. In addition, a recent meta-analysis on probiotics for children with FC also indicated [31] that probiotic use can increase fecal frequency. However, conflicting results suggesting ineffectiveness have been reported in other studies [32]. Considering the specificity of the strains, we believe that this “ineffectiveness” may be attributed to the specific strains used in these studies and the heterogeneity in research subjects, and may not extend to all other probiotics.
The increased fecal frequency may be attributed to two factors: the time effect and the intervention effect. The weekly fecal frequency in both groups significantly increased with the extension of treatment time, indicating the effectiveness of the treatment protocols in both groups. Additionally, the probiotic intervention exhibited additional treatment effects in alleviating FC after accounting for the time effect. However, no interaction between the time effect and intervention effect was found. Besides the increased fecal frequency, the probiotic significantly improved the fecal consistency in children with FC. Nevertheless, in contrast to the changes in fecal frequency, the interaction effect indicated that with the extension of treatment time, the improvement in fecal consistency in the probiotic intervention group was more pronounced.
The alteration in gut microbiota composition within the intestinal micro-ecology and the influence of probiotics on this micro-ecology are closely related to the therapeutic effect and clinical progression of gastrointestinal diseases. Recent studies investigating the use of Bifidobacterium animalis as an adjuvant therapy for gastrointestinal diseases in both children and adults have shown that such usage can regulate the composition of intestinal micro-ecology and enhance prognosis. Examples include Bifidobacterium animalis subsp. lactis BB-12 for infants with colic [33] and individuals receiving antibiotics [34], Bifidobacterium lactis Probio-M8 for asthmatic patients [35], and Bifidobacterium animalis subsp. lactis BL04 for microbiota regulation in healthy adults [36]. The findings of the present study align closely with those mentioned above. Although both groups of children with FC achieved clinical cure outcomes through different treatment methods, the recovery duration and the dynamics of changes in fecal frequency and consistency were not entirely similar. This discrepancy might be partially attributed to distinct alterations in the intestinal microbiome observed in the two groups of children.
In our study, it is reasonable to infer that the intestinal immune function and the incidence rate of gastrointestinal diseases during the follow-up of children in the IG will likely be superior to those of children in the CG due to the distinct changes in gut microbiota composition after intervention. This inference can be validated through the long-term follow-up of participating children. Considering the high diversity of gut microbiota of infants as a characteristic following probiotic administration and the reduced risk of gastrointestinal diseases due to increased variety and abundance of dominant or beneficial bacteria, the high diversity of gut microbiota after the use of XLTG11 might be a manifestation of the microbiota regulation of XLTG11. Although infants in the CG also experienced recovery from constipation after treatment, their gut microbiota composition differed significantly from that of infants in the IG. Not only did the diversity of gut microbiota increase, but the abundance of some potential pathogens also increased. Whether the different gut microbiota composition between the two groups will affect the incidence rate of diarrhea in a later period warrants a long-term follow-up study.
According to the functional gene prediction analysis, XLTG11 treatment might upregulate functional genes involved in short-chain fatty acid (SCFA) metabolism. SCFAs are major fermentation products of the gut microbiota [37]. They may play a role in the pathogenesis of FC by regulating colonic electrolyte absorption and secretion, mucin secretion, and colonic motility. Previous studies on a similar strain, Bifidobacterium animalis subsp. lactis HN019, have demonstrated its ability to reduce intestinal transit time and increase bowel movement frequency in FC, possibly through SCFAs derived from microbial fermentation [38]. Therefore, the upregulated SCFAs metabolism could contribute to the amelioration of FC by regulating intestinal function. Moreover, bacterial motility is facilitated in most bacterial species through the flagellar apparatus [39], which consists of numerous bacterial motility proteins with thousands of individual subunits. Notably, bacterial flagella are potent inflammatory structures [40]. Hence, alterations in the functional predicted genes related to bacterial motility proteins could play a role in alleviating the symptoms experienced by patients with FC. It is also worth noting that after XLTG11 treatment, the functional gene prediction of methane metabolism was downregulated. Methane has been implicated as a factor that decreases ileal and colon transit time, raises the amplitude of contraction, slows peristalsis, and leads to constipation [41]. Studies have shown that targeting methanogenesis directly can relieve symptoms in constipation-predominant irritable bowel syndrome [41]. Therefore, it is reasonable to infer that one possible mechanism of XLTG11 in improving constipation symptoms might be related to its influence on intestinal methanogenesis. However, further research is needed to elucidate the role of XLTG11 in methanogenesis. While these results suggest that XLTG11 treatment might regulate SCFAs and methane metabolism–related genes in the gut microbiota to alleviate FC symptoms, additional clinical trials, animal studies, and in vitro research are necessary to confirm this conclusion.
Mechanistic research on the impact of probiotics on FC have showed that specific probiotics can influence microbiota composition, SCFA production, inflammatory cytokines, bile acid metabolism, the mucus layer, and components of the immune system that could potentially affect gut motility [6]. This study provides a preliminary demonstration that XLTG11 may, at least partially, affect the adjuvant treatment efficiency and long-term prognosis of constipation through these mechanisms. However, further research is required to comprehensively understand the intricate interactions involving specific strain like XLTG11 and constipation.
Limitation analysis
Firstly, the use of a single dose of XLTG11 at 1 × 1010 CFU/day limits our ability to investigate the optimal dose–response relationship of XLTG11 strain in the adjuvant treatment of FC. Secondly, due to the limitations of the study’s primary objectives and design, the probiotic intervention and clinical symptom observation were restricted to a duration of only 4 weeks. Therefore, the potential long-term effects of XLTG11 on children’s health and gut microbiota cannot be adequately observed. Lastly, the effect of exogenous probiotic supplementation on intestinal function is influenced by various bias factors, such as the mode of delivery, feeding method, and allergic disease. Although some of these factors have been considered, it is possible that the intervention effect of XLTG11 may be compromised. Future studies could benefit from expanding the sample size and extending the observation time to enhance the reliability and representativeness of the research conclusions. Additionally, further exploration of the long-term effects of XLTG11 on children’s health would be valuable.
Conclusions
In summary, no adverse effects of XLTG11 intervention were observed during our study period, indicating the safety of XLTG11 for children. The administration of Bifidobacterium animalis subsp. lactis XLTG11 at a dose of 1 × 1010 CFU/day to children aged 0–6 years can increase fecal frequency, improve fecal consistency, induce beneficial changes in intestinal microbiota composition, and regulate SCFs and methane metabolism-related genes in the gut microbiota of children with FC.
Data Availability
The data are available from the corresponding author on reasonable request.
References
Ho JMD, How CH (2020) Chronic constipation in infants and children. Singapore Med J 61(2):63–68
Huang Y, Tan SY, Parikh P et al (2021) Prevalence of functional gastrointestinal disorders in infants and young children in China. BMC Pediatr 21(1):131
Vriesman MH, Rajindrajith S, Koppen IJN et al (2019) Quality of life in children with functional constipation: a systematic review and meta-analysis. J Pediatr 214:141–50
Çağan Appak Y, Yalin Sapmaz Ş, Doğan G et al (2017) Clinical findings, child and mother psychosocial status in functional constipation. Turkish J Gastroenterol Off J Turkish Soc Gastroenterol 28(6):465–470
Bharucha AE, Lacy BE (2020) Mechanisms, evaluation, and management of chronic constipation. Gastroenterology 158(5):1232–49.e3
Dimidi E, Christodoulides S, Scott SM et al (2017) Mechanisms of action of probiotics and the gastrointestinal microbiota on gut motility and constipation. Advances in nutrition (Bethesda, Md) 8(3):484–494
Pan R, Wang L, Xu X et al (2022) Crosstalk between the gut microbiome and colonic motility in chronic constipation: potential mechanisms and microbiota modulation. Nutrients 14(18):3407
Martoni CJ, Evans M, Chow CT et al (2019) Impact of a probiotic product on bowel habits and microbial profile in participants with functional constipation: a randomized controlled trial. J Dig Dis 20(9):435–446
Indrio F, Di Mauro A, Riezzo G et al (2014) Prophylactic use of a probiotic in the prevention of colic, regurgitation, and functional constipation: a randomized clinical trial. JAMA Pediatr 168(3):228–233
Sanctuary MR, Kain JN, Chen SY et al (2019) Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms. PLoS One 14(1):e0210064
Kubota M, Ito K, Tomimoto K et al (2020) Lactobacillus reuteri DSM 17938 and magnesium oxide in children with functional chronic constipation: a double-blind and randomized clinical trial. Nutrients 12(1):225
Mcfarland LV, Evans CT, Goldstein EJC (2018) Strain-specificity and disease-specificity of probiotic efficacy: a systematic review and meta-analysis. Front Med 5:124
Chapman C M C, Gibson G R, Rowland I (2011) Health benefits of probiotics: are mixtures more effective than single strains? Eur J Nutr 50(1):1–17
Collado MC, Isolauri E, Salminen S (2008) Specific probiotic strains and their combinations counteract adhesion of Enterobacter sakazakii to intestinal mucus. FEMS Microbiol Lett 285(1):58–64
Drago L, De Vecchi E, Gabrieli A et al (2015) Immunomodulatory effects of Lactobacillus salivarius LS01 and Bifidobacterium breve BR03, alone and in combination, on peripheral blood mononuclear cells of allergic asthmatics. Allergy Asthma Immunol Res 7(4):409–413
Drago L, Gismondo MR, Lombardi A et al (1997) Inhibition of in vitro growth of enteropathogens by new Lactobacillus isolates of human intestinal origin. FEMS Microbiol Lett 153(2):455–463
Ibarra A, Latreille-Barbier M, Donazzolo Y et al (2018) Effects of 28-day Bifidobacterium animalis subsp. lactis HN019 supplementation on colonic transit time and gastrointestinal symptoms in adults with functional constipation: a double-blind, randomized, placebo-controlled, and dose-ranging trial. Gut Microbes 9(3):236–51
Wang R, Sun J, Li G et al (2021) Effect of Bifidobacterium animalis subsp. lactis MN-Gup on constipation and the composition of gut microbiota. Benefic Microbes 12(1):31–42
De Paula JA, Carmuega E, Weill R (2008) Effect of the ingestion of a symbiotic yogurt on the bowel habits of women with functional constipation. Acta Gastroenterol Latinoam 38(1):16–25
Hyams JS, Di Lorenzo C, Saps M et al (2016) Functional disorders: children and adolescents. Gastroenterology S0016-5085(16):00181–00185
Mulhem E, Khondoker F, Kandiah S (2022) Constipation in children and adolescents: evaluation and treatment. Am Fam Physician 105(5):469–478
Tabbers MM, Dilorenzo C, Berger MY et al (2014) Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr 58(2):258–274
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10(10):996–998
Kuczynski J, Stombaugh J, Walters WA et al (2012) Using QIIME to analyze 16S rRNA gene sequences from microbial communities. Curr Protoc Microbiol Chapter 1:Unit 1E.5
Cole JR, Tiedje JM (2014) History and impact of RDP: a legacy from Carl Woese to microbiology. RNA Biol 11(3):239–243
Langille MG, Zaneveld J, Caporaso JG et al (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31(9):814–821
Wang N, Wang S, Xu B et al (2021) Alleviation effects of Bifidobacterium animalis subsp. lactis XLTG11 on dextran sulfate sodium-induced colitis in mice. Microorganisms 9(10):2093
Xu B, Liang S, Zhao J et al (2022) subsp XLTG11 improves antibiotic-related diarrhea by alleviating inflammation, enhancing intestinal barrier function and regulating intestinal flora. Food Funct 13(11):6404–18
Ma W, Li W, Ma Q et al (2022) Effects of Bifidobacterium lactis XLTG11 on immune function in mice. Food Fermentation Ind 48(13):103–107
Du Juan Xu, Li M-N et al (2021) Effects of Bifidobacterium lactis Bb12 in the adjuvant treatment of functional constipation in children. Mil Med J Southeast China 23(06):610–613
Southwell BR (2020) Treatment of childhood constipation: a synthesis of systematic reviews and meta-analyses. Expert Rev Gastroenterol Hepatol 14(3):163–174
Wang J, Chen T, Li F et al (2014) Efficacy of probiotic supplementation for treatment of functional constipation in children and adolescents: Meta-analysis. J Bio- education 2(03):174–179
Nocerino R, De Filippis F, Cecere G et al (2020) The therapeutic efficacy of Bifidobacterium animalis subsp. lactis BB-12 in infant colic: a randomised, double blind, placebo-controlled trial. Aliment Pharmacol Ther 51(1):110–20
Merenstein D, Fraser CM, Roberts RF et al (2021) subsp. BB-12 protects against antibiotic-induced functional and compositional changes in human fecal microbiome. Nutrients 13(8):2814
Liu A, Ma T, Xu N et al (2021) Adjunctive probiotics alleviates asthmatic symptoms via modulating the gut microbiome and serum metabolome. Microbiol Spectr 9(2):e0085921
Grubb DS, Wrigley SD, Freedman KE et al (2020) PHAGE-2 study: supplemental bacteriophages extend subsp. BL04 benefits on gut health and microbiota in healthy adults. Nutrients 12(8):2474
Zhang S, Wang R, Li D et al (2021) Role of gut microbiota in functional constipation. Gastroenterol Rep 9(5):392–401
Cheng J, Laitila A, Ouwehand AC (2021) Bifidobacterium animalis subsp lactis HN019 effects on gut health: a review. Front Nutr 8:790561
Rajagopala SV, Titz B, Goll J et al (2007) The protein network of bacterial motility. Mol Syst Biol 3:128
Al-Hebshi NN, Nasher AT, Maryoud MY et al (2017) Inflammatory bacteriome featuring Fusobacterium nucleatum and Pseudomonas aeruginosa identified in association with oral squamous cell carcinoma. Sci Rep 7(1):1834
Bin Waqar SH, Rehan A (2019) Methane and constipation-predominant irritable bowel syndrome: entwining pillars of emerging neurogastroenterology. Cureus 11(5):e4764
Acknowledgements
We thank all the parents or main caregivers and their children for their participation in the study. The healthcare workers in the field trial whose names are not mentioned are thanked for their diligent assistance. We also thank Mrs. Meiru Chen and Ms. Youxi Chen who have provided enthusiastic help for the operation of the study.
Funding
This study was supported by Dipro Medical Research Fund (DiPRO/2021/11/H311) and Scientific Research Project of Sichuan Maternal and Child Health Care Association (22FXYB15).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Roxane M Piazza
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, K., Zhou, Z., Nie, Y. et al. Adjunctive efficacy of Bifidobacterium animalis subsp. lactis XLTG11 for functional constipation in children. Braz J Microbiol 55, 1317–1330 (2024). https://doi.org/10.1007/s42770-024-01276-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-024-01276-3